Академический Документы
Профессиональный Документы
Культура Документы
Kunci Jawaban (xx47xx) PDF
Загружено:
Sana YabadaОригинальное название
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
Kunci Jawaban (xx47xx) PDF
Загружено:
Sana YabadaАвторское право:
Доступные форматы
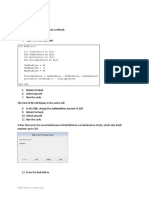
By : hendrik -_1. b.
7 carbon chain, ketone function group in C3 so the IUPAC Name is 3-heptanone
d. there is a double bond in C2 and C3, the subtituent in C2 is H (top) and C in CH3 (bottom), the priority of subtituents are I, Cl, Br, F, O, N, C, H, so, the priority in C2 is top. The subtituent in C3 is C4H9 (top) and CH3 (bottom), C4H9 is a priority subtituent because the second bond is C whereas CH3 second bond is H, so the priority in C3 is bottom. Because the priority isnt at the same side, hte isomer is Entgegen (E) so the name of the molecule is (E) 3-methyl-2-heptene e. the function group of the molecule is ester, (alkyl alcanoate) alkyl is a naming for subtituent beside O. So the name is 1-ethenyl ethanoate or 1-vinyl ethanoate or 1-vinyl acetate f. the function group is ketone, we must naming the complex subtituent first. The cyclohexene subtituent name is cyclohexa-1,3-dienyl. The carbon chain is 7, and we are naming from the nearest end of function group (C2), so the double bounds are in C3 and C5. So the name is 7(cyclohexa-1,3-dienyl)-hepta-3,5-dien-2-one
2. look at the double bond carbon C1 and C2, that is SP2 Hybridization, because there are 3 Hydrogen atom, it must lie in the same plane to be stable, so the atom is C, C, H, H, H, O 3. there are 5 double bond in molecule (f), the phi electron exist in the top and bottom of the double bonds, so, the electron phi total is 5x2=10 electron phi 4. a. the Central Carbons is carbon with the most subtituents, so the hibridization is SP3 and the bond is 109,28 because it is tetrahedral b. hibridization of carbon and oxygen atoms in molecule *(e) : C1 : SP2 C2 : SP2 O : SP3 C3 : SP2 C4 : SP3 O2 (the double bond O) : SP2
By : hendrik -_5. Formal charge in Cation *(i) and carbanion *(j) :
H H N
+
+
H
..
So, all the H is 1 0 (2) = 0 N is 5 0 (8) = +1 C is 4 2 (6) = -1
6. the most stable carbocation is molecule (a) because it is tertiary and allylic. 7. a.
b. the mayor product is 3-bromo-2,2 dimethylbutane
By : hendrik -_8.
Вам также может понравиться
- The Yellow House: A Memoir (2019 National Book Award Winner)От EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Рейтинг: 4 из 5 звезд4/5 (98)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeОт EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeРейтинг: 4 из 5 звезд4/5 (5795)
- 2spheres1 0Документ2 страницы2spheres1 0Sana YabadaОценок пока нет
- Code Snippets: in The Table That Follows You Will Find VBA Code SnippetsДокумент3 страницыCode Snippets: in The Table That Follows You Will Find VBA Code SnippetsSana YabadaОценок пока нет
- Add User CommentДокумент3 страницыAdd User CommentSana YabadaОценок пока нет
- The Centre For Education in Mathematics and Computing: Cemc - Uwaterloo.caДокумент4 страницыThe Centre For Education in Mathematics and Computing: Cemc - Uwaterloo.caSana YabadaОценок пока нет
- Form Controls: Control Name DescriptionДокумент1 страницаForm Controls: Control Name DescriptionSana YabadaОценок пока нет
- Using Byte VariablesДокумент1 страницаUsing Byte VariablesSana YabadaОценок пока нет
- Accessing Macros Through Shapes, or ImagesДокумент1 страницаAccessing Macros Through Shapes, or ImagesSana YabadaОценок пока нет
- These Questions May Be Suitable For Students Who Are Between 2 To 4 Years Away From Going To UniversityДокумент1 страницаThese Questions May Be Suitable For Students Who Are Between 2 To 4 Years Away From Going To UniversitySana YabadaОценок пока нет
- StatДокумент2 страницыStatSana YabadaОценок пока нет
- Test Your Skills - User InteractionsДокумент3 страницыTest Your Skills - User InteractionsSana YabadaОценок пока нет
- 002-Denah Lakespra STSДокумент1 страница002-Denah Lakespra STSSana YabadaОценок пока нет
- Using Date Variables: 1. Type The Following CodeДокумент1 страницаUsing Date Variables: 1. Type The Following CodeSana YabadaОценок пока нет
- TEST 0 (Diagnosis Exam) Basic Basic Basic Algebra For Life Duration: 3600 SecondsДокумент2 страницыTEST 0 (Diagnosis Exam) Basic Basic Basic Algebra For Life Duration: 3600 SecondsSana YabadaОценок пока нет
- Geometry 5 - Congruent Triangles - 1Документ3 страницыGeometry 5 - Congruent Triangles - 1Sana Yabada100% (1)
- (H) Is A Special Question. Do Not Worry If You Cannot AnswerДокумент4 страницы(H) Is A Special Question. Do Not Worry If You Cannot AnswerSana YabadaОценок пока нет
- PART I Arithmetic Sequence and SeriesДокумент2 страницыPART I Arithmetic Sequence and SeriesSana YabadaОценок пока нет
- H N Yields NHДокумент6 страницH N Yields NHSana YabadaОценок пока нет
- P&IDДокумент3 страницыP&IDSana YabadaОценок пока нет
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureОт EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureРейтинг: 4.5 из 5 звезд4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryОт EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryРейтинг: 3.5 из 5 звезд3.5/5 (231)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceОт EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceРейтинг: 4 из 5 звезд4/5 (895)
- Never Split the Difference: Negotiating As If Your Life Depended On ItОт EverandNever Split the Difference: Negotiating As If Your Life Depended On ItРейтинг: 4.5 из 5 звезд4.5/5 (838)
- The Little Book of Hygge: Danish Secrets to Happy LivingОт EverandThe Little Book of Hygge: Danish Secrets to Happy LivingРейтинг: 3.5 из 5 звезд3.5/5 (400)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersОт EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersРейтинг: 4.5 из 5 звезд4.5/5 (345)
- The Unwinding: An Inner History of the New AmericaОт EverandThe Unwinding: An Inner History of the New AmericaРейтинг: 4 из 5 звезд4/5 (45)
- Team of Rivals: The Political Genius of Abraham LincolnОт EverandTeam of Rivals: The Political Genius of Abraham LincolnРейтинг: 4.5 из 5 звезд4.5/5 (234)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyОт EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyРейтинг: 3.5 из 5 звезд3.5/5 (2259)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaОт EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaРейтинг: 4.5 из 5 звезд4.5/5 (266)
- The Emperor of All Maladies: A Biography of CancerОт EverandThe Emperor of All Maladies: A Biography of CancerРейтинг: 4.5 из 5 звезд4.5/5 (271)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreОт EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreРейтинг: 4 из 5 звезд4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)От EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Рейтинг: 4.5 из 5 звезд4.5/5 (121)