Академический Документы
Профессиональный Документы
Культура Документы
PHY3 CJune 2003
Загружено:
api-3726022Оригинальное название
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
PHY3 CJune 2003
Загружено:
api-3726022Авторское право:
Доступные форматы
PHY3 JUNE 2003 - TOPIC C - NUCLEAR & PARTICLE PHYSICS 1
Topic C - Nuclear and Particle Physics
3. (a) The diagram shows part of the range of unit prefixes.
Copy and complete the diagram below by filling in the empty boxes. (4)
(b) What is meant by the term binding energy?
You may be awarded a mark for the clarity of your answer. (3)
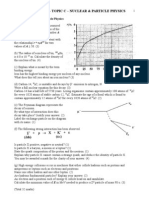
On a copy of the axes opposite sketch a graph of binding energy
per nucleon against nucleon number. (2)
The binding energy of 168O is 123.45 MeV and the binding energy
of 178O is 126.43 MeV.
Which of these two isotopes of oxygen would you expect to be more stable?
Explain your answer. (3)
(c) Add the quark content of the proton to column (ii)
in a copy of of the table opposite.
Complete column (iii) to give the antiparticles of
the particles in column (i).
Complete column (iv) to show the quark content
of the antiparticles. (5)
(d) The Feynman diagram opposite shows the decay of a neutral
lambda particle (Λ) to a proton and a negative pion (π- ).
Show that the strange quark has a charge of - 1/3. (1)
Which of the following words which can be used to describe a π- :
hadron, lepton, meson, baryon
What type of particle is the W - ? (3)
Write down an equation for the decay of the lambda particle. (1)
Show that both charge and baryon number are conserved in this decay. (2)
Explain why this cannot be a strong interaction. (1)
(e) A radioactive isotope of carbon, 146C, is continuously produced in the upper atmosphere when neutrons ejected from
nuclei by cosmic rays collide with atmospheric nitrogen 147N.
Copy and complete the nuclear equation below to show the production of radioactive carbon in the upper atmosphere.
Hence identify X, the other product of the reaction. (3)
Living plants take up carbon dioxide from the atmosphere and have a normal activity
of 15.3 counts per minute per gram of carbon due to absorption of some carbon dioxide containing carbon-14.
On death, the plant no longer takes in carbon-14 and the amount already taken up decays with a half-life of 5730 years.
Estimate the age of an archaeological specimen with an activity of 1.9 counts per minute per gram of carbon. (3)
Suggest one problem in measuring an activity as low as 1.9 counts per minute per gram of carbon. (1)
Total 32 marks
Вам также может понравиться
- The Yellow House: A Memoir (2019 National Book Award Winner)От EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Рейтинг: 4 из 5 звезд4/5 (98)
- Biojune 2008 U4aqДокумент24 страницыBiojune 2008 U4aqapi-3726022100% (1)
- PHY3 DJune 2004Документ1 страницаPHY3 DJune 2004api-3726022Оценок пока нет
- 6102 01 Que 20080109Документ20 страниц6102 01 Que 20080109Inas ManurungОценок пока нет
- Biology Jan 2008 6101 QuestionДокумент20 страницBiology Jan 2008 6101 QuestionAdrees MeahОценок пока нет
- Biojune 2008 U1qДокумент16 страницBiojune 2008 U1qapi-3726022Оценок пока нет
- PHY3 DJanuary 2005Документ1 страницаPHY3 DJanuary 2005api-3726022Оценок пока нет
- PHY3 DJune 2002Документ1 страницаPHY3 DJune 2002api-3726022Оценок пока нет
- PHY3 DJune 2006Документ1 страницаPHY3 DJune 2006api-3726022Оценок пока нет
- PHY3 DJune 2005Документ1 страницаPHY3 DJune 2005api-3726022Оценок пока нет
- PHY3 DJanuary 2004Документ1 страницаPHY3 DJanuary 2004api-3726022Оценок пока нет
- PHY3 AJune 2006Документ1 страницаPHY3 AJune 2006api-3726022Оценок пока нет
- PHY3 CJanuary 2006Документ1 страницаPHY3 CJanuary 2006api-3726022Оценок пока нет
- PHY3 DJanuary 2002Документ1 страницаPHY3 DJanuary 2002api-3726022Оценок пока нет
- PHY3 CJune 2006Документ1 страницаPHY3 CJune 2006api-3726022Оценок пока нет
- PHY3 CJanuary 2005Документ1 страницаPHY3 CJanuary 2005api-3726022Оценок пока нет
- PHY3 CJune 2002Документ1 страницаPHY3 CJune 2002api-3726022Оценок пока нет
- PHY3 CJune 2004Документ1 страницаPHY3 CJune 2004api-3726022Оценок пока нет
- PHY3 CJanuary 2003Документ1 страницаPHY3 CJanuary 2003api-3726022Оценок пока нет
- PHY3 AJune 2004Документ1 страницаPHY3 AJune 2004api-3726022Оценок пока нет
- PHY3 AJune 2005Документ1 страницаPHY3 AJune 2005api-3726022Оценок пока нет
- PHY3 AJune 2003Документ1 страницаPHY3 AJune 2003api-3726022Оценок пока нет
- PHY3 AJanuary 2006Документ1 страницаPHY3 AJanuary 2006api-3726022Оценок пока нет
- PHY3 AJanuary 2004Документ1 страницаPHY3 AJanuary 2004api-3726022Оценок пока нет
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeОт EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeРейтинг: 4 из 5 звезд4/5 (5795)
- Never Split the Difference: Negotiating As If Your Life Depended On ItОт EverandNever Split the Difference: Negotiating As If Your Life Depended On ItРейтинг: 4.5 из 5 звезд4.5/5 (838)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceОт EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceРейтинг: 4 из 5 звезд4/5 (895)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersОт EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersРейтинг: 4.5 из 5 звезд4.5/5 (345)
- The Little Book of Hygge: Danish Secrets to Happy LivingОт EverandThe Little Book of Hygge: Danish Secrets to Happy LivingРейтинг: 3.5 из 5 звезд3.5/5 (400)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureОт EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureРейтинг: 4.5 из 5 звезд4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryОт EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryРейтинг: 3.5 из 5 звезд3.5/5 (231)
- The Emperor of All Maladies: A Biography of CancerОт EverandThe Emperor of All Maladies: A Biography of CancerРейтинг: 4.5 из 5 звезд4.5/5 (271)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaОт EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaРейтинг: 4.5 из 5 звезд4.5/5 (266)
- The Unwinding: An Inner History of the New AmericaОт EverandThe Unwinding: An Inner History of the New AmericaРейтинг: 4 из 5 звезд4/5 (45)
- Team of Rivals: The Political Genius of Abraham LincolnОт EverandTeam of Rivals: The Political Genius of Abraham LincolnРейтинг: 4.5 из 5 звезд4.5/5 (234)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyОт EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyРейтинг: 3.5 из 5 звезд3.5/5 (2259)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreОт EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreРейтинг: 4 из 5 звезд4/5 (1091)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)От EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Рейтинг: 4.5 из 5 звезд4.5/5 (121)