Академический Документы
Профессиональный Документы
Культура Документы
Water
Загружено:
nofacejackИсходное описание:
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
Water
Загружено:
nofacejackАвторское право:
Доступные форматы
WATER
a medium in which biochemical reactions can go on for transporting substances in and out of cells for maintaining temperature for producing digestive fluids and secretions solvent for excreted waste products
Properties
geometry bent polar high heat capacity and heat of vaporization for maintaining body high dielectric constant supports existence of an ionic environment
in the cell electrical conductivity for transmission of nerve impulses temperature
solvent properties forms H bonds interactions with solutes also involve ion dipole and dipole dipole
bonds
self dissociation
BUFFERS
Major intercellular buffer: H2PO4 - - HPO42Major extracellular buffer: H2CO3 HCO3 Importance of buffers in biology
ability to prevent large changes in pH (important property of most cytoplasmic fluids containing dissolved proteins, organic substances
intact biological organisms) and inorganic salts excessive changes in pH blood plasma is a highly effective buffer solution (ideally designed to keep the pH of blood at 7.2 to 7.3; values outside this range are not compatible with life) many of the metabolites being constantly produced and utilized in the cell are weak Bronsted acids enzymes responsible for the catalysis of reactions in which these metabolites participate exhibit their maximum catalytic action at some definite pH
Вам также может понравиться
- 14 April IntroductionДокумент15 страниц14 April IntroductionRuth Danielle GasconОценок пока нет
- BIT18R432Документ56 страницBIT18R432Dinesh JeyakumarОценок пока нет
- Biochemistrylecture1 120615032009 Phpapp01Документ56 страницBiochemistrylecture1 120615032009 Phpapp01Biyaya San PedroОценок пока нет
- Microbial Nutrition and Growth: - Predictive MicrobiologyДокумент23 страницыMicrobial Nutrition and Growth: - Predictive MicrobiologyNivashini VindhyaОценок пока нет
- Chap I, Part 3Документ54 страницыChap I, Part 3angel roseОценок пока нет
- 1 - Foundations of Biochemistry: © 2013 W. H. Freeman and CompanyДокумент63 страницы1 - Foundations of Biochemistry: © 2013 W. H. Freeman and CompanyMoo NaaОценок пока нет
- 1.water, Acids, Bases, Buffer Solutions in BiochemistryДокумент53 страницы1.water, Acids, Bases, Buffer Solutions in BiochemistryÇağlaОценок пока нет
- Pharmaceutical BiochemistryДокумент7 страницPharmaceutical BiochemistryHelping HandsОценок пока нет
- Biochemistry: Study of The Substances (Elements & The Chemical Reactions Underlying Life ProcessesДокумент35 страницBiochemistry: Study of The Substances (Elements & The Chemical Reactions Underlying Life ProcessesRoelin MinozaОценок пока нет
- Lehninger Ch2 WaterДокумент36 страницLehninger Ch2 WaterIsmael ChableОценок пока нет
- Introduction To Biochemistry.-16!10!22Документ72 страницыIntroduction To Biochemistry.-16!10!22Sanjeev walvekarОценок пока нет
- AdaptationДокумент25 страницAdaptationMOHD ZAINI NAWAHWIОценок пока нет
- BCH 2333 - DGD 1Документ19 страницBCH 2333 - DGD 1Simon HagosОценок пока нет
- Chapter 1 CELL ORGANELLES AND MACROMOLECULESДокумент23 страницыChapter 1 CELL ORGANELLES AND MACROMOLECULESAdelette Delos ReyesОценок пока нет
- New WaterДокумент55 страницNew Watergostrider0093sОценок пока нет
- Dent 201 MB Microbial Nutrition Week 2.Документ22 страницыDent 201 MB Microbial Nutrition Week 2.sima mhammedОценок пока нет
- 4.1 WaterДокумент22 страницы4.1 WaterWONG LI KING MoeОценок пока нет
- MC3 Lec Midterm TransesДокумент26 страницMC3 Lec Midterm Transescathryna gaylanОценок пока нет
- Food BiochemistryДокумент48 страницFood BiochemistryFombang AtamОценок пока нет
- Abdul - Bacterial Nutrition and GrowthДокумент71 страницаAbdul - Bacterial Nutrition and GrowthPrincewill SeiyefaОценок пока нет
- Lecture 7 - Class-Biomass Energy Conversion Technologies and Processes - Anaerobic DigestionДокумент51 страницаLecture 7 - Class-Biomass Energy Conversion Technologies and Processes - Anaerobic DigestionBenard KalamboОценок пока нет
- General Biology: Dr. Hoda ParsaДокумент42 страницыGeneral Biology: Dr. Hoda ParsasadeghОценок пока нет
- 2 - Blood and ImmunologyДокумент66 страниц2 - Blood and Immunologyharami666Оценок пока нет
- What Is Life?Документ39 страницWhat Is Life?Kristel AbbyОценок пока нет
- Chapter Two:: Factors That Affect and Favor Microbial Growth in FoodsДокумент30 страницChapter Two:: Factors That Affect and Favor Microbial Growth in FoodsReta megersaОценок пока нет
- BiochemistryДокумент6 страницBiochemistryBlaine Rogalski100% (14)
- Biochem 1st Year College ReviewerДокумент16 страницBiochem 1st Year College ReviewerMeteor 858100% (2)
- Properties of Water STUDENT'sДокумент36 страницProperties of Water STUDENT'sKim TangoОценок пока нет
- Introduction To BiochemistryДокумент32 страницыIntroduction To BiochemistryHazel LouОценок пока нет
- 1 Water Its Properties PDFДокумент27 страниц1 Water Its Properties PDFAllyssa Lorraine PrudencioОценок пока нет
- Water: PH and BuffersДокумент63 страницыWater: PH and BuffersSoffa ShmuelОценок пока нет
- Biochem Midterm ReviewerДокумент22 страницыBiochem Midterm Reviewerrentachin18Оценок пока нет
- Water in FoodsДокумент40 страницWater in FoodsMatíasОценок пока нет
- 9 Wastewater Treatment: Basic Processes of Water TreatmentДокумент94 страницы9 Wastewater Treatment: Basic Processes of Water TreatmentSayan BiswasОценок пока нет
- WATERДокумент30 страницWATERPrakash KhadkaОценок пока нет
- Lecture BIOCHEMISTRY of CYTOSOL AlfiДокумент56 страницLecture BIOCHEMISTRY of CYTOSOL Alfisennaavia12Оценок пока нет
- Protiensppt 219Документ84 страницыProtiensppt 219AHMED RAZAОценок пока нет
- Water, PH and BuffersДокумент43 страницыWater, PH and BuffersDaniel LuchendoОценок пока нет
- IntroductionДокумент15 страницIntroductionDeepak SahОценок пока нет
- Chemistry Biochemistry PresentationДокумент43 страницыChemistry Biochemistry PresentationIon BarboiОценок пока нет
- WaterДокумент24 страницыWaterAshley M NcubeОценок пока нет
- Water and PHДокумент154 страницыWater and PHIMDCBiochemОценок пока нет
- Concept of PH and BufferДокумент27 страницConcept of PH and BufferRolling Coast100% (1)
- Chapter 2 - Chemical and Functional Properties of Food ComponentsДокумент61 страницаChapter 2 - Chemical and Functional Properties of Food Components22125327Оценок пока нет
- BIOCHEMISTRY 106 Lecture RevisedДокумент46 страницBIOCHEMISTRY 106 Lecture Revisedjoanbless79Оценок пока нет
- BIO 203 Biochemistry I by Seyhun YURDUGÜL, PH.D.: What Is Biochemistry? Historical and Cellular AspectsДокумент68 страницBIO 203 Biochemistry I by Seyhun YURDUGÜL, PH.D.: What Is Biochemistry? Historical and Cellular AspectsYasin Çağrı KılıçerОценок пока нет
- PDF SAT Biology TextbookДокумент97 страницPDF SAT Biology TextbookSai SagireddyОценок пока нет
- Module 1 3 NotesДокумент4 страницыModule 1 3 NotesvotwgsidimhhdbujfgОценок пока нет
- Lect 5 CH 6Документ70 страницLect 5 CH 6alexОценок пока нет
- Eksu Lectures 2Документ92 страницыEksu Lectures 2Aina AdesolaОценок пока нет
- Biochemistry ReviewerДокумент20 страницBiochemistry ReviewerJhae Zharie Delasan PanosoОценок пока нет
- Biological Treatment of Hazardous WasteДокумент47 страницBiological Treatment of Hazardous Wastesamson meseretОценок пока нет
- Chapt02 - Lecture - 6eLM FALL 2016Документ51 страницаChapt02 - Lecture - 6eLM FALL 2016yutdefertyОценок пока нет
- Chemicals of LifeДокумент26 страницChemicals of LifeDenise CampuedОценок пока нет
- Microbial NutritionДокумент21 страницаMicrobial NutritionHemay Said MaghdidyОценок пока нет
- Lehninger - PPT - ch02 BetaДокумент52 страницыLehninger - PPT - ch02 Betamasdl100% (1)
- The Biochemistry of WaterДокумент54 страницыThe Biochemistry of WaterIrene BungariaОценок пока нет
- Engineering Processes for BioseparationsОт EverandEngineering Processes for BioseparationsLAURENCE R. WEATHERLEYРейтинг: 4 из 5 звезд4/5 (1)
- Biotransfrmtns Prepartv Organic Chemistry: The Use of Isolated Enzymes and Whole Cell Systems in SynthesisОт EverandBiotransfrmtns Prepartv Organic Chemistry: The Use of Isolated Enzymes and Whole Cell Systems in SynthesisОценок пока нет
- A-level Biology Revision: Cheeky Revision ShortcutsОт EverandA-level Biology Revision: Cheeky Revision ShortcutsРейтинг: 5 из 5 звезд5/5 (5)
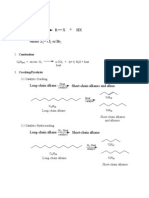
- Reactions of AlkanesДокумент1 страницаReactions of AlkanesnofacejackОценок пока нет
- Intsta1 Lecture5Документ19 страницIntsta1 Lecture5nofacejackОценок пока нет
- 5-AAS SДокумент39 страниц5-AAS SnofacejackОценок пока нет
- 3-Light - Matter - SДокумент32 страницы3-Light - Matter - SnofacejackОценок пока нет
- Intsta1 Lecture8Документ51 страницаIntsta1 Lecture8nofacejackОценок пока нет
- Special Continuous Probability DistributionsДокумент11 страницSpecial Continuous Probability DistributionsnofacejackОценок пока нет
- 1-Chemical Analysis - Quantitation - SДокумент39 страниц1-Chemical Analysis - Quantitation - SnofacejackОценок пока нет
- Pericyclic Reactions PDFДокумент41 страницаPericyclic Reactions PDFnofacejack67% (3)
- Intsta1 Lecture2Документ18 страницIntsta1 Lecture2nofacejackОценок пока нет
- Intsta1 Lecture4Документ40 страницIntsta1 Lecture4nofacejackОценок пока нет
- Intsta1 Lecture6Документ26 страницIntsta1 Lecture6nofacejackОценок пока нет
- Intsta1 Lecture3Документ45 страницIntsta1 Lecture3nofacejackОценок пока нет
- Hard QuestionДокумент183 страницыHard QuestionWidodo Muis100% (1)
- Intsta1 Lecture1Документ18 страницIntsta1 Lecture1nofacejackОценок пока нет
- Rev Bio 4th 10-11 (Part 1)Документ8 страницRev Bio 4th 10-11 (Part 1)nofacejackОценок пока нет
- Rev Bio 4th 10-11 (Part 2)Документ4 страницыRev Bio 4th 10-11 (Part 2)nofacejackОценок пока нет
- Lesson 9: Rectangular Coordinate PlaneДокумент1 страницаLesson 9: Rectangular Coordinate PlanenofacejackОценок пока нет
- The Big Book of Mind-Bending PuzzlesДокумент338 страницThe Big Book of Mind-Bending PuzzlesAustin Albert93% (14)
- WATERДокумент2 страницыWATERnofacejackОценок пока нет
- Introduction To AlgebraДокумент1 страницаIntroduction To AlgebranofacejackОценок пока нет
- Carbohydrates 2 UpdatedДокумент7 страницCarbohydrates 2 UpdatednofacejackОценок пока нет
- 3T06Bioorg11Physical Properties of AlkanesДокумент1 страница3T06Bioorg11Physical Properties of AlkanesnofacejackОценок пока нет
- Revised Naming and Chemical Formula WritingДокумент47 страницRevised Naming and Chemical Formula WritingnofacejackОценок пока нет
- Monosaccharides (Aldoses) : Biochem Structure Sheet 1. CarbohydratesДокумент1 страницаMonosaccharides (Aldoses) : Biochem Structure Sheet 1. CarbohydratesnofacejackОценок пока нет
- Molecular Logic of Life Student NotesДокумент4 страницыMolecular Logic of Life Student Notesnofacejack67% (3)
- Glycosaminoglycan Monsaccharide Components Function: LinkageДокумент1 страницаGlycosaminoglycan Monsaccharide Components Function: LinkagenofacejackОценок пока нет
- Enzymes Updated Mar 2010Документ6 страницEnzymes Updated Mar 2010nofacejackОценок пока нет
- Amino Acids: C COO H N H CHДокумент2 страницыAmino Acids: C COO H N H CHnofacejackОценок пока нет
- Lipids NotesДокумент5 страницLipids NotesnofacejackОценок пока нет
- Enzymes Updated Mar 2010Документ6 страницEnzymes Updated Mar 2010nofacejackОценок пока нет