Академический Документы
Профессиональный Документы
Культура Документы
Iitjee Chemistry Sample Paper - Iv: Solutions
Загружено:
NinderИсходное описание:
Оригинальное название
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
Iitjee Chemistry Sample Paper - Iv: Solutions
Загружено:
NinderАвторское право:
Доступные форматы
www.EntrancesofIndia.
com
www.EntrancesofIndia.com
IITJEE CHEMISTRY SAMPLE PAPER IV
SOLUTIONS
SECTION I
Straight Objective Type
1.
(c)
As the size of F is very small compared to that of Cl, bond length dominates over
electro-negativity difference. Hence, dipole moment of CH3Cl > CH3F.
As F is more electro-negative than Cl, in CH3F C is more electron deficient than
that of the carbon is CH3Cl. So shared pairs of CH bond are staying more closer to
C in case of CH3F and more bond pair-bond pair electron repulsion and more will be
the HCH bond angle.
2.
(b)
Lassaignes test for nitrogen fails if the nitrogen containing compound does not
contain carbon.
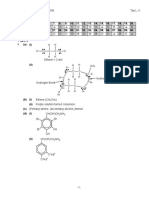
Br
OCH3
3.
(b)
CH2CH2
CH3OH
CH2CH2
Br
Br
CH2CH2
Br
Br
O
4.
(c)
The compound (A) is CH3CCH(OCH3)2 because it contains CH3C group which
responds to iodoform test. It does not react with Tollens reagent, because it does not
contain CHO group.
O
On hydrolysis, it converts to CH3CCH2CHO , which can react with Tollens
reagent. The compound (a) is not possible because hemiacetals can give Tollens
reagent test.
5.
(d)
CaCO 3
CaO CO 2
(X)
CaO H 2 O
Ca (OH) 2
CaCO 3
6.
(c)
1.6 10
=
7.
(b)
X(s)
Y(s)
Ca (OH) 2
(Y)
CO 2
CO 2
19
10 4
CaCO 3
H 2O
H 2O
Ca (HCO 3 ) 2
( Z)
1
mv 2
2
h
0.123
mv
A(g) + 2B(g)
p1
(2p1 + 2p2)
2B(g) + C(g)
Downloaded From www.EntrancesofIndia.com
For any other Engineering Entrance exam paper, Check out our Website.
Since
(2p1 + 2p2)
Kp
p
1
KP
p2
p1
4.5
8.
(a)
p2
2p 2
103 = p2.4(3p2)2 = 36 p23
4.5
1
p23
10 3
10 3
36
8
p2 = 0.05 atm
P1 = 2 .05 = 0.10 atm
p t p1 2p1 2p 2 p 2 3(p1
p 2 ) = 3(0.10 + 0.05) = 0.45 atm.
A
D C
D A
SECTION II
Reasoning Type
9.
(d)
[Cu(NH3)4]+2 has square planar geometry.
10.
(c)
CH3CH2CH2CH3 + Cl2
hv
*
CH3CH2CHCH3
Cl
11.
(c)
CH3
CH3CH2CHOCH3 + H+
CH3CH2CHOCH3
CH3 H
12.
(d)
ln
k2
k1
Ea 1
R T2
Ea 1
R T1
CH3Br + CH3CH2CHCH3
OH
1
T1
1
T2
Higher the value of Ea, greater is the
in rate.
Br
k2
ratio and hence, greater will be the increase
k1
www.EntrancesofIndia.com
www.EntrancesofIndia.com
SECTION III
Linked Comprehension Type
PassageI
15.
(d)
16.
(b)
The feasible reaction is Cu (s) 2Ag (aq)
E 0cell
0.8 0.34
Cu (aq) 2Ag (s) as
ve
17.
(d)
18.
(d)
19.
(b)
20.
(b)
1.
(A) 2 ; (B) 3 ; (C) 2, 3 ; (D) 1, 4
2.
(A) 2, 4 ; (B) 1, 2, 3, 4 ; (C) 1, 3, 4 ; (D) 3
PassageII
NaNO2/conc. HCl at 05C results in diazonium salt formation which forms 1, 2, 3tribromobenzene with H3PO2.
SECTION IV
Matrix Match Type
SECTION V
Subjective or Numerical Type
1.
1.86 = Kf(1)
0.02046 = (1 + ) 1.86 0.01
0.02046
1
1.1
0.0186
= 0.1
[H+] = C = 0.01 0.1 = 103
pH = 3.
2.
x
m
log
1
n
kP
x
m
log k
Now, tan
1
log P
n
1
1
n
n
n=1
Intercept = log k
k 2
x
2(2) 4 .
m
tan 45
log 2
Вам также может понравиться
- Iitjee Chemistry Sample Paper - Iv: SolutionsДокумент3 страницыIitjee Chemistry Sample Paper - Iv: SolutionsAbhishek KumarОценок пока нет
- Chemistry PQMSДокумент10 страницChemistry PQMSprincesingh052005Оценок пока нет
- Sample Paper 1 (Solutions Only) - IsC Chemistry 2024Документ17 страницSample Paper 1 (Solutions Only) - IsC Chemistry 2024Dia SureshОценок пока нет
- G12 - Sample Paper #1 Chemistry SolutionsДокумент9 страницG12 - Sample Paper #1 Chemistry Solutionsrajprince8818Оценок пока нет
- 2013 Jan Unit 5 Chemistry A Level EdexcelДокумент24 страницы2013 Jan Unit 5 Chemistry A Level EdexcelJames KingОценок пока нет
- Fiitjee: Solutions To AIEEE-2007-CHEMISTRY Paper Code (O) - 1Документ9 страницFiitjee: Solutions To AIEEE-2007-CHEMISTRY Paper Code (O) - 1Lokesh KumarОценок пока нет
- Chem 36: General ChemistryДокумент13 страницChem 36: General ChemistryAbdulhakeemSolimanОценок пока нет
- Coordination Compounds - JEE Mains PYQ 2020-2022Документ214 страницCoordination Compounds - JEE Mains PYQ 2020-2022AustinОценок пока нет
- Combined OrganicДокумент82 страницыCombined OrganicSachin KumarОценок пока нет
- CHEM 1000 Mid-Year Exam December 2002: Part A. 60 Marks. Answer Each Question (5 Marks Each)Документ7 страницCHEM 1000 Mid-Year Exam December 2002: Part A. 60 Marks. Answer Each Question (5 Marks Each)Geleni Shalaine BelloОценок пока нет
- Chemistry Paper With Answer SolutionДокумент11 страницChemistry Paper With Answer SolutionNahasОценок пока нет
- Final Exam Study GuideДокумент15 страницFinal Exam Study Guidekramark808Оценок пока нет
- Nov 2008Документ13 страницNov 2008dharshanaabОценок пока нет
- Iit Physic Question PapersДокумент18 страницIit Physic Question PapersSunil PandeyОценок пока нет
- 2019 JC1 H2 MYE Sections A and C - Mark Scheme With Examiners CommentsДокумент18 страниц2019 JC1 H2 MYE Sections A and C - Mark Scheme With Examiners CommentsTimothy HandokoОценок пока нет
- Edexcel GCE Chemistry Unit-5 June 2014 Question PaperДокумент28 страницEdexcel GCE Chemistry Unit-5 June 2014 Question PaperAvrinoxОценок пока нет
- Narayana... Iit Jee PaperДокумент26 страницNarayana... Iit Jee PaperAbhishek KumarОценок пока нет
- STPM Trial 2012 Chemistry Qa KelantanДокумент42 страницыSTPM Trial 2012 Chemistry Qa Kelantanteoh6234100% (2)
- Class 11 Chemistry Topperlearning Sample Paper3Документ23 страницыClass 11 Chemistry Topperlearning Sample Paper3phultushiblsОценок пока нет
- CHEM101 051 Old-Exam Second-Major Master-KeyДокумент10 страницCHEM101 051 Old-Exam Second-Major Master-KeyalwafiОценок пока нет
- Final Selection Examination For The 2004 Australian Chemistry Olympiad TeamДокумент6 страницFinal Selection Examination For The 2004 Australian Chemistry Olympiad Teamrajeswar royОценок пока нет
- 6CH01 01R Que 20140523Документ28 страниц6CH01 01R Que 20140523Celinne TehОценок пока нет
- AIPMT 2015 Sample PaperДокумент26 страницAIPMT 2015 Sample PaperFirdosh Khan100% (3)
- Class - Xii Chemistry Sample Paper - 3 Time: Three Hours Max. Marks: 70 General InstructionsДокумент17 страницClass - Xii Chemistry Sample Paper - 3 Time: Three Hours Max. Marks: 70 General Instructionssoumya mazumdarОценок пока нет
- STPM Trial 2012 Chemistry Qa SmkSeafield SJДокумент27 страницSTPM Trial 2012 Chemistry Qa SmkSeafield SJVitez RaoОценок пока нет
- 2013 Alkane Tutorial (Solutions)Документ7 страниц2013 Alkane Tutorial (Solutions)Pinzhen ChenОценок пока нет
- NEET 2019 Question Paper With Answers and Solution ChemistryДокумент11 страницNEET 2019 Question Paper With Answers and Solution Chemistryashutosh singh pariharОценок пока нет
- Concerto in E Sharp Minor by Alfred ReedДокумент62 страницыConcerto in E Sharp Minor by Alfred ReedMalcolm TanОценок пока нет
- Class XI Chem SAMPLEДокумент4 страницыClass XI Chem SAMPLEFIITJEE DPSОценок пока нет
- Compartment 2 Chem QPДокумент5 страницCompartment 2 Chem QPAAKASH BHATTОценок пока нет
- HCI 2021 Prelim Paper 1 SolutionsДокумент18 страницHCI 2021 Prelim Paper 1 Solutions4A730RudhreshОценок пока нет
- XIIth ChemistryДокумент7 страницXIIth ChemistryRiya MalikОценок пока нет
- Chemistry Practise QuestionДокумент12 страницChemistry Practise Questiong24n3950Оценок пока нет
- Nov 2015Документ54 страницыNov 2015dharshanaabОценок пока нет
- A Level Chemistry Paper 1 Set 31marking GuideДокумент14 страницA Level Chemistry Paper 1 Set 31marking GuidekitookebarnabasОценок пока нет
- Crclho Clho: Material Downloaded From and Portal For Cbse Notes, Test Papers, Sample Papers, Tips and TricksДокумент12 страницCrclho Clho: Material Downloaded From and Portal For Cbse Notes, Test Papers, Sample Papers, Tips and TricksChandan PatraОценок пока нет
- QP 3 Xi Chem Paper 3Документ5 страницQP 3 Xi Chem Paper 3technical SiteОценок пока нет
- 2018 A Level H2 CM Suggested SolutionДокумент19 страниц2018 A Level H2 CM Suggested SolutionabishekksivarajОценок пока нет
- 11 Chem Hy Qp-Set 2Документ5 страниц11 Chem Hy Qp-Set 2jameslebronhadi2005Оценок пока нет
- 3R SolutionsДокумент64 страницы3R SolutionsMichelleHanОценок пока нет
- Mahesh Janmanchi Iit 2010 Paper 1Документ15 страницMahesh Janmanchi Iit 2010 Paper 1janmanchiОценок пока нет
- MCQs 1Документ6 страницMCQs 1VVA. .S0603Оценок пока нет
- XI Chemistry QPДокумент6 страницXI Chemistry QPuddyan TripathiОценок пока нет
- Sankalp Sanjeevani NEET: Chemical BondingДокумент10 страницSankalp Sanjeevani NEET: Chemical BondingKey RavenОценок пока нет
- KCET 2020 Chemistry Paper Questions and SolutionsДокумент33 страницыKCET 2020 Chemistry Paper Questions and SolutionsSaroja RajuОценок пока нет
- 235practice Exam 2 AnswerДокумент9 страниц235practice Exam 2 Answernbobs7Оценок пока нет
- 2009 RI Prelims Chem H2 P1 QPДокумент16 страниц2009 RI Prelims Chem H2 P1 QPniveumaОценок пока нет
- MCAT Review SilberbergДокумент26 страницMCAT Review SilberbergGuy La100% (1)
- 2010 NYJC 9647 H2 Chem Paper 3 AnswersДокумент25 страниц2010 NYJC 9647 H2 Chem Paper 3 AnswersYeeloong YlОценок пока нет
- Asm1 Chemistry 253147Документ6 страницAsm1 Chemistry 253147deek_jОценок пока нет
- 2015 NYJC H2 Chem PrelimДокумент55 страниц2015 NYJC H2 Chem PrelimTan Jia YiОценок пока нет
- Chemistry - Sample Question Paper - 9Документ6 страницChemistry - Sample Question Paper - 9Mohd AdilОценок пока нет
- Practice Makes Perfect in Chemistry: Oxidation-ReductionОт EverandPractice Makes Perfect in Chemistry: Oxidation-ReductionРейтинг: 5 из 5 звезд5/5 (1)
- Practice Makes Perfect in Chemistry: Oxidation-Reduction with AnswersОт EverandPractice Makes Perfect in Chemistry: Oxidation-Reduction with AnswersОценок пока нет
- Computational Methods in Lanthanide and Actinide ChemistryОт EverandComputational Methods in Lanthanide and Actinide ChemistryMichael DolgОценок пока нет
- Introduction?: Who Invented The Microwave Oven?Документ10 страницIntroduction?: Who Invented The Microwave Oven?NinderОценок пока нет
- Chemistry Project On Salivary AmylaseДокумент16 страницChemistry Project On Salivary AmylaseSurjyasnata Rath100% (2)
- DemoДокумент1 страницаDemoNinderОценок пока нет
- Che-110Документ3 страницыChe-110NinderОценок пока нет
- N 57298 C 0257363Документ2 страницыN 57298 C 0257363NinderОценок пока нет
- Saint Fateh Singh Convent School, Maur Mandi: Paper: Chemistry Practical 10+2 30 MarksДокумент5 страницSaint Fateh Singh Convent School, Maur Mandi: Paper: Chemistry Practical 10+2 30 MarksNinderОценок пока нет
- Presentation 1Документ1 страницаPresentation 1NinderОценок пока нет
- 01 PhysicsДокумент8 страниц01 PhysicsNinderОценок пока нет
- 03 ChemistryДокумент8 страниц03 ChemistryNinderОценок пока нет
- Iitjee Mathematics Sample Paper - Iii: SolutionsДокумент4 страницыIitjee Mathematics Sample Paper - Iii: SolutionsNinderОценок пока нет
- 05 Maths PDFДокумент8 страниц05 Maths PDFNinderОценок пока нет
- 03 Chemistry PDFДокумент7 страниц03 Chemistry PDFNinderОценок пока нет
- Iitjee Physics Sample Paper - Iii: SolutionsДокумент3 страницыIitjee Physics Sample Paper - Iii: SolutionsNinderОценок пока нет
- 01 Physics PDFДокумент8 страниц01 Physics PDFNinderОценок пока нет
- 05 Maths PDFДокумент8 страниц05 Maths PDFNinderОценок пока нет
- Iitjee Mathematics Sample Paper - Iii: SolutionsДокумент4 страницыIitjee Mathematics Sample Paper - Iii: SolutionsNinderОценок пока нет
- Iitjee Physics Sample Paper - Iii: SolutionsДокумент3 страницыIitjee Physics Sample Paper - Iii: SolutionsNinderОценок пока нет
- Iitjee Chemistry Sample Paper - Iii: SolutionsДокумент3 страницыIitjee Chemistry Sample Paper - Iii: SolutionsNinderОценок пока нет
- 01 Physics PDFДокумент8 страниц01 Physics PDFNinderОценок пока нет
- Iitjee Chemistry Sample Paper - Iii: SolutionsДокумент3 страницыIitjee Chemistry Sample Paper - Iii: SolutionsNinderОценок пока нет
- 01 PhysicsДокумент9 страниц01 PhysicsNinderОценок пока нет
- 03 ChemistryДокумент8 страниц03 ChemistryNinderОценок пока нет
- Short Tricks of Maths For Iit-JeeДокумент38 страницShort Tricks of Maths For Iit-JeePraveen Kaushik73% (51)
- 03 ChemistryДокумент8 страниц03 ChemistryNinderОценок пока нет
- Iitjee Mathematics Sample Paper - Iv: SolutionsДокумент3 страницыIitjee Mathematics Sample Paper - Iv: SolutionsNinderОценок пока нет
- 05 MathsДокумент8 страниц05 MathsNinderОценок пока нет
- Iitjee Physics Sample Paper - Iv: SolutionsДокумент6 страницIitjee Physics Sample Paper - Iv: SolutionsNinderОценок пока нет
- Problems in Calculus of One Variable - I. A. Maron - DjvuДокумент694 страницыProblems in Calculus of One Variable - I. A. Maron - DjvuNinderОценок пока нет
- 01 Physics PDFДокумент10 страниц01 Physics PDFNinderОценок пока нет