Академический Документы
Профессиональный Документы
Культура Документы
BPFK Reg Flow Chart 2a
Загружено:
Raja Afiq Raja AhmadИсходное описание:
Оригинальное название
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
BPFK Reg Flow Chart 2a
Загружено:
Raja Afiq Raja AhmadАвторское право:
Доступные форматы
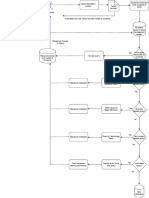
Data Entry by Applicant Applicant to re-enter data
Yes Submission made within 30 days
No
Data deleted entirely from database
Screening by No
Unit Head
Yes Incomplete Submission
Complete Submission
Yes
First Time?
Approved for payment
No
Payment made No Application Applicant
within 14 days? rejected No Yes
correspond
within 30 days
Yes
Applicant to re-submit data
Type of
All applications Application Full evaluation or traditional product only
Tray of Unit Head Laboratory Services Unit
(Centre for Quality Control)
Assign to evaluator
Pharmaceutical Traditional
Evaluation in process Correspondence
/ Traditional? Sample Testing
Yes
Fail Yes
First Applicant
Pharmaceutical Result
Data time appeals
No
submitted is Applicant Assign to evaluators from
complete?
Additional appropriate units
Information
Yes
Evaluation:
Pass No
Approval by Unit Head 1. Analytical Validation Data
2. Protocol of Analysis
Applicant 3. Certificate of Analysis
Compile report of evaluation
dossier and results of
laboratory sample testing Data
and evaluation No
submitted is
Inform applicant Result is
complete?
released to
Centre for
Registration of
Decision of the
Yes
Re-evaluate Products via
Product
QUEST3
Evaluation
Committee
Satisfactory
Meeting of Application
Re-evaluate
the Drug Approved Generate MAL Print product
Control number certificate
Authority
Rejected
Applicant can appeal to
the Minister
The National Pharmaceutical Control Bureau, Ministry of Health Malaysia
Lot 36, Jalan Universiti, 46200 Petaling Jaya, Selangor, Malaysia
Tel : + 603 7883 5400 Fax : + 603 7956 2924 www.bpfk.gov.my
Вам также может понравиться
- Customer Service Block DiagramДокумент1 страницаCustomer Service Block DiagramTushar IyerОценок пока нет
- Cta-861-F Final Revised 2017Документ188 страницCta-861-F Final Revised 2017yinning zengОценок пока нет
- Rpi RGB Led Matrix - WordДокумент95 страницRpi RGB Led Matrix - WordAbhiОценок пока нет
- Led Display Board ProjectДокумент17 страницLed Display Board ProjectRoshanОценок пока нет
- Exploring Application Portfolio Management in Indonesia: A Case Study of The Indonesia Agency For The Assessment and Application of TechnologyДокумент9 страницExploring Application Portfolio Management in Indonesia: A Case Study of The Indonesia Agency For The Assessment and Application of TechnologyCSIT iaesprimeОценок пока нет
- Edid GuideДокумент18 страницEdid GuideexeterbjОценок пока нет
- Information Technology Director in San Francisco CA Resume Ray HallДокумент3 страницыInformation Technology Director in San Francisco CA Resume Ray HallRayHall1Оценок пока нет
- FPT IS overview and business domainsДокумент61 страницаFPT IS overview and business domainsDương ZeeОценок пока нет
- Audio Visual Aspect Ratios - CTS PrepДокумент66 страницAudio Visual Aspect Ratios - CTS PrepOctavio NietoОценок пока нет
- Mfa Quick Admin GuideДокумент26 страницMfa Quick Admin GuideJosh WhiteОценок пока нет
- TAI - MAL Handbook PDFДокумент25 страницTAI - MAL Handbook PDFIssaka Ouedraogo100% (1)
- 已经整理 以色列LED Panel LightДокумент14 страниц已经整理 以色列LED Panel LightLeo SungОценок пока нет
- Project Stage ImplementationДокумент27 страницProject Stage ImplementationDicky AfrizalОценок пока нет
- Creating Awareness of Ict To End UsersДокумент22 страницыCreating Awareness of Ict To End Usersyann_1021Оценок пока нет
- Introduction To Service ManagementДокумент216 страницIntroduction To Service ManagementvuОценок пока нет
- Kansai Nerolac Annual Report 2018Документ205 страницKansai Nerolac Annual Report 2018TejdeepОценок пока нет
- DataVision International Limited - Company ProfileДокумент11 страницDataVision International Limited - Company ProfileWilliam MgayaОценок пока нет
- Get Cts D Exam Guide PDF Save Your 20 On Cts D Questions PDFДокумент7 страницGet Cts D Exam Guide PDF Save Your 20 On Cts D Questions PDFJorge CabelloОценок пока нет
- Support Terms and Service Level Agreements (SLA) of The OutSystems Software - OutSystemsДокумент8 страницSupport Terms and Service Level Agreements (SLA) of The OutSystems Software - OutSystemssaracaro13Оценок пока нет
- Data Integrity Guide en 17Документ28 страницData Integrity Guide en 17UnknownОценок пока нет
- ACL Care Customer Support OverviewДокумент5 страницACL Care Customer Support OverviewMohammad Mahmudur RahmanОценок пока нет
- HL7 Interface Specification - Rev9Документ32 страницыHL7 Interface Specification - Rev9adelabraОценок пока нет
- Strategic Management ToolsДокумент31 страницаStrategic Management ToolsMuhammad AzimОценок пока нет
- Techniques For Measuring Quality of ExperienceДокумент13 страницTechniques For Measuring Quality of ExperienceBen DoverОценок пока нет
- Unit 1 Part AДокумент42 страницыUnit 1 Part AShanthiОценок пока нет
- OpenText Product Compatibility Matrix (Current Maintenance)Документ870 страницOpenText Product Compatibility Matrix (Current Maintenance)Sunil KumarОценок пока нет
- Merchant Banking and Financial Services AssignmentsДокумент8 страницMerchant Banking and Financial Services AssignmentsAnkit JugranОценок пока нет
- S4hana Cloud Top Value AreasДокумент6 страницS4hana Cloud Top Value AreasTUI ThaweesakОценок пока нет
- Genesys Inbound VoiceДокумент6 страницGenesys Inbound VoiceparidimalОценок пока нет
- Marlabs 15Документ32 страницыMarlabs 15nishmehtaОценок пока нет
- Automated Tool for Regression Testing of Active Safety FeaturesДокумент58 страницAutomated Tool for Regression Testing of Active Safety FeaturesB VAMSI KRISHNA REDDY100% (1)
- OSC Logistics - C&F Refined Foundation Design Document v.1.1Документ66 страницOSC Logistics - C&F Refined Foundation Design Document v.1.1En KirukalgalОценок пока нет
- Manage and Optimize Datasets in Power BIДокумент41 страницаManage and Optimize Datasets in Power BIJYОценок пока нет
- Measuring The Cost Effectiveness of Confluent Cloud: White PaperДокумент10 страницMeasuring The Cost Effectiveness of Confluent Cloud: White PaperDeni DianaОценок пока нет
- Cost Model For Outsourcing 2010Документ23 страницыCost Model For Outsourcing 2010shrikantsubsОценок пока нет
- Lecture 0 INT306Документ38 страницLecture 0 INT306Joy BoyОценок пока нет
- Full Stack Developer Profiles - Capgemini - 12182023Документ7 страницFull Stack Developer Profiles - Capgemini - 12182023Arghya KusumОценок пока нет
- Magic Quadrant For Business Continuity Management Planning SoftwareДокумент20 страницMagic Quadrant For Business Continuity Management Planning SoftwareSantanu LodhОценок пока нет
- TeamViewer API Documentation SummaryДокумент40 страницTeamViewer API Documentation SummaryjonasoutlawОценок пока нет
- MLOPs Artem KovalДокумент38 страницMLOPs Artem KovalchОценок пока нет
- IT StratPlanДокумент36 страницIT StratPlanJum F. AmerinОценок пока нет
- Scalable Wage Management Program to Close Living Wage GapДокумент6 страницScalable Wage Management Program to Close Living Wage GapShweta IyerОценок пока нет
- Information Systems and Technology in BusinessДокумент11 страницInformation Systems and Technology in BusinessZamzam AbdelazimОценок пока нет
- CBV StandardДокумент62 страницыCBV StandardAthanassios HatzisОценок пока нет
- Marlabs FeaturesДокумент3 страницыMarlabs FeaturesGyanesh kumarОценок пока нет
- Kavita Shukla CRM PROJECTДокумент30 страницKavita Shukla CRM PROJECTYogendra Pratap SinghОценок пока нет
- Application Performance Management Advanced For Saas Flyer PDFДокумент7 страницApplication Performance Management Advanced For Saas Flyer PDFIrshad KhanОценок пока нет
- Hid Support HandbookДокумент46 страницHid Support HandbookChance Christian100% (1)
- Chapter 2 - E-Commerce - MechanismsДокумент49 страницChapter 2 - E-Commerce - MechanismsThuong Vy MinhОценок пока нет
- Promantia Profile 2023Документ21 страницаPromantia Profile 2023Prashant A UОценок пока нет
- Priya 732-675-7779 SummaryДокумент6 страницPriya 732-675-7779 Summarysahastra001Оценок пока нет
- DSS components and security questionsДокумент49 страницDSS components and security questionsMiralkhan003 Miralkhan003Оценок пока нет
- Change SAP PM Functional LocationДокумент8 страницChange SAP PM Functional LocationAbdou AbdouОценок пока нет
- BA TrainingДокумент3 страницыBA TrainingChinmay KumarОценок пока нет
- Truecaller SRS overviewДокумент26 страницTruecaller SRS overviewDiksha LomteОценок пока нет
- Bosch Sensortec Product OverviewДокумент16 страницBosch Sensortec Product OverviewAmador Garcia III100% (1)
- Merritt Morning Market 3812 - Mar 31Документ2 страницыMerritt Morning Market 3812 - Mar 31Kim LeclairОценок пока нет
- AX2012 Financials L10 AccountsPayable LabsДокумент73 страницыAX2012 Financials L10 AccountsPayable LabsSky Boon Kok LeongОценок пока нет
- UntitledДокумент39 страницUntitledAvnish ThakkarОценок пока нет
- Flowchart Program ProcessДокумент1 страницаFlowchart Program ProcessJohnjohn MateoОценок пока нет
- French 24Документ54 страницыFrench 24nuckcheddyОценок пока нет
- CPG Management of Type 2 Diabetes Mellitus (4th Edition)Документ84 страницыCPG Management of Type 2 Diabetes Mellitus (4th Edition)apalaginih100% (3)
- Management of Heart FailureДокумент62 страницыManagement of Heart Failureapi-13265958Оценок пока нет
- CPG Management of Type 2 Diabetes Mellitus (4th Edition)Документ84 страницыCPG Management of Type 2 Diabetes Mellitus (4th Edition)apalaginih100% (3)
- Max Heap C ImplementationДокумент11 страницMax Heap C ImplementationKonstantinos KonstantopoulosОценок пока нет
- Question Total Quality ManagementДокумент4 страницыQuestion Total Quality ManagementAztvОценок пока нет
- Foundations of Information Systems in BusinessДокумент28 страницFoundations of Information Systems in BusinessRaheel PunjwaniОценок пока нет
- Script (English)Документ7 страницScript (English)Jona Mae CamachoОценок пока нет
- SQL Introduction and Practical ExamplesДокумент97 страницSQL Introduction and Practical ExamplesRajiv Kumar100% (1)
- Types of Prose Lesson PlanДокумент2 страницыTypes of Prose Lesson PlanChun Sa ParkОценок пока нет
- Chapter 14 SFCДокумент48 страницChapter 14 SFCSiswo Ponco RahardjoОценок пока нет
- Ssp393 - Audi A5 - Convenience Electronics and Driver Assist SystemsДокумент56 страницSsp393 - Audi A5 - Convenience Electronics and Driver Assist SystemsCyberemuleОценок пока нет
- Mary PowellДокумент53 страницыMary PowellAnonymous HZgwzwОценок пока нет
- Alert Broadcasting Conferencing: Broadcasts and Alert NotificationsДокумент4 страницыAlert Broadcasting Conferencing: Broadcasts and Alert Notificationsdaniel.bpmОценок пока нет
- Battle of The Giants - Comparing Kimball and InmonДокумент15 страницBattle of The Giants - Comparing Kimball and InmonFelipe Oliveira GutierrezОценок пока нет
- AI QuestionsДокумент2 страницыAI QuestionsNarender SinghОценок пока нет
- V Flower Vocaloid Wiki FandomДокумент1 страницаV Flower Vocaloid Wiki FandomFlower chanОценок пока нет
- ABB ROBOT Training IRC5 Hardware PDFДокумент97 страницABB ROBOT Training IRC5 Hardware PDFTensaigaОценок пока нет
- Marantz SR 4000 User GuideДокумент30 страницMarantz SR 4000 User Guidekeerthipinnawala6498100% (1)
- SQL (Structured Query Language)Документ35 страницSQL (Structured Query Language)Chemutai JoyceОценок пока нет
- 04 SQLQueriesДокумент18 страниц04 SQLQueriescccc gggg oooОценок пока нет
- Bell Canada's Journey with SAP BPCДокумент20 страницBell Canada's Journey with SAP BPCPraveenОценок пока нет
- Click To Edit Master Title Style Crawl, Walk, RunДокумент43 страницыClick To Edit Master Title Style Crawl, Walk, RunFlavio XongasОценок пока нет
- Fertilizer Information System For Banana PlantatioДокумент5 страницFertilizer Information System For Banana PlantatioHazem EmadОценок пока нет
- Transport Layer: Unit - IVДокумент19 страницTransport Layer: Unit - IVHarishmaОценок пока нет
- End of Chapter 8 (p.606) Questions 1,2,4,8,14.: Short AnswerДокумент4 страницыEnd of Chapter 8 (p.606) Questions 1,2,4,8,14.: Short AnswerTung VanОценок пока нет
- Sunnxt AppДокумент8 страницSunnxt ApppsiphoniphoneОценок пока нет
- Professional PractiseДокумент24 страницыProfessional PractisemarksahaОценок пока нет
- BN20 e PreДокумент59 страницBN20 e PreTuan DinhОценок пока нет
- Compact NSX - SchneiderДокумент296 страницCompact NSX - SchneiderNicolás Santiago UgarteОценок пока нет
- Ghid Automatizari SchneiderДокумент372 страницыGhid Automatizari SchneidervalicanОценок пока нет
- Ict-Webpage 10 q1 w3 Mod3Документ12 страницIct-Webpage 10 q1 w3 Mod3Lemuel Ramos RempilloОценок пока нет
- LoRaWan BookДокумент132 страницыLoRaWan BookHợpですОценок пока нет
- The Rope Memory-A Permanent Storage DeviceДокумент14 страницThe Rope Memory-A Permanent Storage DevicePaul CultreraОценок пока нет