Академический Документы
Профессиональный Документы
Культура Документы
K Solubility of Organic (G Solubility of Water (G: 100 ML) 100 ML)
Загружено:
Caroline H David0 оценок0% нашли этот документ полезным (0 голосов)
41 просмотров2 страницыLLE Chemistry
Оригинальное название
LLE
Авторское право
© © All Rights Reserved
Доступные форматы
DOCX, PDF, TXT или читайте онлайн в Scribd
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документLLE Chemistry
Авторское право:
© All Rights Reserved
Доступные форматы
Скачайте в формате DOCX, PDF, TXT или читайте онлайн в Scribd
0 оценок0% нашли этот документ полезным (0 голосов)
41 просмотров2 страницыK Solubility of Organic (G Solubility of Water (G: 100 ML) 100 ML)
Загружено:
Caroline H DavidLLE Chemistry
Авторское право:
© All Rights Reserved
Доступные форматы
Скачайте в формате DOCX, PDF, TXT или читайте онлайн в Scribd
Вы находитесь на странице: 1из 2
What is liquid-liquid extraction?
Also known as solvent extraction
Separation of analytes from a liquid matrix by the use of another liquid
based upon selective partitioning.
Analytes are separated based on their different solubility in different
liquids.
Thus, the success of LLE depends upon the difference in solubility of an
analyte in various solvents.
How it works?
It consists of two solvents that do not mix (diff solubility)
o First solvent contains the analyte of interest
o Second solvent acts to extract the analyte
Since the two solvents do not mix, they can be separated in a separatory
funnel providing a very quick and easy way to separate compounds.
Solvent system
As mentioned earlier, Solvent system must comprise of two immiscible
solvents
Usually one phase is a water or water-based (aqueous) solution and the
other an immiscible organic solvent (often methylene chloride, diethyl
ether, or ethyl acetate).
o Most common solvent pairs are water-hexane, waterdichloromethane, water-ether
The analyte must be soluble in organic solvent but insoluble in water
Distribution
When an analyte is shaken in a separatory funnel with two immiscible
solvents, the analyte will distribute itself between the two solvents.
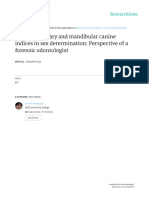
Distribution coefficient
o A quantitative measure of how an organic compound (analyte) will
distribute between aqueous and organic phases.
o K = distribution coefficient
g
)
100 mL
K=
g
solubility of water (
)
100 mL
solubility of organic(
o
o
Efficiency
It is the ratio of the concentration of solute distributed in the two
different solvent once the system reached equilibrium.
Distribution coefficient, K is independent of the actual amounts of
the two solvents mixed.
Вам также может понравиться
- 2210 - ExtractionДокумент13 страниц2210 - ExtractionChandra Icha KurniauanОценок пока нет
- 09 01 24 CHE 411 Extraction-1Документ27 страниц09 01 24 CHE 411 Extraction-1olawale.olamide19Оценок пока нет
- Liquid Liquid ExtractionДокумент20 страницLiquid Liquid ExtractionRavi JoshiОценок пока нет
- Separating Acids and Neutral Compounds by Solvent Extraction 705Документ11 страницSeparating Acids and Neutral Compounds by Solvent Extraction 705irohanihoheto12350% (2)
- Liquid Liquid ExtractionДокумент15 страницLiquid Liquid ExtractionDrAlok GargОценок пока нет
- P1W3 - Solvent ExrtractionДокумент42 страницыP1W3 - Solvent ExrtractionJoshua Richard Jr Tubiano PayopaninОценок пока нет
- Liquid-Liquid Extraction (LLE)Документ26 страницLiquid-Liquid Extraction (LLE)soran najebОценок пока нет
- Chem 301 Lab Report 2Документ4 страницыChem 301 Lab Report 2katlegoОценок пока нет
- Solvents Used in Liquid-Liquid ExtractionДокумент3 страницыSolvents Used in Liquid-Liquid ExtractionJohn Andrew GonzalesОценок пока нет
- Lecture 07 CH 419 Spring 2023Документ14 страницLecture 07 CH 419 Spring 2023Isha latifОценок пока нет
- ExtractionДокумент5 страницExtractionRiya VishwakarmaОценок пока нет
- Unit 4 - Obtention of Active CompoundsДокумент19 страницUnit 4 - Obtention of Active CompoundsAelita RuseelОценок пока нет
- Solvent Extraction Updated 14.02.2012Документ5 страницSolvent Extraction Updated 14.02.2012Loveena SteadmanОценок пока нет
- Is0l4tion of 4lk4l0ids fr0m Te4Документ9 страницIs0l4tion of 4lk4l0ids fr0m Te4Timothy DrakeОценок пока нет
- Liquid Liquid ExtractionДокумент25 страницLiquid Liquid ExtractionsyafiqОценок пока нет
- Chem Lab 6Документ3 страницыChem Lab 6Sean Rupert Luces PanuncioОценок пока нет
- Extraction: 8.1 Simple Liquid-Liquid ExtractionДокумент14 страницExtraction: 8.1 Simple Liquid-Liquid ExtractionclodinaОценок пока нет
- ChemДокумент19 страницChemHannah Valerie NuqueОценок пока нет
- Mubeen PPT 2Документ5 страницMubeen PPT 2Asad KhanОценок пока нет
- Separating Mixtures: Key Separation MethodsДокумент5 страницSeparating Mixtures: Key Separation MethodsJoelle SwaisОценок пока нет
- Introduction to the Extraction ProcessДокумент2 страницыIntroduction to the Extraction ProcessSun Jacob Javier CapundagОценок пока нет
- Liquid Liquid ExtractionДокумент40 страницLiquid Liquid ExtractionApurba Sarker Apu93% (29)
- Extraction With Acid and AlkalineДокумент2 страницыExtraction With Acid and AlkalineChris Mark50% (2)
- FractionationДокумент19 страницFractionationnovitariaОценок пока нет
- Industrial Pharmacy (3) - Solutions as a Dosage FormДокумент31 страницаIndustrial Pharmacy (3) - Solutions as a Dosage FormSaraОценок пока нет
- Solvent Extraction Experiment Analyzes Metal Ion SeparationДокумент12 страницSolvent Extraction Experiment Analyzes Metal Ion SeparationKelven LeeОценок пока нет
- Leaching Vs ExtractionДокумент4 страницыLeaching Vs ExtractionGilead ObasОценок пока нет
- Table 1: Comparing Extraction and DistillationДокумент23 страницыTable 1: Comparing Extraction and DistillationAnonymous QM0NLqZOОценок пока нет
- Extraction TheoryДокумент32 страницыExtraction TheoryFida RoinikaОценок пока нет
- Liquid-Liquid ExtractionДокумент5 страницLiquid-Liquid ExtractionFahad ShakeelОценок пока нет
- Experiment No. 8 - 203144254 - TIBДокумент4 страницыExperiment No. 8 - 203144254 - TIBBokutoОценок пока нет
- Dasar Dasar Kromatografi Dalam 40 KarakterДокумент14 страницDasar Dasar Kromatografi Dalam 40 Karaktersuraiya dwana24Оценок пока нет
- Organic Chemistry: Basra University College of Science and Technology Pharmacy DepartmentДокумент10 страницOrganic Chemistry: Basra University College of Science and Technology Pharmacy DepartmentcrtgyhujikОценок пока нет
- Liquid-Liquid Extraction TheoryДокумент6 страницLiquid-Liquid Extraction Theorybakhtyar21Оценок пока нет
- Lab LleДокумент20 страницLab LleMuhamad Baihakhi Shamsudin100% (1)
- Partition ExtractionДокумент5 страницPartition ExtractionWhoZz DizОценок пока нет
- Distribution of Acetic Acid Between Two Immiscible Solution by Simple Simple Methods PapooДокумент11 страницDistribution of Acetic Acid Between Two Immiscible Solution by Simple Simple Methods PapooHasnain SaifiОценок пока нет
- Liquid ChromatographyДокумент2 страницыLiquid ChromatographyalexpharmОценок пока нет
- Separation PDFДокумент25 страницSeparation PDFaenidrisОценок пока нет
- Separatory Funnel Extraction BackgroundДокумент3 страницыSeparatory Funnel Extraction BackgroundCelrose FernandezОценок пока нет
- TI - Liquid-Liquid Extraction (LLE) PDFДокумент32 страницыTI - Liquid-Liquid Extraction (LLE) PDF08Leonardo Demas Krisna Satria WijayaОценок пока нет
- SolutionДокумент38 страницSolutionhaithemОценок пока нет
- Liquid LiquidДокумент8 страницLiquid LiquidAnonymous b9fcR5Оценок пока нет
- BSC 821 CH 1Документ31 страницаBSC 821 CH 1PaxChem Ltd.Оценок пока нет
- Extraction in Chemical Technology PrincipleДокумент24 страницыExtraction in Chemical Technology PrincipleFatima ZaharaОценок пока нет
- Exp 6 Lab ReportДокумент15 страницExp 6 Lab ReportNur Syuhaidah100% (1)
- Liquid-Liquid ExtractionДокумент50 страницLiquid-Liquid ExtractionWILLIE JR. GATUSОценок пока нет
- Module 6 1Документ5 страницModule 6 1Ericka LouiseОценок пока нет
- Liquid Liquid ExtractionДокумент16 страницLiquid Liquid ExtractionShahrizatSmailKassimОценок пока нет
- This Study Resource WasДокумент8 страницThis Study Resource WasDaniellaОценок пока нет
- Solutions - Active Pharmaceutical IngredientsДокумент4 страницыSolutions - Active Pharmaceutical IngredientsMXLTRОценок пока нет
- CheДокумент15 страницChenelsonОценок пока нет
- Liquid Liquid ExtractionДокумент12 страницLiquid Liquid ExtractionKhalil Lasfer100% (1)
- SolutoinДокумент144 страницыSolutoinrandatagОценок пока нет
- Liquid Liquid ExtractionДокумент33 страницыLiquid Liquid ExtractionDrAlok GargОценок пока нет
- Liquid/Liquid Extraction HandoutДокумент28 страницLiquid/Liquid Extraction HandoutTINOTENDA TERAОценок пока нет
- Expt1 CaffeineДокумент8 страницExpt1 CaffeineCjES EvaristoОценок пока нет
- Oil and Water Won't Mix and Other Mixture Separation Techniques - Chemistry Book for Kids 8-10 | Children's Chemistry BooksОт EverandOil and Water Won't Mix and Other Mixture Separation Techniques - Chemistry Book for Kids 8-10 | Children's Chemistry BooksОценок пока нет
- Solution Chemistry: Essential Chemistry Self-Teaching GuideОт EverandSolution Chemistry: Essential Chemistry Self-Teaching GuideОценок пока нет
- Mader's Understanding Human Anatomy & Physiology 9th Edition PDFДокумент513 страницMader's Understanding Human Anatomy & Physiology 9th Edition PDFCaroline H David90% (30)
- Canine IndicesДокумент5 страницCanine IndicesCaroline H DavidОценок пока нет
- Kelantan SKEMA PDFДокумент17 страницKelantan SKEMA PDFCaroline H DavidОценок пока нет
- Exercise 1Документ5 страницExercise 1Caroline H DavidОценок пока нет
- Canine Index-A Tool For Sex Determination - BakkanwharДокумент5 страницCanine Index-A Tool For Sex Determination - BakkanwharCaroline H David100% (1)
- Gantt ChartДокумент1 страницаGantt ChartCaroline H DavidОценок пока нет
- Acharya 2009Документ3 страницыAcharya 2009Caroline H DavidОценок пока нет
- Broyles2e ch3Документ96 страницBroyles2e ch3swareshОценок пока нет
- PT3 Kelantan PDFДокумент27 страницPT3 Kelantan PDFCaroline H DavidОценок пока нет
- BFS Thesis & Proposal GuidelinesДокумент34 страницыBFS Thesis & Proposal GuidelinesCaroline H DavidОценок пока нет
- Antianginal Drugs FinalДокумент38 страницAntianginal Drugs FinalCaroline H DavidОценок пока нет
- Paper Additives FillerДокумент4 страницыPaper Additives FillerCaroline H DavidОценок пока нет
- Antianginal Drugs FinalДокумент38 страницAntianginal Drugs FinalCaroline H DavidОценок пока нет
- Experiment 2Документ2 страницыExperiment 2Caroline H David100% (1)
- Form4 ExerciseДокумент9 страницForm4 ExerciseCaroline H DavidОценок пока нет
- Experiment 1Документ3 страницыExperiment 1Caroline H DavidОценок пока нет
- Lab Practical 3: DNA EXTRACTIONДокумент2 страницыLab Practical 3: DNA EXTRACTIONCaroline H DavidОценок пока нет
- Lab Practical 4:gel ElectrophoresisДокумент2 страницыLab Practical 4:gel ElectrophoresisCaroline H David100% (5)
- Form4 NOTES-Nervous SystemДокумент9 страницForm4 NOTES-Nervous SystemCaroline H DavidОценок пока нет