Академический Документы
Профессиональный Документы
Культура Документы
Reaction Kinetics
Загружено:
dimasaditya28Оригинальное название
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
Reaction Kinetics
Загружено:
dimasaditya28Авторское право:
Доступные форматы
1
Kinetika Reaksi
2
Kinetika Reaksi
) ( ) (
2
3
) (
2
1
3 2 2
g NH g H g N +
Contoh
) ( ) (
2
1
) (
2 2 2
l O H g O g H +
1
/
A mol kJ G
o
r
-16.63
-237.19
Termodinamik: energi dan spontanitas reaksi
Termodinamik: tidak dapat menjawab (1) bagaimana reaksi
berlangsung (2) berapa cepat reaksi berlangsung (3) mekanisme
reaksi
Reaction
Kinetics
3
Kinetika Reaksi
) ( ) (
2
3
) (
2
1
3 2 2
g NH g H g N +
) ( ) (
2
1
) (
2 2 2
l O H g O g H +
T, P, katalis
T, katalis
Kinetika kimia:
Kinetika kimia
Mempelajari laju dan mekanisme reaksi dari reaksi kimia
Reaction
Kinetics
4
Laju reaksi
Reaksi homogen:
b
a
dt dn
dt dn
B
A
=
/
/
+ + + + fF eE bB aA
Laju konversi
dt
dn
f dt
dn
e dt
dn
b dt
dn
a
J
F E B A
1 1 1 1
= = = =
dt
dn
b dt
dn
a
B A
1 1
=
Reaction
Kinetics
5
Laju Reaksi
|
.
|
\
|
=
dt
dn
a V V
J
r
A
1 1
= = = = = =
dt
F d
f dt
E d
e dt
B d
b dt
A d
a
r
] [ 1 ] [ 1 ] [ 1 ] [ 1
Laju reaksi, r
mol dm
-3
s
-1
mol cm
-3
s
-1
Reaction
Kinetics
6
Hukum Laju
Untuk beberapa
reaksi
| | | | | |
| o
L B A k r =
k: konstanta laju, k = f (T, P)
o, |, .: orde reaksi
o+| + . + n : orde keseluruhan
Reaction
Kinetics
7
Orde Reaksi
o
k r =
| | A k r =
| |
2
A k r =
| || | B A k r =
| | | | B A k r
2
=
| || |
2
B A k r =
| || |
2
= B A k r
| || |
2 / 1
B A k r =
| || | | | ( )
2 / 1
1 / B B A k r =
Zero-order
First-order
Second-order
Third-order
Negative First-order
1.5-order
No simple order
Reaction
Kinetics
8
Hukum laju
Reaksi Hukum Laju
HBr Br H 2
2 2
+
| || |
| | | |
2
2 / 1
2 2
/ 1 Br HBr j
Br H k
r
+
=
2 2 5 2
4 2 O NO O N +
HI I H 2
2 2
+ | || |
2 2
I H k r =
| |
5 2
O N k r =
CO CH CHO CH +
4 3
| |
2 / 3
3
CHO CH k r =
Reaction
Kinetics
9
Mekanisme Reaksi
3 2 2
SO NO SO NO + +
2 2
2 2 NO NO O +
3 2 2
2 2 SO O SO
NO
+
intermediate
2 2 5 2
4 2 O NO O N +
3 2 5 2
NO NO O N +
2 3
2NO NO NO +
2 2 3 2
NO O NO NO NO + + +
Tahap (a)
Tahap(b)
Tahap (c)
Elementary
reaction
Reaction
Kinetics
10
Pengukuran Laju Reaksi
Metode Kimia
The concentration of a reactant or product as a function of time
Reaction vessels
At constant T
At intervals
Slows down or stop
the reaction
Rapidly analyze chemical
compositions of the mixture
Cooling the sample
removing a catalyst
Diluting the mixture
Adding a species
Reaction
Kinetics
11
Metode Fisika
Measures a physical property of the reaction system as a
function of time. This allows the reaction to be followed
continuously as it proceeds.
More accurate and less tedious
Reaction
Kinetics
12
Integrasi Laju Reaksi
Assumptions
(a) The reaction is carried out at constant temperature (k is constant)
(b) The volume is constant (r is given by (17.4))
(c) The reaction is irreversible (k
o
is very large)
= = = = = =
dt
F d
f dt
E d
e dt
B d
b dt
A d
a
r
] [ 1 ] [ 1 ] [ 1 ] [ 1
Integrate it to find [A] as a function of time: [A] = g(t)
Reaction
Kinetics
13
Reaksi Orde Satu
Suppose the reaction
P aA
is first-order with
| | A k r =
= = = = = =
dt
F d
f dt
E d
e dt
B d
b dt
A d
a
r
] [ 1 ] [ 1 ] [ 1 ] [ 1
| | | | | |
| o
L B A k r =
| |
| | A k
dt
A d
a
r = =
1
define
ak k
A
| | | | A k dt A d
A
= /
| | | | dt k A A d
A
= /
| | | |
}
=
}
2
1
2
1
/ dt k A A d
A
Reaction
Kinetics
14
| | | |
}
=
}
2
1
2
1
/ dt k A A d
A
| | | | ( ) ) ( / ln
1 2
1 2
t t k A A
A
=
| |
| |
t k
A
A
A
o
= ln
if
t t =
2
| | | |
o
A A =
1
at
0
1
= t | | | | A A =
2
at and
| | | |
t k
o
A
e A A
=
The concentration of A (and r) decreases exponentially with
time for a first-order reaction.
| |
| | A k
dt
A d
a
r = =
1
Integrated rate law
Reaction
Kinetics
15
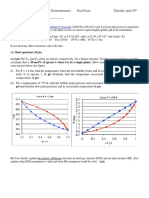
First-order reaction: ln[A] vs t
| | | |
t k
o
A
e A A
=
| | | | t k A A
A
o
= ln ln
[
A
]
/
[
A
]
o
t
First-order reaction: [A]/[A]
o
vs t
| |
| |
t k
o
A
e
A
A
=
L
n
[
A
]
t
| | | | t k A A
A
o
= / ln
Reaction
Kinetics
16
2 / 1
t
Half-life:
2 / 1
t t =
o
A A ] [ 2 / 1 ] [ =
| |
| |
t k
A
A
A
o
= ln
2 / 1
] [
] [ 2 / 1
ln t k
A
A
A
o
o
=
693 . 0 2 ln
2 / 1
= = t k
A
First-order reaction
2 / 1
2 / 1 ln t k
A
=
The time needed for [A] to drop to half its value
A
k t / 693 . 0
2 / 1
=
Independent of [A]
o
A useful indication for the chemical reaction rate
Reaction
Kinetics
17
Second-Order Reactions
2
2
2
] [A P 2A ) 2 (
A][B] [ P B A ) 1 (
k r
k r
=
= +
| |
| |
2
1
A k
dt
A d
a
r = =
define
ak k
A
| | | |
2
/ A k dt A d
A
=
| |
| |
2
A k
dt
A d
A
=
| |
| |
}
=
}
2
1
2
1
2
1
dt k A d
A
A
Reaction
Kinetics
18
Second-Order Reactions
| | | |
) (
1 1
1 2
2 1
t t k
A A
A
=
A plot of 1/[A] versus t gives a strait line of slope k
A
if
ak k
A
| |
| |
| |
o
A
o
A t k
A
A
+
=
1
| |
| |
}
=
}
2
1
2
1
2
1
dt k A d
A
A
| | | |
t k
A A
A
o
=
1 1
| |
2
A k r =
Reaction
Kinetics
19
{[A]
-1
}
{t}
Second-order reaction: 1/[A] vs t
Second-Order Reactions
| | | |
t k
A A
A
o
=
1 1
| | | |
t k
A A
A
o
+ =
1 1
2 / 1
t
Half-life:
2 / 1
t t =
o
A A ] [ 2 / 1 ] [ =
A o
k A
t
] [
1
2 / 1
=
Second-order reaction with
r = k[A]
2
Reaction
Kinetics
20
Second-Order Reactions
A o
k A
t
] [
1
2 / 1
=
Second-order reaction with r = k[A]
2
A
k
t
2 ln
2 / 1
=
First-order reaction with r = k[A]
products bB aA +
| || | B A k r =
| |
| || | B A k
dt
A d
a
=
1
Three variables
Reaction
Kinetics
21
Second-Order Reactions
products B A +
0 = t
| |
| || | B A k
dt
A d
=
x=0 when t=0
| |
o
A | |
o
B
t t = | | x A
o
| | x B
o
0
x
| |
| || | | | ( ) | | ( ) x B x A k B A k
dt
A d
o o
= =
| |
dt
dx
dt
A d
=
| | ( ) | | ( ) x B x A k
dt
dx
o o
=
| | ( ) | | ( )
kdt
x B x A
dx
o o
=
| | ( ) | | ( )
}
=
}
t x
o o
kdt
x B x A
dx
0 0
Reaction
Kinetics
22
Second-Order Reactions
| | ( ) | | ( )
kt kdt
x B x A
dx
t x
o o
=
}
=
}
0 0
| | ( ) | | ( ) | | | | | | ( ) | | ( )
dx
x B x A A B x B x A
dx
x
o o o o
x
o o
}
)
`
=
}
0 0
1 1 1
| | | |
| |
| |
| |
| |
)
|
|
.
|
\
|
|
|
.
|
\
|
=
x B
B
x A
A
A B
o
o
o
o
o o
ln ln
1
| | | |
| |
| |
| |
| |
kt
x B
B
x A
A
A B
o
o
o
o
o o
=
|
|
.
|
\
|
|
|
.
|
\
|
ln ln
1
| | | |
| | | |
| | | |
kt
A A
B B
A B
o
o
o o
=
|
|
.
|
\
|
/
/
ln
1
| | | | x A A
o
=
| | | | x B B
o
=
Reaction
Kinetics
23
Second-Order Reactions
| | ( ) | | ( )
| | ( )
kt kdt
x A
dx
x B x A
dx
t x
o
x
o o
=
}
=
}
=
}
0 0
2
0
| | ( )
| | | |
kt
A x A
x A
dx
o o
x
o
=
=
}
1 1
0
2
If [A]
o
=[B]
o
| | | |
kt
A A
o
=
1 1
2 / 1
t
Half-life:
2 / 1
t t =
o
A A ] [ 2 / 1 ] [ =
| |
o
A k
t
1
2 / 1
=
Reaction
Kinetics
24
Third-Order Reactions
| |
| |
3
A ak
dt
A d
=
| |
| |
dt k
A
A d
A
=
3
| | | |
t k
A A
A
o
2
1 1
2 2
=
| |
| | | | B A ak
dt
A d
2
=
| |
| || |
2
B A ak
dt
A d
=
| |
| || || | C B A ak
dt
A d
=
(Problems 17.17 and 17.24)
| |
| |
| | ( )
2 / 1
2
2 1
o
o
A kt
A
A
+
=
Reaction
Kinetics
25
nth-Order Reaction
Consider
| |
1
) 1 (
n
o
A n
| |
| |
n
A
A k
dt
A d
=
Integration gives
| |
| |
}
=
}
2
1
2
1
dt k
A
A d
A
n
| | | |
t k
n
A A
A
n
o
n
=
+
+ +
1
1 1
For n = 1
| |
| |
| | t k n A
A
A
A
n
o
n
o
) 1 ( 1
1
1
+ =
|
|
.
|
\
|
For n = 1
Reaction
Kinetics
26
nth-Order Reaction
| |
A
n
o
n
k A n
t
1
1
2 / 1
) 1 (
1 2
=
For n = 1
| | | |
t k
o
A
e A A
= For n = 1
2 / 1
t
Half-life:
2 / 1
t t =
o
A A ] [ 2 / 1 ] [ =
A
k
t
2 ln
2 / 1
=
| |
| |
}
=
}
2
1
2
1
dt k
A
A d
A
n
| | | | t k A A
A o
=
| |
A
o
k
A
t
2
2 / 1
=
For n = 0
Reaction
Kinetics
Вам также может понравиться
- Chemical Reaction Kinetics: Concepts, Methods and Case StudiesОт EverandChemical Reaction Kinetics: Concepts, Methods and Case StudiesОценок пока нет
- Chemical Kinetics: Peter Atkins, Physical Chemistry, 7 EditionДокумент38 страницChemical Kinetics: Peter Atkins, Physical Chemistry, 7 EditionalchemiyОценок пока нет
- Ramon Lu Gerico Alforja Lorenzo AramilДокумент55 страницRamon Lu Gerico Alforja Lorenzo Aramilramonlu05Оценок пока нет
- Chemical Kinetics1Документ59 страницChemical Kinetics1farooq_bagbanОценок пока нет
- X. Reactions x.1 Order of Reactions x.1.01 Zero Order ReactionsДокумент28 страницX. Reactions x.1 Order of Reactions x.1.01 Zero Order ReactionsJon Bisu DebnathОценок пока нет
- Physical Chemistry - KineticsДокумент66 страницPhysical Chemistry - KineticsarieleliannasternОценок пока нет
- Week 2. Chemical Kinetics Analysis of Rate EquationДокумент31 страницаWeek 2. Chemical Kinetics Analysis of Rate EquationYuni ApriyaniОценок пока нет
- 2.kinetics Homogenous ReactionsДокумент33 страницы2.kinetics Homogenous ReactionsArief Al Imam HidayatullahОценок пока нет
- Chapter 2-Mass Reactor Model (102 P)Документ102 страницыChapter 2-Mass Reactor Model (102 P)shardulkaviОценок пока нет
- Reaction KineticsДокумент37 страницReaction KineticsNurshuhada NordinОценок пока нет
- Kinetics: Reaction Rates, Orders, Half Lives: Aa + BB CC + DDДокумент8 страницKinetics: Reaction Rates, Orders, Half Lives: Aa + BB CC + DDsgyblee100% (1)
- Solutions To Home Work Test/Chemistry: Chemical Equilibrium HWT - 1Документ5 страницSolutions To Home Work Test/Chemistry: Chemical Equilibrium HWT - 1varunkohliinОценок пока нет
- Unit 15 Kinetics: ChemicalДокумент75 страницUnit 15 Kinetics: Chemicalpetercyh175Оценок пока нет
- Content Marketed & Distributed By: Chemical KineticsДокумент9 страницContent Marketed & Distributed By: Chemical KineticsPrithviraj NetkeОценок пока нет
- Chemical Kinetics: Describing The Rate of A ReactionДокумент29 страницChemical Kinetics: Describing The Rate of A ReactionIrfan ShafiqОценок пока нет
- Condensation: - in The Continuum Regime, Diffusion Theory Is Used. at Steady StateДокумент28 страницCondensation: - in The Continuum Regime, Diffusion Theory Is Used. at Steady StateJorn DoeОценок пока нет
- FORMULARIO 2012icДокумент2 страницыFORMULARIO 2012icWildor Cordova SanchezОценок пока нет
- Chemical Kinetics: Chung (Peter) Chieh Professor of Chemistry University of Waterloo Waterloo, Ontario, CanadaДокумент34 страницыChemical Kinetics: Chung (Peter) Chieh Professor of Chemistry University of Waterloo Waterloo, Ontario, Canadadescar84Оценок пока нет
- Chem Chapt13 PractiseДокумент5 страницChem Chapt13 PractiseqwerОценок пока нет
- Lec 16Документ28 страницLec 16Khai HuynhОценок пока нет
- Cre Ii - 28Документ37 страницCre Ii - 28Mehul VarshneyОценок пока нет
- Chemical Equilibrium: Ideal GasesДокумент6 страницChemical Equilibrium: Ideal GasessgybleeОценок пока нет
- L - 21 External Mass Transfer Effects: Prof. K.K.Pant Department of Chemical Engineering IIT DelhiДокумент57 страницL - 21 External Mass Transfer Effects: Prof. K.K.Pant Department of Chemical Engineering IIT DelhiMehul VarshneyОценок пока нет
- Heat and Mass Transfer ResistancesДокумент51 страницаHeat and Mass Transfer ResistancesSidОценок пока нет
- Chemistry 18 NotesДокумент63 страницыChemistry 18 NotesJara RogacionОценок пока нет
- Harmonically Excited VibrationДокумент61 страницаHarmonically Excited VibrationSiddharth MohanОценок пока нет
- RT Solutions-08!05!2011 XII ABCD Paper II Code AДокумент13 страницRT Solutions-08!05!2011 XII ABCD Paper II Code Avishal27042233Оценок пока нет
- ChE441 Analysis of Rate Data-1Документ28 страницChE441 Analysis of Rate Data-1Xnd3RОценок пока нет
- Physical Chemistry I For Biochemists: Chem340 Chem340 Lecture 13 (2/11/11)Документ5 страницPhysical Chemistry I For Biochemists: Chem340 Chem340 Lecture 13 (2/11/11)Dulce Wendolyn BollásОценок пока нет
- Jam 2Документ17 страницJam 2Adam AbuОценок пока нет
- Chemical Kinetics Type 1Документ32 страницыChemical Kinetics Type 1Sudhakar ChollangiОценок пока нет
- Sinusoidal Response.: S A S XДокумент15 страницSinusoidal Response.: S A S XManidhar ThulaОценок пока нет
- Chemical Reaction Engineering (CRE)Документ29 страницChemical Reaction Engineering (CRE)Phuong PhamОценок пока нет
- SCH 305Документ34 страницыSCH 305Qwin NajyaОценок пока нет
- Chemical Kinetics Part IДокумент45 страницChemical Kinetics Part IKarthikanAmirthalingamОценок пока нет
- Chemical KineticsДокумент7 страницChemical Kineticsthinkiit100% (1)
- EquationsДокумент5 страницEquationsBernadette Bithao AnonoyОценок пока нет
- Non-Linear Methods 4.1. Asymptotic Analysis: 4.1.2. Stochastic RegressorsДокумент73 страницыNon-Linear Methods 4.1. Asymptotic Analysis: 4.1.2. Stochastic RegressorsDОценок пока нет
- Kinetika KimiaДокумент39 страницKinetika KimiaMia YukimuraОценок пока нет
- 13 KineticsДокумент43 страницы13 KineticsharriolaОценок пока нет
- 6 - Chemical Kinetics PDFДокумент16 страниц6 - Chemical Kinetics PDFthinkiit100% (1)
- Basic Differentiation RulesДокумент3 страницыBasic Differentiation RulesHannah VisitacionОценок пока нет
- Communication IITN Review1Документ28 страницCommunication IITN Review1Pankaj MeenaОценок пока нет
- Instantaneous Frac. Yield Overall Fractional Yield: Max. Mix. ModelДокумент2 страницыInstantaneous Frac. Yield Overall Fractional Yield: Max. Mix. ModelElena TodorovskaОценок пока нет
- Circular FunctionsДокумент44 страницыCircular Functionsrica_marquezОценок пока нет
- Physics Formula UtpДокумент4 страницыPhysics Formula UtpRubin TasnimОценок пока нет
- Nonideal Behavior: ITK-234 Termodinamika Teknik Kimia IIДокумент65 страницNonideal Behavior: ITK-234 Termodinamika Teknik Kimia IIAdhiaRieyanasariОценок пока нет
- Term Odin A MikaДокумент27 страницTerm Odin A MikaAnonymous GTCOMvОценок пока нет
- IIT JEE Main Advanced Physical Chemistry 12th Chemical KineticsДокумент44 страницыIIT JEE Main Advanced Physical Chemistry 12th Chemical KineticsT sidharth100% (3)
- Transform Analysis of LTI SystemsДокумент117 страницTransform Analysis of LTI SystemsreneeshczОценок пока нет
- 2 Plug Flow Reactor AdiabaticДокумент33 страницы2 Plug Flow Reactor Adiabaticbian_cool88Оценок пока нет
- Due Mar 7Документ14 страницDue Mar 7Keith Joseph JrОценок пока нет
- CBE3508 Sp21 FinalДокумент6 страницCBE3508 Sp21 Finalsasuke uchihaОценок пока нет
- Fundamentals of Electronics 3: Discrete-time Signals and Systems, and Quantized Level SystemsОт EverandFundamentals of Electronics 3: Discrete-time Signals and Systems, and Quantized Level SystemsОценок пока нет
- Ten-Decimal Tables of the Logarithms of Complex Numbers and for the Transformation from Cartesian to Polar Coordinates: Volume 33 in Mathematical Tables SeriesОт EverandTen-Decimal Tables of the Logarithms of Complex Numbers and for the Transformation from Cartesian to Polar Coordinates: Volume 33 in Mathematical Tables SeriesОценок пока нет
- Computational Methods in Lanthanide and Actinide ChemistryОт EverandComputational Methods in Lanthanide and Actinide ChemistryMichael DolgОценок пока нет
- Logical progression of twelve double binary tables of physical-mathematical elements correlated with scientific-philosophical as well as metaphysical key concepts evidencing the dually four-dimensional basic structure of the universeОт EverandLogical progression of twelve double binary tables of physical-mathematical elements correlated with scientific-philosophical as well as metaphysical key concepts evidencing the dually four-dimensional basic structure of the universeОценок пока нет
- Kinetics FR WorksheetДокумент10 страницKinetics FR WorksheetCynОценок пока нет
- Attainable RegionДокумент23 страницыAttainable RegionSaurabh SumanОценок пока нет
- Mechanisms of Antioxidant Action of Phosphite and Phosphonite EstersДокумент38 страницMechanisms of Antioxidant Action of Phosphite and Phosphonite EstersDuy Khánh ĐỗОценок пока нет
- Atomic StructureДокумент7 страницAtomic Structureneetisharma2010Оценок пока нет
- Modeling and Simulation of CSTR For Manufacture of Propylene GlycolДокумент6 страницModeling and Simulation of CSTR For Manufacture of Propylene Glycolantoojacome100% (1)
- STPM 962/2: (40 Marks) Answer All QuestionsДокумент7 страницSTPM 962/2: (40 Marks) Answer All QuestionsLim Tze ChuenОценок пока нет
- Cell Banks ModelДокумент20 страницCell Banks ModelCristian CaamañoОценок пока нет
- First-Order Kinetics: Whose I.E. Greater The Concentration, Faster The ReactionДокумент12 страницFirst-Order Kinetics: Whose I.E. Greater The Concentration, Faster The ReactionPrashant307Оценок пока нет
- CHEM 26.1 ReviewerДокумент6 страницCHEM 26.1 ReviewerClara MirabuenoОценок пока нет
- Reaction Rate: A+B ABДокумент5 страницReaction Rate: A+B ABFaisal Mohad Al SakhenОценок пока нет
- CHE171 - Kinetics 1Документ1 страницаCHE171 - Kinetics 1JannineОценок пока нет
- PREBOARD Class 12 CHEMISTRY 2022Документ4 страницыPREBOARD Class 12 CHEMISTRY 2022Parth SharmaОценок пока нет
- 9701 s10 QP 42Документ20 страниц9701 s10 QP 42Hubbak Khan100% (1)
- Quiz 12Документ5 страницQuiz 12Hằng ThanhОценок пока нет
- Kinetics of Liquid - Phase Hydrogenation of DiolefinДокумент17 страницKinetics of Liquid - Phase Hydrogenation of DiolefinSoroush KaramianОценок пока нет
- FarmakokinetikaДокумент142 страницыFarmakokinetikaAstrid Bernadette Ulina PurbaОценок пока нет
- Board Que 2023Документ19 страницBoard Que 2023HeerОценок пока нет
- PDF Langford Gray PDFДокумент54 страницыPDF Langford Gray PDFangeklicaОценок пока нет
- Question Paper Class 12 Set 1Документ4 страницыQuestion Paper Class 12 Set 1praveen bhatiОценок пока нет
- Chemical Kinetics Part 1Документ35 страницChemical Kinetics Part 1RRОценок пока нет
- Kinetics and Mechanism of Iodide Oxidation by Iron (III), A Clock Reaction Approach PDFДокумент3 страницыKinetics and Mechanism of Iodide Oxidation by Iron (III), A Clock Reaction Approach PDFJeffersonVillegasОценок пока нет
- Gec 534Документ154 страницыGec 534Daniel OmolewaОценок пока нет
- Reaction Rates C12-3-01-03Документ6 страницReaction Rates C12-3-01-03kerriena mcdonaldОценок пока нет
- 1.0 Reaction Kinetic 22 - 23 (REVIEWED)Документ115 страниц1.0 Reaction Kinetic 22 - 23 (REVIEWED)alyaainsyirah04Оценок пока нет
- CHNG 3004 - 2019-2020 AssignmentsДокумент26 страницCHNG 3004 - 2019-2020 AssignmentsXheikhKaleem100% (1)
- Chapter Eiaght - Chemical EquiДокумент33 страницыChapter Eiaght - Chemical EquiAhmed Saeed100% (2)
- Practice Test 7Документ65 страницPractice Test 7The LightОценок пока нет
- Chemistry - Paper - 1 - TZ1 - HL 2Документ97 страницChemistry - Paper - 1 - TZ1 - HL 2pablinsky05Оценок пока нет