Академический Документы
Профессиональный Документы
Культура Документы
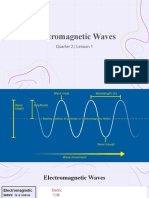
Ultraviolet (UV) Light Is
Загружено:
parin advaniОригинальное название
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
Ultraviolet (UV) Light Is
Загружено:
parin advaniАвторское право:
Доступные форматы
Ultraviolet (UV) light is electromagnetic radiation with a wavelength from 400 nm to 10 nm, shorter
than that of visible light but longer than X-rays. Though usually invisible, under some conditions
children and young adults can see ultraviolet down to wavelengths of about 310 nm,[1][2] and people
with aphakia (missing lens) can also see some UV wavelengths. Near-UV is visible to a number of
insects and birds.
UV radiation is present in sunlight, and is produced by electric arcs and specialized lights such
as mercury-vapor lamps,tanning lamps, and black lights. Although lacking the energy to ionize
atoms, long-wavelength ultraviolet radiation can cause chemical reactions, and causes many
substances to glow or fluoresce. Consequently, biological effects of UV are greater than simple
heating effects, and many practical applications of UV radiation derive from its interactions with
organic molecules.
Suntan and sunburn are familiar effects of over-exposure, along with higher risk of skin cancer.
Living things on dry land would be severely damaged by ultraviolet radiation from the sun if most of it
were not filtered out by the Earth's atmosphere, particularly the ozone layer.[3] More-energetic,
shorter-wavelength "extreme" UV below 121 nm ionizes air so strongly that it is absorbed before it
reaches the ground.[4] Ultraviolet is also responsible for the formation of bone-strengthening vitamin
D in most land vertebrates, including humans. The UV spectrum thus has effects both beneficial and
harmful to human health.
Вам также может понравиться
- Ultraviolet: "UV" Redirects Here. For Other Uses, See - For Other Uses For "Ultraviolet", SeeДокумент2 страницыUltraviolet: "UV" Redirects Here. For Other Uses, See - For Other Uses For "Ultraviolet", SeeGemma CanicosaОценок пока нет
- Ultraviolet Light Presentation PDFДокумент16 страницUltraviolet Light Presentation PDFParis ArcillaОценок пока нет
- UltravioletДокумент9 страницUltravioletKevinОценок пока нет
- Ultraviolet RadiationДокумент3 страницыUltraviolet RadiationbirlacementОценок пока нет
- Group 5 Physics 10 UV ScriptДокумент3 страницыGroup 5 Physics 10 UV ScriptEmilrie RogeroОценок пока нет
- UltravioletДокумент17 страницUltravioletDhanmeet KaurОценок пока нет
- Ultraviolet RaysДокумент24 страницыUltraviolet RayschrisshitОценок пока нет
- UvraysДокумент14 страницUvraysReviews Benchmarks & TipsОценок пока нет
- Effects of Electromagnetic Radiation To Our EnvironmentДокумент11 страницEffects of Electromagnetic Radiation To Our EnvironmentKrislyn Bibar PereaОценок пока нет
- The Effects of EM Radiation On Living ThingsДокумент23 страницыThe Effects of EM Radiation On Living ThingsCharm VergaraОценок пока нет
- Ultraviolet (UV)Документ7 страницUltraviolet (UV)mariaОценок пока нет
- S10 - Q2 - Week 5Документ9 страницS10 - Q2 - Week 5Evefel Ruth SanchezОценок пока нет
- Biology Project On OzoneДокумент26 страницBiology Project On OzoneAkansha Agarwal33% (3)
- Document (6 Sci Presentation)Документ5 страницDocument (6 Sci Presentation)Ivan UmaliОценок пока нет
- Electromagnetic WavesДокумент21 страницаElectromagnetic WavesCrisanta GanadoОценок пока нет
- Effects of Electromagnetic Radiation - 095059Документ19 страницEffects of Electromagnetic Radiation - 095059Jocidelle LubricoОценок пока нет
- Uv RadiationДокумент3 страницыUv RadiationcesarОценок пока нет
- The Effects of EM Radiations On Living Things and EnvironmentДокумент30 страницThe Effects of EM Radiations On Living Things and EnvironmentJhunner Buan100% (1)
- Ultraviolet LightДокумент25 страницUltraviolet LightHyojin100% (2)
- Electromagnetic SpectrumДокумент56 страницElectromagnetic Spectrumkylle amigoОценок пока нет
- Visible Light Is One Way Energy Moves AroundДокумент2 страницыVisible Light Is One Way Energy Moves AroundNash Gemar Braga EvangelistaОценок пока нет
- EM SpectrumДокумент6 страницEM SpectrumKaren100% (1)
- ToxicologyДокумент118 страницToxicologyAnand DadkeОценок пока нет
- Non Ionizing RadiationДокумент6 страницNon Ionizing RadiationtechzonesОценок пока нет
- Ultraviolet Radiation and The Eye - An Epidemiologic StudyДокумент52 страницыUltraviolet Radiation and The Eye - An Epidemiologic StudyMaria Gerber RozaОценок пока нет
- Ultraviolet RaysДокумент37 страницUltraviolet RaysJerome CatacutanОценок пока нет
- Investigatory Project in BiologyДокумент8 страницInvestigatory Project in BiologyHarpreet SinghОценок пока нет
- Team Leader - Ibasan, Michael Ellise: I. Cover Page II. Members and Assigned TasksДокумент12 страницTeam Leader - Ibasan, Michael Ellise: I. Cover Page II. Members and Assigned TasksJOHN ASHLEY RANIN CARIAGAОценок пока нет
- 300-Word Essay Expressing Your Opinions and Assertion On The Effects of Electromagnetic Radiation On Living Things and The EnvironmentДокумент1 страница300-Word Essay Expressing Your Opinions and Assertion On The Effects of Electromagnetic Radiation On Living Things and The EnvironmentELISHA RUTH BARAOIDANОценок пока нет
- Science10 Research PresentationДокумент13 страницScience10 Research PresentationIvan UmaliОценок пока нет
- Ultraviolet Radiation Group 5Документ30 страницUltraviolet Radiation Group 5Blessie Zyren BasinangОценок пока нет
- ULTRAVIOLETДокумент2 страницыULTRAVIOLETYerduah LopezОценок пока нет
- Light and LuminescenceДокумент10 страницLight and LuminescenceJoseGarciaRuizОценок пока нет
- Untitled DocumentДокумент6 страницUntitled DocumentMichaela CuarteroОценок пока нет
- Technology in Education Technology Presentation in Blue Peach Illustrative StyleДокумент7 страницTechnology in Education Technology Presentation in Blue Peach Illustrative StyleAlyzzaОценок пока нет
- Em SpectrumДокумент24 страницыEm SpectrumFender CoomstoneОценок пока нет
- Biology Project On Ultraviolet RaysДокумент14 страницBiology Project On Ultraviolet Raysokesh27Оценок пока нет
- Filtre Organice !!Документ15 страницFiltre Organice !!Lazar CrinaОценок пока нет
- Ultraviolet: Ultraviolet (UV) Light IsДокумент17 страницUltraviolet: Ultraviolet (UV) Light Ismike vidalОценок пока нет
- Sia83 3notesДокумент5 страницSia83 3notesAmaechi ZalОценок пока нет
- Investigatory Project in BiologyДокумент17 страницInvestigatory Project in Biologyrajath96100% (2)
- Week3Q2 RLAS Science10 FinalДокумент8 страницWeek3Q2 RLAS Science10 FinalericaОценок пока нет
- Uv Rays SciДокумент10 страницUv Rays SciMichaela CasiquinОценок пока нет
- UV Radiation: Did You Know?Документ2 страницыUV Radiation: Did You Know?Ahtarunnisa Fauzia HanifaОценок пока нет
- Uvradiation PDFДокумент2 страницыUvradiation PDFCemaraSedanaОценок пока нет
- The Electromagnetic Spectrum 3Документ7 страницThe Electromagnetic Spectrum 3Nur-shima SakilinОценок пока нет
- The Full Electromagnetic Spectrum Is Shown in The Following ImageДокумент3 страницыThe Full Electromagnetic Spectrum Is Shown in The Following ImageJhade Kianne Mendoza BarzaОценок пока нет
- EM WavesДокумент11 страницEM WavesHomok Noki100% (1)
- Biology InvestigatoryДокумент13 страницBiology Investigatorykalai prasathОценок пока нет
- Ultraviolet RadiationДокумент2 страницыUltraviolet RadiationMarcus PiroteОценок пока нет
- Non Ionizing RadiationДокумент3 страницыNon Ionizing RadiationArun437Оценок пока нет
- Electromagnetic Radiation Frequency Wavelength Light Ultraviolet RadiationДокумент3 страницыElectromagnetic Radiation Frequency Wavelength Light Ultraviolet RadiationCamille Galam duquezОценок пока нет
- Integrantes:: Laura María Baquero Cuéllar Joan Camilo Caicedo Daniela Delmar Vásquez Ingrid Jhoana Sánchez CharrupiДокумент49 страницIntegrantes:: Laura María Baquero Cuéllar Joan Camilo Caicedo Daniela Delmar Vásquez Ingrid Jhoana Sánchez CharrupiFabricio Echeverri LondoñoОценок пока нет
- U V Procted Textile PDFДокумент15 страницU V Procted Textile PDFMusa EltayebОценок пока нет
- Cancer CausesДокумент36 страницCancer Causessufian abdo jiloОценок пока нет
- Uses of EM WavesДокумент3 страницыUses of EM WavesPallika DhingraОценок пока нет
- Sci 10Документ7 страницSci 10John Marithe PutunganОценок пока нет
- UltravioletДокумент154 страницыUltravioletAnonymous gUjimJKОценок пока нет
- Small Scale ReviewДокумент14 страницSmall Scale Reviewparin advaniОценок пока нет
- AsteroidsДокумент1 страницаAsteroidsparin advaniОценок пока нет
- Techno-Economic Analysis of A Small-Scale Biomass-to-Energy BFB Gasification-Based SystemДокумент17 страницTechno-Economic Analysis of A Small-Scale Biomass-to-Energy BFB Gasification-Based Systemblack KNIGHTОценок пока нет
- JungleJuice FeedChartДокумент1 страницаJungleJuice FeedChartparin advaniОценок пока нет
- SolidWize CSWP Sample Exam 2 Segment 3 PDFДокумент16 страницSolidWize CSWP Sample Exam 2 Segment 3 PDFparin advani100% (3)
- BAДокумент1 страницаBAparin advaniОценок пока нет
- EnthuДокумент1 страницаEnthuparin advaniОценок пока нет
- Gravity or Gravitation Is AДокумент1 страницаGravity or Gravitation Is Aparin advaniОценок пока нет
- MissileДокумент1 страницаMissileparin advaniОценок пока нет
- ScramjetДокумент1 страницаScramjetparin advaniОценок пока нет
- SupernovaДокумент1 страницаSupernovaparin advaniОценок пока нет
- SpacetimeДокумент2 страницыSpacetimeparin advaniОценок пока нет
- Shock Waves Sound Explosion BulletДокумент1 страницаShock Waves Sound Explosion Bulletparin advaniОценок пока нет
- RocketДокумент1 страницаRocketparin advaniОценок пока нет
- Giant Star Solar Masses Stellar Evolution Spectral Types Carbon StarsДокумент1 страницаGiant Star Solar Masses Stellar Evolution Spectral Types Carbon Starsparin advaniОценок пока нет
- Longitudinal and Transverse WavesДокумент1 страницаLongitudinal and Transverse Wavesparin advaniОценок пока нет
- Astrophysics (from Greek astron, στρον "star", andДокумент1 страницаAstrophysics (from Greek astron, στρον "star", andparin advaniОценок пока нет
- Wave Speed of Sound Liquid Gas Plasma Fluid Pressure Temperature Density Expansion Fan Solitons Sonic BoomДокумент1 страницаWave Speed of Sound Liquid Gas Plasma Fluid Pressure Temperature Density Expansion Fan Solitons Sonic Boomparin advaniОценок пока нет
- FlutterДокумент2 страницыFlutterparin advaniОценок пока нет
- Supersonic Speed Is A Rate of Travel of An Object That Exceeds TheДокумент1 страницаSupersonic Speed Is A Rate of Travel of An Object That Exceeds Theparin advaniОценок пока нет
- Physics Mass Velocity State of Rest Accelerate Magnitude Direction Vector SI Unit Newtons Newton's Second Law Rate Momentum Acceleration MassДокумент1 страницаPhysics Mass Velocity State of Rest Accelerate Magnitude Direction Vector SI Unit Newtons Newton's Second Law Rate Momentum Acceleration Massparin advaniОценок пока нет
- Fluid Resistance Shear Stress Tensile Stress Honey WaterДокумент1 страницаFluid Resistance Shear Stress Tensile Stress Honey Waterparin advaniОценок пока нет
- Fluid Dynamics Friction Fluid Friction Forces Solid FrictionДокумент1 страницаFluid Dynamics Friction Fluid Friction Forces Solid Frictionparin advaniОценок пока нет
- StringДокумент1 страницаStringparin advaniОценок пока нет
- EnergyДокумент2 страницыEnergyparin advaniОценок пока нет
- Wind Is The Flow of Gases On A Large Scale. On The Surface of The Earth, Wind Consists of The BulkДокумент2 страницыWind Is The Flow of Gases On A Large Scale. On The Surface of The Earth, Wind Consists of The Bulkparin advaniОценок пока нет
- Science (From: Laws of NatureДокумент1 страницаScience (From: Laws of Natureparin advaniОценок пока нет
- The Four Fundamental States of Matter Solid Liquid Plasma Noble Gas Neon Elemental Oxygen Compound Carbon Dioxide Mixture AirДокумент1 страницаThe Four Fundamental States of Matter Solid Liquid Plasma Noble Gas Neon Elemental Oxygen Compound Carbon Dioxide Mixture Airparin advaniОценок пока нет
- White Dwarf Degenerate DwarfДокумент1 страницаWhite Dwarf Degenerate Dwarfparin advaniОценок пока нет
- A Brief History of Time: From the Big Bang to Black HolesОт EverandA Brief History of Time: From the Big Bang to Black HolesРейтинг: 4 из 5 звезд4/5 (2193)
- Dark Matter and the Dinosaurs: The Astounding Interconnectedness of the UniverseОт EverandDark Matter and the Dinosaurs: The Astounding Interconnectedness of the UniverseРейтинг: 3.5 из 5 звезд3.5/5 (69)
- A Beginner's Guide to Constructing the Universe: The Mathematical Archetypes of Nature, Art, and ScienceОт EverandA Beginner's Guide to Constructing the Universe: The Mathematical Archetypes of Nature, Art, and ScienceРейтинг: 4 из 5 звезд4/5 (51)
- Knocking on Heaven's Door: How Physics and Scientific Thinking Illuminate the Universe and the Modern WorldОт EverandKnocking on Heaven's Door: How Physics and Scientific Thinking Illuminate the Universe and the Modern WorldРейтинг: 3.5 из 5 звезд3.5/5 (64)
- Giza: The Tesla Connection: Acoustical Science and the Harvesting of Clean EnergyОт EverandGiza: The Tesla Connection: Acoustical Science and the Harvesting of Clean EnergyОценок пока нет
- Quantum Spirituality: Science, Gnostic Mysticism, and Connecting with Source ConsciousnessОт EverandQuantum Spirituality: Science, Gnostic Mysticism, and Connecting with Source ConsciousnessРейтинг: 4 из 5 звезд4/5 (6)
- Summary and Interpretation of Reality TransurfingОт EverandSummary and Interpretation of Reality TransurfingРейтинг: 5 из 5 звезд5/5 (5)
- Packing for Mars: The Curious Science of Life in the VoidОт EverandPacking for Mars: The Curious Science of Life in the VoidРейтинг: 4 из 5 звезд4/5 (1395)
- Lost in Math: How Beauty Leads Physics AstrayОт EverandLost in Math: How Beauty Leads Physics AstrayРейтинг: 4.5 из 5 звезд4.5/5 (125)
- The Power of Eight: Harnessing the Miraculous Energies of a Small Group to Heal Others, Your Life, and the WorldОт EverandThe Power of Eight: Harnessing the Miraculous Energies of a Small Group to Heal Others, Your Life, and the WorldРейтинг: 4.5 из 5 звезд4.5/5 (54)
- Midnight in Chernobyl: The Story of the World's Greatest Nuclear DisasterОт EverandMidnight in Chernobyl: The Story of the World's Greatest Nuclear DisasterРейтинг: 4.5 из 5 звезд4.5/5 (410)
- The Beginning of Infinity: Explanations That Transform the WorldОт EverandThe Beginning of Infinity: Explanations That Transform the WorldРейтинг: 5 из 5 звезд5/5 (60)
- Let There Be Light: Physics, Philosophy & the Dimensional Structure of ConsciousnessОт EverandLet There Be Light: Physics, Philosophy & the Dimensional Structure of ConsciousnessРейтинг: 4.5 из 5 звезд4.5/5 (57)
- The Tao of Physics: An Exploration of the Parallels between Modern Physics and Eastern MysticismОт EverandThe Tao of Physics: An Exploration of the Parallels between Modern Physics and Eastern MysticismРейтинг: 4 из 5 звезд4/5 (500)
- Chasing Heisenberg: The Race for the Atom BombОт EverandChasing Heisenberg: The Race for the Atom BombРейтинг: 4.5 из 5 звезд4.5/5 (8)
- The End of Everything: (Astrophysically Speaking)От EverandThe End of Everything: (Astrophysically Speaking)Рейтинг: 4.5 из 5 звезд4.5/5 (157)
- Black Holes: The Key to Understanding the UniverseОт EverandBlack Holes: The Key to Understanding the UniverseРейтинг: 4.5 из 5 звезд4.5/5 (13)
- Quantum Physics: What Everyone Needs to KnowОт EverandQuantum Physics: What Everyone Needs to KnowРейтинг: 4.5 из 5 звезд4.5/5 (49)
- The Simulated Multiverse: An MIT Computer Scientist Explores Parallel Universes, The Simulation Hypothesis, Quantum Computing and the Mandela EffectОт EverandThe Simulated Multiverse: An MIT Computer Scientist Explores Parallel Universes, The Simulation Hypothesis, Quantum Computing and the Mandela EffectРейтинг: 4.5 из 5 звезд4.5/5 (20)
- The Magick of Physics: Uncovering the Fantastical Phenomena in Everyday LifeОт EverandThe Magick of Physics: Uncovering the Fantastical Phenomena in Everyday LifeОценок пока нет