Академический Документы
Профессиональный Документы
Культура Документы
Fission and Fusion Worksheet
Загружено:
black bettyАвторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
Fission and Fusion Worksheet
Загружено:
black bettyАвторское право:
Доступные форматы
Date:
Name:
Class:
Reinforcement
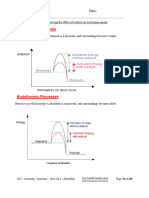
Fission and Fusion
Chapter 21
BLM 21-1
Goal
Show your understanding of concepts related to fission and fusion.
Procedure
Answer the following questions on a separate page. Show your calculations where
applicable.
1. Compare and contrast fission reactions and fusion reactions. Include nuclear
equations to show an example of each type of reaction.
2. In a certain nuclear reaction, a slow neutron collides with a uranium-235 atom.
The collision results in one rubidium-90 atom, one cesium-144 atom, two
neutrons, and thermal energy.
(a) Write an equation to represent this nuclear reaction.
(b) What type of nuclear reaction is this? Explain how you know.
(c) Compare the mass of the uranium-235 nucleus to the combined mass of the
rubidium-90 atom and the cesium-144 atom. Explain your answer in detail.
3. Consider the following equation:
235
92
U 01 n
140
54
Xe
2 01 n
(a) What type of nuclear reaction is shown in the equation?
(b) Complete the equation.
4. (a) For nuclides with atomic mass numbers larger than 60, what happens to the
binding energy per nucleon as the atomic mass number increases? How does
this trend relate to the energy produced by fission?
(b) Why do only very large nuclei undergo fission?
5. Explain why fission products tend to be very radioactive.
6. In a fission reaction, the loss of mass was 4.32 105 g. How much energy was
released? Show your calculations.
7. What conditions are necessary for fusion reactions to take place? Why are these
conditions necessary?
Copyright 2003 McGraw-Hill Ryerson Limited.
Вам также может понравиться
- Chapter 12 - Radioactivity Excercise Ex. 12BДокумент32 страницыChapter 12 - Radioactivity Excercise Ex. 12Brohan udupaОценок пока нет
- Nuclei and Nuclear EnergyДокумент2 страницыNuclei and Nuclear EnergyankitОценок пока нет
- IB Assessment Statements, Topic 7.3: Nuclear ReactionsДокумент65 страницIB Assessment Statements, Topic 7.3: Nuclear ReactionsArun MuddamsettyОценок пока нет
- Nuclear PhysicsДокумент16 страницNuclear PhysicsSprabhu ParamОценок пока нет
- ws23 4Документ9 страницws23 4Louis Fetilo FabunanОценок пока нет
- Nuclear Reactors: Factsheet PhysicsДокумент4 страницыNuclear Reactors: Factsheet PhysicsSarwar MahmoodОценок пока нет
- Science Department: Topic 7.1 - Discrete Energy & Radioactivity Topic 7.2 - Nuclear Reactions 12 Grade (Physics SL)Документ8 страницScience Department: Topic 7.1 - Discrete Energy & Radioactivity Topic 7.2 - Nuclear Reactions 12 Grade (Physics SL)ananОценок пока нет
- Nuclear Reactions, Nuclear EnergeticsДокумент30 страницNuclear Reactions, Nuclear Energeticsmelodi1453Оценок пока нет
- MIT22 01F16 ProblemSet1Документ4 страницыMIT22 01F16 ProblemSet1koitaabdoulaye12.maОценок пока нет
- Fission and FusionДокумент6 страницFission and FusionShikhar SinghОценок пока нет
- g485 5 3 4 Fission and FusionДокумент14 страницg485 5 3 4 Fission and Fusionapi-236179294Оценок пока нет
- 8.3.2 Nuclear PowerДокумент8 страниц8.3.2 Nuclear PowerBosco Merino EchevarriaОценок пока нет
- ACT. 12 Answer DliДокумент3 страницыACT. 12 Answer DliDexter DizonОценок пока нет
- Chemistry Crunch #13.2: Nuclear Fission & Fusion KEY Why?Документ5 страницChemistry Crunch #13.2: Nuclear Fission & Fusion KEY Why?kmantoineОценок пока нет
- Factors Affecting Rates of Reaction: Chemguide - QuestionsДокумент2 страницыFactors Affecting Rates of Reaction: Chemguide - QuestionsFatma MoustafaОценок пока нет
- Chapter 1Документ8 страницChapter 1zjutt sabОценок пока нет
- PHYSICSДокумент2 страницыPHYSICSlkОценок пока нет
- Note 28 Oct 2023Документ21 страницаNote 28 Oct 2023Jihan Abou GhaddaraОценок пока нет
- Nuclear Tutorial SolutionДокумент19 страницNuclear Tutorial SolutionGordon GohОценок пока нет
- LecPPT4 ModerationДокумент8 страницLecPPT4 ModerationVishal MeenaОценок пока нет
- (Principles of Nuclear Energy) - Modified - AyubДокумент40 страниц(Principles of Nuclear Energy) - Modified - AyubMuzammil KhalilОценок пока нет
- Nuclear Reactions by UlfatДокумент20 страницNuclear Reactions by Ulfataiman javaidОценок пока нет
- Unit 5 - Nuclear Fission, Chain Reaction, Estimation of EnergyДокумент18 страницUnit 5 - Nuclear Fission, Chain Reaction, Estimation of EnergyLinОценок пока нет
- Science Department: Topic 7.1 - Discrete Energy & Radioactivity Topic 7.2 - Nuclear Reactions 12 Grade (Physics SL)Документ8 страницScience Department: Topic 7.1 - Discrete Energy & Radioactivity Topic 7.2 - Nuclear Reactions 12 Grade (Physics SL)ananОценок пока нет
- The Fires of Nuclear FissionДокумент27 страницThe Fires of Nuclear FissionJayant NaharОценок пока нет
- Nuclear Energy.: LESSON 4 - UnderstandingДокумент6 страницNuclear Energy.: LESSON 4 - UnderstandingTS ShongОценок пока нет
- Ahmed PerbhizДокумент2 страницыAhmed PerbhizahmedОценок пока нет
- 2 - Nuc ChemДокумент44 страницы2 - Nuc ChemRENIER JANE GAIDОценок пока нет
- Nuclear FissionДокумент46 страницNuclear Fissionpaganag333Оценок пока нет
- CBSE TERM2 - NucleiДокумент50 страницCBSE TERM2 - Nucleivenom eОценок пока нет
- Year 13 Physics Topic 7.2 Exam-Style Questions: (82 Marks)Документ13 страницYear 13 Physics Topic 7.2 Exam-Style Questions: (82 Marks)Johan SwartzОценок пока нет
- Worksheet 30 PDFДокумент4 страницыWorksheet 30 PDFVijay Bhaskar100% (3)
- SR Inter IPE Question Bank Chapter-XIV (NUCLEI)Документ18 страницSR Inter IPE Question Bank Chapter-XIV (NUCLEI)sojakoj867Оценок пока нет
- Atoms and NucleiДокумент18 страницAtoms and NucleihumanruhulОценок пока нет
- N ReactionДокумент38 страницN ReactionRizal Shobirin S. E100% (1)
- Energy From Nuclear FissionДокумент22 страницыEnergy From Nuclear FissionBijli ಪಟಾಕಿОценок пока нет
- Fission and FusionДокумент9 страницFission and FusionAlexandra VeresОценок пока нет
- Unit 3.2: Unit 3.2 Mass-Energy RelationshipДокумент33 страницыUnit 3.2: Unit 3.2 Mass-Energy RelationshipGabriel FungОценок пока нет
- Multiple Choice Questions (MCQ) On Nuclear PhysicsДокумент150 страницMultiple Choice Questions (MCQ) On Nuclear PhysicsChudaman MahajanОценок пока нет
- 5.3 Nuclear EnergyДокумент49 страниц5.3 Nuclear EnergyCardry MusicОценок пока нет
- Topic 7 Problem Set 2016Документ9 страницTopic 7 Problem Set 2016Paul AmezquitaОценок пока нет
- Atoms & NucleiДокумент15 страницAtoms & NucleixkryxxzОценок пока нет
- 22.01 Fall 2015, Problem Set 7 (Normal Version) : 1 Conceptual/Analytical QuestionsДокумент8 страниц22.01 Fall 2015, Problem Set 7 (Normal Version) : 1 Conceptual/Analytical QuestionsDANIELA FORERO RAMÍREZОценок пока нет
- Lesson 0103Документ5 страницLesson 0103Suheel AhmedОценок пока нет
- 7.2 Nuclear Reactions 2022Документ7 страниц7.2 Nuclear Reactions 2022ananОценок пока нет
- NucleiДокумент24 страницыNucleihembrampriyanka07Оценок пока нет
- 23 Nuclear PhysicsДокумент52 страницы23 Nuclear Physicsdayana hanis hassanОценок пока нет
- Nuclear Physics Notes For A-Level by HubbakДокумент33 страницыNuclear Physics Notes For A-Level by HubbakHubbak Khan100% (1)
- LecPPT3 - Neutron Interactions, Cross-Section and ExamplesДокумент32 страницыLecPPT3 - Neutron Interactions, Cross-Section and ExamplesVishal MeenaОценок пока нет
- Binding Energy 1Документ1 страницаBinding Energy 1karim adelОценок пока нет
- MODULE 3 Nuclear ChemistryДокумент28 страницMODULE 3 Nuclear ChemistryILIVEFOR MONSTA7Оценок пока нет
- Fission and FusionДокумент8 страницFission and FusionTanmoy BanerjeeОценок пока нет
- UNIT 8: Atoms & Nuclei: Question BankДокумент3 страницыUNIT 8: Atoms & Nuclei: Question BankNathanianОценок пока нет
- Nuclei: 29. A nucleus undergoes through α-decay and transforms to thorium. What isДокумент3 страницыNuclei: 29. A nucleus undergoes through α-decay and transforms to thorium. What isShrvan SudhakarОценок пока нет
- Knief Chapter 2 FinalДокумент49 страницKnief Chapter 2 FinalJohn W HollandОценок пока нет
- Sri Bhagawan Mahaveer Jain College Physics Ii Pu Mock Paper-I Part-AДокумент2 страницыSri Bhagawan Mahaveer Jain College Physics Ii Pu Mock Paper-I Part-AChiranjivi ChiruОценок пока нет
- Nuclei QB XiiДокумент23 страницыNuclei QB XiiToshani GuptaОценок пока нет
- Jonish Class 12 Physics InvestДокумент22 страницыJonish Class 12 Physics Investjeba kingОценок пока нет
- Chemical Kinetics and ElectrochemistryДокумент3 страницыChemical Kinetics and ElectrochemistryB NithuОценок пока нет
- Certified Ethical Hacking Module 5 MapДокумент1 страницаCertified Ethical Hacking Module 5 MapRismal Ray VaughanОценок пока нет
- CEHv9 Instructor GuideДокумент112 страницCEHv9 Instructor Guideblack bettyОценок пока нет
- CEH Exam Blueprint v2.0Документ3 страницыCEH Exam Blueprint v2.0aqil_shamsiОценок пока нет
- Sample ExamДокумент38 страницSample ExamjayarajanОценок пока нет
- Hacking - CEH Cheat Sheet ExercisesДокумент49 страницHacking - CEH Cheat Sheet ExercisesTetuan AzlanОценок пока нет
- Seed Disc Assembly SchemeДокумент1 страницаSeed Disc Assembly Schemeblack bettyОценок пока нет
- MindCert CEH Ethical Hacking MindMapДокумент1 страницаMindCert CEH Ethical Hacking MindMapyki01Оценок пока нет
- W164131 WfeДокумент1 страницаW164131 Wfeblack bettyОценок пока нет
- MG2B - Rev 01 - 2012-11-16Документ1 страницаMG2B - Rev 01 - 2012-11-16black bettyОценок пока нет
- W 2045002 SaleДокумент1 страницаW 2045002 Saleblack bettyОценок пока нет
- W2045002 SalДокумент1 страницаW2045002 Salblack bettyОценок пока нет
- W225001 SaleДокумент1 страницаW225001 Saleblack bettyОценок пока нет
- W225002 SaleДокумент1 страницаW225002 Saleblack bettyОценок пока нет
- W2045002 SalДокумент1 страницаW2045002 Salblack bettyОценок пока нет
- W2045002 - SAL - Rev 06-08Документ1 страницаW2045002 - SAL - Rev 06-08black bettyОценок пока нет
- W184001WFDMEДокумент1 страницаW184001WFDMEblack bettyОценок пока нет
- W 174001 WfeДокумент1 страницаW 174001 Wfeblack bettyОценок пока нет
- MG4A-MG4B - Rev 01 - 2012-11-16Документ1 страницаMG4A-MG4B - Rev 01 - 2012-11-16black bettyОценок пока нет
- 8" Disk Assembly (Nichols PN 08035DYCAB) 540500: Revisions Zone Rev. Description Date ApprovedДокумент1 страница8" Disk Assembly (Nichols PN 08035DYCAB) 540500: Revisions Zone Rev. Description Date Approvedblack bettyОценок пока нет
- 24X14FC12SДокумент1 страница24X14FC12Sblack bettyОценок пока нет
- Mg4a MG4BДокумент1 страницаMg4a MG4Bblack bettyОценок пока нет
- UWQC7 Blank LayoutДокумент1 страницаUWQC7 Blank Layoutblack bettyОценок пока нет
- G9a G9BДокумент1 страницаG9a G9Bblack bettyОценок пока нет
- 22X14FC18SДокумент1 страница22X14FC18Sblack bettyОценок пока нет
- 22X7FC18SДокумент1 страница22X7FC18Sblack bettyОценок пока нет
- 24X14FC12RДокумент1 страница24X14FC12Rblack bettyОценок пока нет
- 22X14FCN18SДокумент1 страница22X14FCN18Sblack bettyОценок пока нет
- 22X14FCN12RДокумент1 страница22X14FCN12Rblack bettyОценок пока нет
- 22X7FCN18SДокумент1 страница22X7FCN18Sblack bettyОценок пока нет
- 22X7FC18DSДокумент1 страница22X7FC18DSblack bettyОценок пока нет
- Pharmacy System Project PlanДокумент8 страницPharmacy System Project PlankkumarОценок пока нет
- Flange CheckДокумент6 страницFlange CheckMohd. Fadhil JamirinОценок пока нет
- Pinterest or Thinterest Social Comparison and Body Image On Social MediaДокумент9 страницPinterest or Thinterest Social Comparison and Body Image On Social MediaAgung IkhssaniОценок пока нет
- Integrated Management System 2016Документ16 страницIntegrated Management System 2016Mohamed HamedОценок пока нет
- Rfis On Formliners, Cover, and EmbedmentsДокумент36 страницRfis On Formliners, Cover, and Embedmentsali tahaОценок пока нет
- Photoshoot Plan SheetДокумент1 страницаPhotoshoot Plan Sheetapi-265375120Оценок пока нет
- Annotated Bibliography 2Документ3 страницыAnnotated Bibliography 2api-458997989Оценок пока нет
- Invenio Flyer enДокумент2 страницыInvenio Flyer enErcx Hijo de AlgoОценок пока нет
- James KlotzДокумент2 страницыJames KlotzMargaret ElwellОценок пока нет
- Jurnal KORELASI ANTARA STATUS GIZI IBU MENYUSUI DENGAN KECUKUPAN ASIДокумент9 страницJurnal KORELASI ANTARA STATUS GIZI IBU MENYUSUI DENGAN KECUKUPAN ASIMarsaidОценок пока нет
- Promoting Services and Educating CustomersДокумент28 страницPromoting Services and Educating Customershassan mehmoodОценок пока нет
- 3-CHAPTER-1 - Edited v1Документ32 страницы3-CHAPTER-1 - Edited v1Michael Jaye RiblezaОценок пока нет
- LhiannanДокумент6 страницLhiannanGreybornОценок пока нет
- D2E133AM4701 Operating Instruction UsДокумент9 страницD2E133AM4701 Operating Instruction UsMohamed AlkharashyОценок пока нет
- Video ObservationДокумент8 страницVideo Observationapi-532202065Оценок пока нет
- Manual de Utilizare HUMAX DIGI TV RDSДокумент116 страницManual de Utilizare HUMAX DIGI TV RDSenamicul50Оценок пока нет
- Advantages of The CapmДокумент3 страницыAdvantages of The Capmdeeparaghu6Оценок пока нет
- -4618918اسئلة مدني فحص التخطيط مع الأجوبة من د. طارق الشامي & م. أحمد هنداويДокумент35 страниц-4618918اسئلة مدني فحص التخطيط مع الأجوبة من د. طارق الشامي & م. أحمد هنداويAboalmaail Alamin100% (1)
- 7 Ways To Support Your Babys Learning Today Monti KidsДокумент19 страниц7 Ways To Support Your Babys Learning Today Monti KidsMareim A HachiОценок пока нет
- Know Your TcsДокумент8 страницKnow Your TcsRocky SinghОценок пока нет
- Mars Atlas MOM 8 13Документ6 страницMars Atlas MOM 8 13aldert_pathОценок пока нет
- By Vaibhav Pandya S R.information Security Consultant M.Tech Solutions (India) PVT - LTDДокумент22 страницыBy Vaibhav Pandya S R.information Security Consultant M.Tech Solutions (India) PVT - LTDtsegay.csОценок пока нет
- Unit 7 - Evolution and Classification: Regents BiologyДокумент24 страницыUnit 7 - Evolution and Classification: Regents BiologyTalijah JamesОценок пока нет
- Vmware It Academy Program May2016Документ26 страницVmware It Academy Program May2016someoneОценок пока нет
- Policy Implementation NotesДокумент17 страницPolicy Implementation NoteswubeОценок пока нет
- ACTIVITY Design - Nutrition MonthДокумент7 страницACTIVITY Design - Nutrition MonthMaria Danica89% (9)
- Language EducationДокумент33 страницыLanguage EducationLaarni Airalyn CabreraОценок пока нет
- Week1 TutorialsДокумент1 страницаWeek1 TutorialsAhmet Bahadır ŞimşekОценок пока нет
- 42ld340h Commercial Mode Setup Guide PDFДокумент59 страниц42ld340h Commercial Mode Setup Guide PDFGanesh BabuОценок пока нет
- Dark Matter and the Dinosaurs: The Astounding Interconnectedness of the UniverseОт EverandDark Matter and the Dinosaurs: The Astounding Interconnectedness of the UniverseРейтинг: 3.5 из 5 звезд3.5/5 (69)
- A Brief History of Time: From the Big Bang to Black HolesОт EverandA Brief History of Time: From the Big Bang to Black HolesРейтинг: 4 из 5 звезд4/5 (2193)
- Giza: The Tesla Connection: Acoustical Science and the Harvesting of Clean EnergyОт EverandGiza: The Tesla Connection: Acoustical Science and the Harvesting of Clean EnergyОценок пока нет
- A Beginner's Guide to Constructing the Universe: The Mathematical Archetypes of Nature, Art, and ScienceОт EverandA Beginner's Guide to Constructing the Universe: The Mathematical Archetypes of Nature, Art, and ScienceРейтинг: 4 из 5 звезд4/5 (51)
- Summary and Interpretation of Reality TransurfingОт EverandSummary and Interpretation of Reality TransurfingРейтинг: 5 из 5 звезд5/5 (5)
- The Beginning of Infinity: Explanations That Transform the WorldОт EverandThe Beginning of Infinity: Explanations That Transform the WorldРейтинг: 5 из 5 звезд5/5 (60)
- The Simulated Multiverse: An MIT Computer Scientist Explores Parallel Universes, The Simulation Hypothesis, Quantum Computing and the Mandela EffectОт EverandThe Simulated Multiverse: An MIT Computer Scientist Explores Parallel Universes, The Simulation Hypothesis, Quantum Computing and the Mandela EffectРейтинг: 4.5 из 5 звезд4.5/5 (20)
- Quantum Spirituality: Science, Gnostic Mysticism, and Connecting with Source ConsciousnessОт EverandQuantum Spirituality: Science, Gnostic Mysticism, and Connecting with Source ConsciousnessРейтинг: 4 из 5 звезд4/5 (6)
- Lost in Math: How Beauty Leads Physics AstrayОт EverandLost in Math: How Beauty Leads Physics AstrayРейтинг: 4.5 из 5 звезд4.5/5 (125)
- Knocking on Heaven's Door: How Physics and Scientific Thinking Illuminate the Universe and the Modern WorldОт EverandKnocking on Heaven's Door: How Physics and Scientific Thinking Illuminate the Universe and the Modern WorldРейтинг: 3.5 из 5 звезд3.5/5 (64)
- Midnight in Chernobyl: The Story of the World's Greatest Nuclear DisasterОт EverandMidnight in Chernobyl: The Story of the World's Greatest Nuclear DisasterРейтинг: 4.5 из 5 звезд4.5/5 (410)
- Packing for Mars: The Curious Science of Life in the VoidОт EverandPacking for Mars: The Curious Science of Life in the VoidРейтинг: 4 из 5 звезд4/5 (1395)
- Let There Be Light: Physics, Philosophy & the Dimensional Structure of ConsciousnessОт EverandLet There Be Light: Physics, Philosophy & the Dimensional Structure of ConsciousnessРейтинг: 4.5 из 5 звезд4.5/5 (57)
- Chasing Heisenberg: The Race for the Atom BombОт EverandChasing Heisenberg: The Race for the Atom BombРейтинг: 4.5 из 5 звезд4.5/5 (8)
- The Power of Eight: Harnessing the Miraculous Energies of a Small Group to Heal Others, Your Life, and the WorldОт EverandThe Power of Eight: Harnessing the Miraculous Energies of a Small Group to Heal Others, Your Life, and the WorldРейтинг: 4.5 из 5 звезд4.5/5 (54)
- Black Holes: The Key to Understanding the UniverseОт EverandBlack Holes: The Key to Understanding the UniverseРейтинг: 4.5 из 5 звезд4.5/5 (13)
- Beyond Weird: Why Everything You Thought You Knew about Quantum Physics Is DifferentОт EverandBeyond Weird: Why Everything You Thought You Knew about Quantum Physics Is DifferentРейтинг: 4 из 5 звезд4/5 (25)
- Quantum Physics: What Everyone Needs to KnowОт EverandQuantum Physics: What Everyone Needs to KnowРейтинг: 4.5 из 5 звезд4.5/5 (49)
- The Tao of Physics: An Exploration of the Parallels between Modern Physics and Eastern MysticismОт EverandThe Tao of Physics: An Exploration of the Parallels between Modern Physics and Eastern MysticismРейтинг: 4 из 5 звезд4/5 (500)
- The Holographic Universe: The Revolutionary Theory of RealityОт EverandThe Holographic Universe: The Revolutionary Theory of RealityРейтинг: 4.5 из 5 звезд4.5/5 (76)
- The Magick of Physics: Uncovering the Fantastical Phenomena in Everyday LifeОт EverandThe Magick of Physics: Uncovering the Fantastical Phenomena in Everyday LifeРейтинг: 4.5 из 5 звезд4.5/5 (3)
- The End of Everything: (Astrophysically Speaking)От EverandThe End of Everything: (Astrophysically Speaking)Рейтинг: 4.5 из 5 звезд4.5/5 (157)