Академический Документы
Профессиональный Документы
Культура Документы
Concentrations of Acids
Загружено:
Havila Saafi0 оценок0% нашли этот документ полезным (0 голосов)
10 просмотров1 страницаAcids concentrations
Авторское право
© © All Rights Reserved
Доступные форматы
DOCX, PDF, TXT или читайте онлайн в Scribd
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документAcids concentrations
Авторское право:
© All Rights Reserved
Доступные форматы
Скачайте в формате DOCX, PDF, TXT или читайте онлайн в Scribd
0 оценок0% нашли этот документ полезным (0 голосов)
10 просмотров1 страницаConcentrations of Acids
Загружено:
Havila SaafiAcids concentrations
Авторское право:
© All Rights Reserved
Доступные форматы
Скачайте в формате DOCX, PDF, TXT или читайте онлайн в Scribd
Вы находитесь на странице: 1из 1
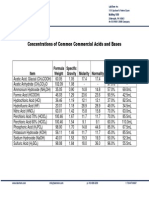
Concentrations of Acids & Bases
Composition of concentrated reagent grade acids, ammonium hydroxide, and sodium and potassium hydroxide solutions (with dilution directions to prepare 1N solution)
Chemical
MF
Approx. Strength of
Concd. Reagenta
Molarity of
Concd. Reagent
Milliliters of Concd. Reagent Necessary
to Prepare 1 Liter of 1 Normal Soln.c
CH3COOH
99.8
17.4
57.5
HCOOH
90.0
23.6
42.5
Hydrochloric Acid
HCl
37.2
12.1
82.5
Hydrofluoric Acid
HF
49.0
28.9
34.5
Nitric Acid
HNO3
70.4
15.9
63.0
Perchloric Acid
HClO4
70.5
11.7
85.5
Perchloric Acid
HClO4
61.3
9.5
105.5
Phosphoric Acid
H3PO4
85.5
14.8
22.5
Sulfuric Acid
H2SO4
96.0
18.0
28.0
Ammonium Hydroxide
NH4OH
56.6b
14.5
69.0
Sodium Hydroxide
NaOH
50.5
19.4
51.5
KOH
45.0
11.7
85.5
Acetic Acid, Glacial
Formic Acid
Potassium Hydroxide
a - Representative value, w/w%.
b - Equivalent to 28.0% w/w NH3.
c - Rounded to nearest 0.5 ml.
Вам также может понравиться
- 355 FC618 D 01Документ3 страницы355 FC618 D 01Mery BladieОценок пока нет
- Molarity of Concentrated Reagents 2Документ2 страницыMolarity of Concentrated Reagents 2srikanthdip007Оценок пока нет
- Concentrations of Common Commercial Acids and BasesДокумент1 страницаConcentrations of Common Commercial Acids and BasesNazimah MaqboolОценок пока нет
- Molarity of Concentrated Acids & BasesДокумент2 страницыMolarity of Concentrated Acids & BasesjacОценок пока нет
- Multiple Choice Questions: AMHS AP Chemistry NameДокумент22 страницыMultiple Choice Questions: AMHS AP Chemistry NameKZS1996Оценок пока нет
- Buffers&titrationsquestions ReviewДокумент6 страницBuffers&titrationsquestions Reviewapi-279595789Оценок пока нет
- 7.0 Ionic Equilibria: TutorialДокумент13 страниц7.0 Ionic Equilibria: Tutorializatirfan00Оценок пока нет
- Lab Report DETERMINATION OF THE CONCENTRATION OF ACETIC ACID IN VINEGARДокумент27 страницLab Report DETERMINATION OF THE CONCENTRATION OF ACETIC ACID IN VINEGARP NDОценок пока нет
- Lab Report Experiment 1Документ12 страницLab Report Experiment 1afifiОценок пока нет
- Acid Base 3 in Class WorksheetДокумент2 страницыAcid Base 3 in Class WorksheetDesiree Thea TaparОценок пока нет
- CHM271 - Tutorial 3 - Ionic EquilibriumДокумент3 страницыCHM271 - Tutorial 3 - Ionic Equilibriumfiefy zmrОценок пока нет
- Lab Report DETERMINATION OF THE CONCENTRATION OF ACETIC ACID IN VINEGARДокумент27 страницLab Report DETERMINATION OF THE CONCENTRATION OF ACETIC ACID IN VINEGARمحمد ازوادي100% (1)
- Chemistry Lab Report1Документ22 страницыChemistry Lab Report1RoseAnne BellaОценок пока нет
- Acid Base HomeworkДокумент5 страницAcid Base HomeworkAriel ChuОценок пока нет
- Exp 1Документ12 страницExp 1abdullahclanmrОценок пока нет
- CHEM 1221: Neutralization Titration Problem SetДокумент2 страницыCHEM 1221: Neutralization Titration Problem SetJohn Kristoffer RoblezaОценок пока нет
- Acid Base - NeutralizationДокумент71 страницаAcid Base - NeutralizationAyen AyieОценок пока нет
- Chapter 12Документ37 страницChapter 12Biotechnology IIUM Kuantan100% (2)
- BuffersДокумент10 страницBuffersMaya FirdaОценок пока нет
- Lab 1 Determination of Acetic Acid in VinegarДокумент20 страницLab 1 Determination of Acetic Acid in Vinegaramiraaikharah100% (1)
- Stoichiometry PDFДокумент7 страницStoichiometry PDFggk2013Оценок пока нет
- SMB 1 Xi Chem Mod8Документ10 страницSMB 1 Xi Chem Mod8Aditya SinghОценок пока нет
- Lab Chemicals - Updated VersionДокумент2 страницыLab Chemicals - Updated VersionWayaya2009Оценок пока нет
- Determinate of The Concentration of Acetic Acid in VinegarДокумент22 страницыDeterminate of The Concentration of Acetic Acid in VinegarSYahira HAzwaniОценок пока нет
- Ole2 BufferДокумент19 страницOle2 BufferKherulJefriJamenОценок пока нет
- Titration Questions Set 1Документ8 страницTitration Questions Set 1danielmahsaОценок пока нет
- Review Question Topic: Buffer Titration and SolubilityДокумент3 страницыReview Question Topic: Buffer Titration and SolubilitySTEPHANUS DARRENОценок пока нет
- Ionic Equilibrium Lecture-14 Notes PDFДокумент14 страницIonic Equilibrium Lecture-14 Notes PDFnimit singhОценок пока нет
- Name: Tooba Class: MSC Final Year Course Code: Chm-642 Lab Presentation Topic: Titrimetric Analysis of MixturesДокумент22 страницыName: Tooba Class: MSC Final Year Course Code: Chm-642 Lab Presentation Topic: Titrimetric Analysis of MixturesABDUL NABEELОценок пока нет
- R-Cooh, R-Co H,: À Ant À VinegarДокумент43 страницыR-Cooh, R-Co H,: À Ant À VinegarArvin MarasiganОценок пока нет
- Volumetric AnalysisДокумент15 страницVolumetric AnalysisSaraОценок пока нет
- Sodium Phosphate: Volume (ML) of 1 M Nah Po Volume (ML) of 1 M Na Hpo Final PHДокумент2 страницыSodium Phosphate: Volume (ML) of 1 M Nah Po Volume (ML) of 1 M Na Hpo Final PHAkash Pagare0% (1)
- Lab Report Acid in VinegarДокумент18 страницLab Report Acid in VinegarAmirah Nadia Mat Lias89% (19)
- No. Pages: Table of ContentДокумент18 страницNo. Pages: Table of ContentAzzian AriffinОценок пока нет
- Quantitative Analysis of H2c2o4 and H2so4Документ32 страницыQuantitative Analysis of H2c2o4 and H2so4Willmann Jimenez MoralesОценок пока нет
- Table of Ka and KBДокумент1 страницаTable of Ka and KBkarimhandoyoОценок пока нет
- Additional Aspects of Aqueous Equilibria (AP MC)Документ4 страницыAdditional Aspects of Aqueous Equilibria (AP MC)Sumolmal SrisukriОценок пока нет
- Tugas Kimia DasarДокумент5 страницTugas Kimia DasarOfficial ProtectionОценок пока нет
- Ionic Equilibrium ProblemsДокумент2 страницыIonic Equilibrium ProblemsNinad Puranik0% (1)
- 1-15 Tutorial Questions: HG HGДокумент4 страницы1-15 Tutorial Questions: HG HGAyez SassinОценок пока нет
- Acid Base CH 16 ComprehensiveДокумент4 страницыAcid Base CH 16 ComprehensiveAidah AmirОценок пока нет
- 17PS2AДокумент4 страницы17PS2ASeamus AlaricОценок пока нет
- 19BCM0032 VL2021220101540 Ast02Документ3 страницы19BCM0032 VL2021220101540 Ast02Ms. ZeynabОценок пока нет
- Manips CalcaireДокумент5 страницManips CalcaireNaztovenОценок пока нет
- Problem Set 17.2: Salts & Polyprotic Calculations: CHEM 10123 General Chemistry Ii Dr. FryДокумент2 страницыProblem Set 17.2: Salts & Polyprotic Calculations: CHEM 10123 General Chemistry Ii Dr. Frykatherine ramirezОценок пока нет
- Chapter 15 - Acid-Base EquilibriaДокумент59 страницChapter 15 - Acid-Base EquilibriaPatel MswaziОценок пока нет
- HW IonicEq G51 (1-64)Документ1 страницаHW IonicEq G51 (1-64)Heart TeppreechaОценок пока нет
- SMB 2 Xii Chem Mod2Документ12 страницSMB 2 Xii Chem Mod2Shubh GuptaОценок пока нет
- Sodium PhosphateДокумент2 страницыSodium PhosphateshivgoldiОценок пока нет
- The Titration of Acetic Acid in Vinegar: CHEM 122L General Chemistry Laboratory Revision 1.4Документ16 страницThe Titration of Acetic Acid in Vinegar: CHEM 122L General Chemistry Laboratory Revision 1.4Nur Najwa YunusОценок пока нет
- Exp 05Документ4 страницыExp 05Hasun MadurangaОценок пока нет
- Chapter 7: Acid and Base 7.4: NeutralisationДокумент30 страницChapter 7: Acid and Base 7.4: NeutralisationNovah GurulooОценок пока нет
- Oxalic Acid Vs NaOH Lab ReportДокумент2 страницыOxalic Acid Vs NaOH Lab ReportAkhil Menon100% (1)
- Grade 11 Chemistry Review With AnswersДокумент15 страницGrade 11 Chemistry Review With AnswersRiham ElhabyanОценок пока нет
- Standardization BuffersДокумент4 страницыStandardization BuffersHoàng TuấnОценок пока нет
- Hydrolysis of Salt and PH of Buffer Solutions.Документ16 страницHydrolysis of Salt and PH of Buffer Solutions.amiraaikharah100% (1)
- StanderizationДокумент1 страницаStanderizationMunazza SohailОценок пока нет
- Determination Acetic AcidДокумент21 страницаDetermination Acetic Acidameyakem100% (1)
- Sulfuric Acid Manufacture: Analysis, Control and OptimizationОт EverandSulfuric Acid Manufacture: Analysis, Control and OptimizationРейтинг: 3.5 из 5 звезд3.5/5 (3)
- Arene Chemistry: Reaction Mechanisms and Methods for Aromatic CompoundsОт EverandArene Chemistry: Reaction Mechanisms and Methods for Aromatic CompoundsОценок пока нет
- Cloning:: Making A Genetic Duplicate of Something (Eg. Gene, Cell or Organism)Документ1 страницаCloning:: Making A Genetic Duplicate of Something (Eg. Gene, Cell or Organism)Havila SaafiОценок пока нет
- Flame TestsДокумент2 страницыFlame TestsHavila SaafiОценок пока нет
- IB Syllabus in WordДокумент16 страницIB Syllabus in WordHavila SaafiОценок пока нет
- Monohybrid CrossДокумент2 страницыMonohybrid CrossHavila SaafiОценок пока нет
- Syllabus Details: Topic 1: CellsДокумент16 страницSyllabus Details: Topic 1: CellsHavila SaafiОценок пока нет
- Kidney / Homeostasis Exam Paper Questions: Word PerfectДокумент7 страницKidney / Homeostasis Exam Paper Questions: Word PerfectHavila SaafiОценок пока нет
- DNA ModelДокумент1 страницаDNA ModelHavila SaafiОценок пока нет
- AnswersДокумент5 страницAnswersHavila Saafi100% (1)
- Year 12 SyllabusДокумент18 страницYear 12 SyllabusHavila SaafiОценок пока нет
- Syllabus Details: Topic 1: CellsДокумент16 страницSyllabus Details: Topic 1: CellsHavila SaafiОценок пока нет
- Topic 1 CellsДокумент13 страницTopic 1 CellsHavila SaafiОценок пока нет
- Command TermsДокумент1 страницаCommand TermsHavila SaafiОценок пока нет
- IB Chemistry Syllabus - Core OnlyДокумент89 страницIB Chemistry Syllabus - Core OnlyHavila SaafiОценок пока нет
- IB Syllabus in WordДокумент16 страницIB Syllabus in WordHavila SaafiОценок пока нет
- IBChemsyllabusДокумент29 страницIBChemsyllabusHavila SaafiОценок пока нет