Академический Документы
Профессиональный Документы
Культура Документы
The Effects of Different Catalysts On The Pyrolysis of Industrial Wastes
Загружено:
imafishИсходное описание:
Оригинальное название
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
The Effects of Different Catalysts On The Pyrolysis of Industrial Wastes
Загружено:
imafishАвторское право:
Доступные форматы
Bioresource Technology 99 (2008) 80028007
Contents lists available at ScienceDirect
Bioresource Technology
journal homepage: www.elsevier.com/locate/biortech
The effects of different catalysts on the pyrolysis of industrial wastes (olive and hazelnut bagasse)
_ Ilknur Demiral *, Sevgi Sensz
Department of Chemical Engineering, Faculty of Engineering and Architecture, Eskisehir Osmangazi University, Meselik Campus, 26480 Eskisehir, Turkey
a r t i c l e
i n f o
a b s t r a c t
Pyrolysis of olive and hazelnut bagasse biomass samples with two selected catalysts, namely activated alumina and sodium feldspar, have been conducted in a xed-bed reactor. Experiments were carried out under certain pyrolysis conditions in a xed-bed Heinze reactor. The catalyst was mixed with feedstock in different percentages. The effects of catalysts and their ratio (10%, 20%, 30% and 40% w/w) on the pyrolysis product yields were investigated and the results were compared with the results of experiments performed without catalyst under the same conditions. The maximum bio-oil yields for the bio-oils obtained from pyrolysis of olive bagasse were found as 37.07% and 36.67% on using activated alumina and sodium feldspar as catalysts, respectively, while these values were 27.64% and 31.68%, respectively, for the bio-oils from hazelnut bagasse. The oxygen contents of the bio-oils were also markedly reduced while the yield of bio-oil was reduced by the use of catalysts. The pyrolysis oils were examined using some spectroscopic and chromatographic analysis techniques. The results were compared with the petroleum fractions and the possibility of being a potential source of bio-oils was investigated. 2008 Elsevier Ltd. All rights reserved.
Article history: Received 12 November 2007 Received in revised form 17 March 2008 Accepted 22 March 2008 Available online 2 May 2008 Keywords: Biomass Olive bagasse Hazelnut bagasse Catalytic pyrolysis Bio-oil
1. Introduction The demand for energy and its resources is increasing day by day due to the rapid outgrowth of population and urbanization. As the major conventional energy resources like coal, petroleum and natural gas are at the verge of getting extinct, biomass can be considered as one of the promising environment friendly renewable energy options (Goyal et al., 2008). Biomass has been used as a source of energy throughout history and remains as an important component of national energy supplies in many countries today (Gercel, 2002; Jones, 1989; Hall et al., 1982). Biomass is the fourth largest source of energy in the world, accounting for about 1415% of the worlds primary energy consumption and about 3843% of the primary energy consumption in developing countries (Hall et al., 1982; Chen et al., 2003; Demirbas, 2001; Scurlock et al., 1993). Biomass energy may also become attractive for economically developed countries, particularly at times when global warming is becoming an issue. If the harvesting and ring the biomass as a fuel makes land available for re-growth, these renewable energy sources are nearly CO2 neutral (Liang and Kozinski, 2000). Biomass conversion methods can be divided into two broad pathways: biological (fermentation and anaerobic digestion) and
* Corresponding author. Tel.: +90 222 2393750/3290; fax: +90 222 2393613. _ E-mail address: idemiral@ogu.edu.tr (I. Demiral). 0960-8524/$ - see front matter 2008 Elsevier Ltd. All rights reserved. doi:10.1016/j.biortech.2008.03.053
thermochemical (combustion, gasication and pyrolysis). Among various thermochemical conversion processes, pyrolysis is considered to be an emerging technology for liquid oil production (Islam et al., 2004; Mohan et al., 2006). The liquid obtained from a pyrolysis process is considered to be a very promising biofuel as it can be easily transported, be burnt directly in thermal power stations, be injected into a conventional petroleum renery, be burnt in a gas turbine or upgraded to obtain a light hydrocarbon fuel (Antonakou et al., 2006). The char may be used as solid fuel or activated carbon and the gas has a high caloric value, sufcient to be used for the total energy requirements of the biomass pyrolysis plant. However, the pyrolysis oil is highly oxygenated, viscous, corrosive, relatively unstable and chemically very complex (Horne and Williams, 1994; Bridgwater and Bridge, 1991). Numerous studies regarding liquid fuel production from various sources of biomass have demonstrated that an oil product can be obtained from biomass in signicant yields (Prasad et al., 1986; Encinar et al., 1997, 1998; Srinivas et al., 2000; Williams and Chishti, 2000; Williams and Nugranad, 2000; Demirbas, 2004; Vitolo et al., 1999; Williams and Horne, 1995). Their direct use as conventional fuels may present some difculties due to their high viscosity, poor heating value, corrosiveness and instability. Upgrading pyrolytic oils, a necessary process before using them as a regular fuel, essentially involves removal of oxygen. Currently, two methods have been proposed for this process. The rst method is a typical catalytic hydrotreatment with hydrogen and carbon monoxide
_ I. Demiral, S. Sensz / Bioresource Technology 99 (2008) 80028007
8003
under high pressure and/or in the presence of hydrogen donor solvents. The second method utilizes cracking catalysts (zeolites, silicaalumina and molecular sieves) under atmospheric pressure without hydrogen (Vitolo et al., 1999; Williams and Horne, 1995; Bridgwater, 1996; Horne and Williams, 1996; Lappas et al., 2002). The products obtained from this process depend on the characteristics of the catalyst used. The different catalysts are characterized by different operating conditions and different product distributions. Catalysis in this context is used mainly to crack higher molecular weight compounds to a lighter more commercially valuable product. Catalysts for use in pyrolysis or gasication processes may be divided into two distinct groups: the rst group of catalysts (primary catalyst) is added directly to the biomass before the experiments. The addition is either by wet impregnation of the raw material or by dry mixing of the catalyst with it. They operate under the same temperature with the reactor and are usually nonrenewable (in-bed mode). This type of operation permits the immediate contact of the evolved pyrolysis vapours with the catalyst. The second group of catalysts is placed in a secondary reactor located downstream from the pyrolysis reactor (Sutton et al., 2001; Samolada et al., 2000). In this study, olive bagasse and hazelnut bagasse were chosen as the renewable energy sources. Hazelnut is Turkeys most important agricultural crop since Turkey is one of the main producers of hazelnuts in the world. Turkey has the largest hazelnut production area with 550600 thousand hectares that includes the 83% of total world area. Our country supplied the 80% of world hazelnut production and 70% of world trade with the capacity of 350,000 tons/year (www.turkstat.gov.tr). Although hazelnut is being raised mainly for its fruit and oil, hazelnut bagasse and hazelnut shells have a great importance as a source of energy. Olive is one of the most important agricultural products of the Mediterranean countries (Yaman et al., 2000; Zabaniotou et al., 2000). For example, the total olive production in Turkey in 2004 was approximately 1.6 106 tons (www.turkstat.gov.tr). Pyrolysis experiments of two biomass samples combined with different catalysts at optimum conditions were presented and pyrolysis product yields were compared in this paper. The obtained bio-oils were analyzed and their usabilities as potential sources of renewable fuels were investigated.
Table 1 Proximate and ultimate analyses of used biomass samples Olive bagasse (Sensz et al., 2006) Moisture content (%) Proximate analysisa (%) Volatiles Fixed C Ash Ultimate analysisb (%) C H N Oc H/C Caloric values (MJ/kg)
a b c
Hazelnut bagasse (Demiral and Sensz, 2006a) 9.97
6.82
67.16 21.60 4.42
68.22 15.25 6.56
53.40 7.46 1.70 37.44 1.676 19.97
43.80 6.29 7.92 41.99 1.723 14.78
Weight percentage on dry basis. Weight percentage on dry and ash-free basis. By difference.
of carbon, hydrogen and nitrogen were determined using LECO CHNS 932 Elemental Analyzer and the weight fraction of oxygen was calculated by difference. Determination of ash and moisture was performed according to ASTM Standards (D-1102-84 and D2016-74). 2.2. Catalysts The activated alumina and sodium feldspar catalysts were selected for the catalytic pyrolysis of biomass. They were activated and kept in a desiccator for the experiments. The surface area, pore volume and average pore diameter of activated alumina were found as 304.7 m2/g, 0.407 ml/g and 53.42 , respectively, while these values were found, respectively, as 1.61 m2/g, 5.43 103 ml/g and 135 for sodium feldspar. Surface area, pore volume and average pore diameter were determined by the N2 adsorption method at 77 K with Autosorb 1 C (Quantachrome). 2.3. Pyrolysis
2. Experimental 2.1. Biomass The hazelnut bagasse sample investigated in this study was taken from hazelnut oil factory around the city of Ordu located in the Black Sea region, in the northern part of Turkey. The olive waste used was composed of a crushed mixture of kernel and pulp, and it is a waste product obtained during vegetable oil production from olives in the Marmara region of Turkey. Before the experiments, the samples were dried, ground in a high-speed rotary cutting mill and sieved to obtain the desired particle size. Table 1 shows the proximate and ultimate analyses of the samples (Sensz et al., 2006; Demiral and Sensz, 2006a). Oil, cellulose, hemicellulose, lig nin, extractives and protein contents of the samples were determined according to Turkish Standarts and ASTM Standarts TS 769 (TSE, 1971), TS 324 3-5, by difference, ASTM D 1106-56, ASTM D 1105-56 and TS 324 1-3 (TSE, 1981a, b), respectively. Olive bagasse was found to consist of 12.8% oil, 31.1% cellulose, 15.6% hemicellulose, 25.2% lignin, 28.1% extractives and 10.5% protein (Sensz et al., 2006). Hazelnut bagasse was found to consist of 1.0% oil, 9.0% cellulose, 35.4% hemicellulose, 8.9% lignin, 46.7% extractives and 41% protein (Demiral and Sensz, 2006a). The weight fractions The pyrolysis experiments were performed by dry mixing of the catalysts with biomass samples at in-bed mode in the pyrolysis atmosphere. The 316 stainless steel retort had a volume of 400 cm3 (70 mm ID) and was externally heated by an electrical furnace in which the temperature is measured by a thermocouple inside the bed. The experiments were conducted to determine the effect of catalyst ratio on the pyrolysis yields for both biomass samples. During experiments, 20 g of sample with an average particle size of 0.4250.600 mm for olive bagasse and hazelnut bagasse was mixed with catalyst (10%, 20%, 30% and 40% w/w) and then placed into the reactor. The optimum experimental conditions determined in a previous study were a nal pyrolysis temperature of 500 C, a heating rate of 10 C/min, a sweeping gas ow rate of 150 ml/min and a mean particle size of 0.425 < Dp < 0.600 mm (Sensz et al., 2006; Demiral and Sensz, 2006a). Experimental apparatus was held at nal pyrolysis temperature for either a minimum of 30 min or until no further signicant release of gas was observed. The ow of gas released was measured using a soap lm owmeter for the duration of experiments. The liquid products (bio-oil + water) were collected by condensing in cold traps maintained at about 0 C and recovered by washing with dichloromethane. The aqueous phase in the liquid product was separated using a
8004
_ I. Demiral, S. Sensz / Bioresource Technology 99 (2008) 80028007
separatory funnel. The bio-oil and solvent mixture was passed over dry sodium sulphate to make it water-free and then the solvent was removed from bio-oil by rotavapour. After pyrolysis, the char was weighed. The gas yield was calculated from the material balance. In this study, all the yields are expressed on a dry ash-free (daf) basis and the average yields of at least three experiments are given within the experimental error of less than 0.5%. 2.4. Characterization The bio-oils characterized in this study were obtained under experimental conditions that gave maximum bio-oil yield. The FTIR spectra of the bio-oils were recorded using a Perkin Elmer 100 Model Fourier transform infrared spectrophotometer. The elemental compositions and caloric values of the pyrolysis oils were determined. The caloric values of the olive bagasse, hazelnut bagasse and bio-oils were determined using Gallenkamp Auto Adiabatic Bomb Calorimeter (ASTM 240). Chemical class composition of bio-oils was determined using liquid column chromatographic fractionation. The glass column used was packed with silica gel (70230 mesh) pretreated at 105 C for 2 h prior to use. The column was eluted successively with n-pentane, toluene and methanol to produce aliphatic, aromatic and polar fractions, respectively. The aliphatic fraction was analyzed with a Hewlett-Packard 6890 Model Gas Chromatograph (Hewlett-Packard Instruments, USA) equipped with a ame ionization detector (FID), with 5 ml/min nitrogen as the carrier gas and a thin lm (30 m 0.32 mm i.d.; 0.25 lm lm thickness), HP-5MS capillary column. The injection port and detector were both operated at 300 C. The GC oven was heated to 30 C for 2 min then to 290 C at a rate of 3 C/min. The injection method was used for analysis of 1 ll samples. 3. Results and discussion 3.1. Product yields The biomass samples were pyrolysed to determine the effect of catalyst ratio (10%, 20%, 30% and 40%) on the pyrolysis yields. The results are given in Figs. 14. The bio-oil yields decreased as the catalyst ratio increased. The bio-oil yield from the olive bagasse, which was 37.68% without cat-
alyst, reached the maximum value of 37.07% on using activated alumina catalyst at 10% by weight. The bio-oil yield decreased about 1.62 wt% at catalytic pyrolysis conditions. The results obtained with sodium feldspar, another catalyst, show that the highest bio-oil yield is 36.67% on using this catalyst at 10% by weight. The bio-oil yield decreased about 2.68 wt% for this catalyst. The gas product yields for these two catalysts increased compared to the non-catalytic pyrolysis. The gas yield, obtained as 18.54% without catalyst, increased to 19.94% with activated alumina (40% w/w) and increased to 31.92% with sodium feldspar (40% w/w) as catalysts. The water yield, which was 17.05% without catalyst, reached the maximum value of 20.43% on using activated alumina catalyst at 30% by weight and it reached the maximum value of 13.48% on using sodium feldspar catalyst at 20% in weight. In the experiments performed with hazelnut bagasse; the biooil yield, which was 34.41% without catalyst, reached the maximum value of 27.64% on using activated alumina catalyst at 30% by weight. The decrease of the bio-oil yield was about 19.67 wt% at catalytic pyrolysis conditions. The results obtained with sodium feldspar showed that the highest bio-oil yield was 31.68% on using this catalyst at 40% by weight. A 7.93 wt% decrease was observed in the bio-oil yield under catalytic pyrolysis conditions. The gas yield obtained as 28.97% without catalyst reached the maximum value of 29.13% with activated alumina (10% w/w) and reached the maximum value of 25.75% with sodium feldspar (10% w/w) as catalysts. Pyrolysis products conrmed that the catalysts promote depolymerization processes to yield a strong carbonization (high solid yields) or a strong liquid phase cracking (low liquid yields), and hence a higher hydrogen formation, since this gas was formed from liquid cracking (Encinar et al., 1997). The water yields for the use of two catalysts increased compared to the non-catalytic experiments. The water yield from hazelnut, which was 9.56% without catalyst, reached the maximum value of 21.90% on using activated alumina catalyst at 40% by weight and it reached the maximum value of 17.85% on using sodium feldspar catalyst at 10% by weight. The water yield from the hazelnut bagasse was increased by using catalyst. While examining the results from the water yield point of view, the liquid product generally contains higher amounts of water when a catalytic material is used in relation to self pyrolysis. According to Williams and Horne (1995) the aqueous product was the main result of oxygen removal from the biomass pyrolysis vapours for the Y-zeolite and activated alumina catalysts. The low bio-oil yields resulted from oxygen removal to water, CO2 and CO, from coke formation on the catalyst and from an increase in hydrocarbon gas yield due to catalysis (Vitolo et al., 1999; Williams and Horne, 1995). Pyrolysis char values did not change signicantly in both samples by using different catalyst ratios. Both catalysts have signicant effects in the view of decreasing the bio-oil yield when it is compared to non-catalytic case. These results are consistent with those obtained from some of the previous studies (Encinar et al., 1997, 1998; Williams and Nugranad, 2000; Lappas et al., 2002; Nokkosmaki et al, 1998a; Garcia et al., 1998). 3.2. Chemical composition The properties of bio-oils derived from olive and hazelnut bagasses are given in Table 2. As can be seen from this table, bio-oils contain less oxygen than the original feedstock. The signicant decrease in oxygen content of the bio-oil is important compared to the original feedstock because the high oxygen content is not attractive for the production of transport fuels. Further comparison of the H/C ratios with conventional fuels indicates that the H/C ratios of the bio-oils are very similar to those of light petroleum
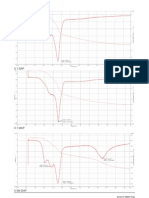
Fig. 1. The change in the product yields from olive bagasse on the activated alumina catalyst (pyrolysis temperature of 500 C, heating rate of 10 C/min, nitrogen ow rate of 150 ml/min).
_ I. Demiral, S. Sensz / Bioresource Technology 99 (2008) 80028007
8005
Fig. 2. The change in the product yields from olive bagasse on the sodium feldspar catalyst (pyrolysis temperature of 500 C, heating rate of 10 C/min, nitrogen ow rate of 150 ml/min).
Fig. 4. The change in the product yields from hazelnut bagasse on the sodium felspar catalyst (pyrolysis temperature of 500 C, heating rate of 10 C/min, nitrogen ow rate of 150 ml/min).
Fig. 3. The change in the product yields from hazelnut bagasse on the activated alumina catalyst (pyrolysis temperature of 500 C, heating rate of 10 C/min, nitrogen ow rate of 150 ml/min).
products. It can be seen in Table 2 that the C and H values increased and oxygen content decreased in the bio-oils obtained by catalytic pyrolysis when compared with non-catalytic experiments. The caloric values of the bio-oils obtained by applying the optimum conditions stated above were determined as 31.80, 33.77 and 32.09 MJ/kg for the products obtained from the pyrolysis of olive bagasse without using a catalyst, with activated alumina catalyst and with sodium feldspar catalyst, respectively, while these values were found, respectively, as 34.57, 34.70 and 35.74 MJ/kg for the bio-oils obtained from the pyrolysis of hazelnut bagasse. When the caloric values of the bio-oils obtained from catalytic pyrolysis are compared with the bio-oils of the non-catalytic process, it can be seen that the addition of activated alumina and sodium feldspar catalysts to raw material provided higher caloric values. As noted in the literature, catalytic treatment caused removal of oxygen, increased heating value and stability (Vitolo et al., 1999). All of the bio-oils were separated into two fractions as n-pentane soluble (deasphalted oil) and insoluble (asphaltenes). The n-pen-
tane soluble materials were further separated by column chromatography as aliphatic, aromatic and polar bases. Overall results are presented in Table 3. Aliphatic fraction contains predominantly parafns and olens. Aromatic fraction contains low molecular weight aromatics, usually benzene or its derivatives, and it contains oxygenated compounds as polar fraction. While n-pentane solubles obtained with non-catalytic pyrolysis of olive bagasse is in the ratio of 80.41%, it reached 77.26% and 74.69% on using activated alumina and sodium feldspar catalysts, respectively. While n-pentane solubles obtained by non-catalytic pyrolysis of hazelnut bagasse is in the ratio of 54.42%, it reached 38.72% and 43.37% on using activated alumina and sodium feldspar catalysts, respectively. In the experiments performed by using olive and hazelnut bagasses, the aliphatic fractions increased to 35.84 and 40.82 wt% for olive bagasse by the use of activated alumina and sodium feldspar catalysts, respectively, while the aliphatic fraction value was 20.63 wt% for non-catalytic process. The aliphatic fractions increased to 33.37 and 36.36 wt% for hazelnut bagasse by the use of activated alumina and sodium feldspar catalysts, respectively, while the aliphatic fraction value was 33.29 wt% for the non-catalytic process. The aromatic fractions, in the respective ratios of 52.13 and 14.81 wt% for olive and hazelnut bagasses for non-catalytic process increased to 18.73 and 22.41 wt% for hazelnut bagasse on the use of activated alumina and sodium feldspar catalysts, respectively. On the other hand, for olive bagasse, the aromatic fraction decreased to 40.45 and 32.03 wt% on the use of activated
Table 2 Elemental composition of bio-oilsa (wt%) Bio-oil source Olive bagasse without catalyst (Sensz et al., 2006) Olive bagasse with activated alumina by 10% Olive bagasse with sodium feldspar by 10% Hazelnut bagasse without catalyst (Demiral and Sensz, 2006b) Hazelnut bagasse with activated alumina by 30% Hazelnut bagasse with sodium feldspar by 40%
a b
C 66.89 68.79 67.83 58.66 68.75 68.17
H 9.18 10.62 9.89 7.06 7.47 8.95
N 2.01 3.02 3.96 8.72 11.20 10.80
Ob 21.92 17.57 18.32 25.56 12.58 12.08
H/C 1.65 1.85 1.75 1.45 1.30 1.57
Obtained at 500 C, 10 C/min, with a nitrogen ow rate of 150 ml/min. By difference.
8006
_ I. Demiral, S. Sensz / Bioresource Technology 99 (2008) 80028007
Table 3 Yields of column chromatographic fractions of bio-oilsa (wt%) Precursor Olive bagasse Olive bagasse Olive bagasse Hazelnut bagasse Hazelnut bagasse Hazelnut bagasse
a
Bio-oil Without catalyst (Sensz et al., 2006) With activated lumina by 10% With sodium feldspar by 10% Without catalyst (Demiral and Sensz, 2006b) With activated alumina by 30% With sodium feldspar by 40%
Pentane soluble oil fraction 80.41 77.26 74.69 54.42 38.72 43.37
Pentane insoluble oil fraction 19.59 22.74 25.31 45.58 61.28 56.63
Pentane (%) 20.63 35.84 40.82 33.29 33.37 36.36
Toluene (%) 52.13 40.45 32.03 14.81 18.73 22.41
Methanol (%) 27.24 23.71 27.15 51.90 48.10 41.23
Obtained at 500 C, 10 C/min, with a nitrogen ow rate of 150 ml/min.
alumina and sodium feldspar as catalysts, respectively. The polar fraction yields, that were 27.24 and 51.90 wt%, respectively, for olive and hazelnut bagasses without using catalysts, decreased to 23.71, 27.15 wt% for olive bagasse and 48.10, 41.23 wt% for hazelnut bagasse with activated alumina and sodium feldspar catalysts, respectively. As noted in the literature, catalytic pyrolysis products of the bio-oils include more aromatics or aliphatics when compared with uncatalysed products (Horne and Williams, 1994; Williams and Brindle, 2002; Nokkosmaki et al., 1998b). When these results are taken into account in the view of the effect of catalysts on the fractions, it is clear that the activated alumina and sodium feldspar catalysts have an important role in affecting the chemical composition when compared with the non-catalytic experimental results in bio-oil. The uncatalysed bio-oil shows a low percentage mass fraction of aliphatic or aromatic compounds but a high concentration of polar material. By using catalysis, the methanol fraction was reduced with a corresponding increase in the other, less polar fraction as can be seen in the literature (Williams and Horne, 1995). The uncatalysed oil is almost entirely composed of oxygenated material which is present in methanol eluates. This is consistent with the results of the elemental analysis. The works of Williams and Nugranad (2000), Williams and Horne (1995), Nokkosmaki et al. (1998a), Williams and Brindle (2002), Vitolo et al. (1999) have all shown that there is a large decrease in the yield of bio-oil after catalysis, while an improved product is obtained with lower oxygen and higher aromatic or aliphatic contents. The FTIR spectra of the catalytic pyrolysis bio-oils were taken for olive and hazelnut bagasses. The FTIR spectra obtained for the bio-oil samples were very similar. The uncataysed pyrolysis bio-oil shows the presence of oxygenated groups. For example, peaks representing OH stretching vibrations between 3050 and 3600 cm1, C@O stretching vibrations between 1650 and 1850 cm1, CO stretching vibrations between 850 and 950 cm1 and CO stretching and OH in-plane deformations between 950 and 1325 cm1 are all present in the uncatalysed bio-oil (Sensz et al., 2006; Demiral and Sensz, 2006b). However, these oxygenated peaks are also present in the catalysed bio-oils suggesting that the catalyst does not completely remove the oxygenated species, as has already been shown by the signicant oxygen content in the bio-oils shown in Table 2. The oxygenated compounds, represented by the functional groups discussed, are represented by a wide range of oxygenated compounds which have been identied in biomass pyrolysis oils. These include primary, secondary and tertiary alcohols such as methanol, propanol, butanol and furfuryl alcohol, carboxylic acids, i.e. formic, acetic, propionic, benzoic and butyric acids and their derivatives, ketones and aldehydes including acetaldehyde, benzaldehydes, acetone, pentanone, indanone and alkylated derivatives, phenol, alkylated phenols and oxyphenols, etc. (Williams and Nugranad, 2000). Some
representatives of nonoxygenated compounds were also present in the catalysed oils. For example, the CH stretching vibrations between 2800 and 3000 cm1 and CH deformation vibrations between 1300 and 1450 cm1 indicate the presence of the functional groups, CH3, CH2 and CH which are characteristic of alkane groups. The adsorption peaks between 675 and 900 cm1 and 1575 and 1625 cm1 are characteristics of single ring aromatic compounds and polycyclic aromatic compounds. The n-pentane subfractions of the bio-oils were identied by gas chromatography using external standards. The n-pentane subfractions consist of normal alkanes, alkenes and branched hydrocarbons for hazelnut bagasse bio-oil with two different catalysts. The straight chain alkanes and alkenes range between C13 and C30 with activated alumina catalyst and between C12 and C30 with sodium feldspar for bagasse bio-oil. The distribution of straight chain alkanes exhibits maxima at C15C17 for bio-oils obtained with catalyst. 4. Conclusion In this study, two biomass samples and two catalysts were investigated from the view of the effects of catalysts on product yields and chemical compositions of the bio-oil products. Bio-oil yield was decreased when compared with non-catalytic experiments for both biomass samples and catalysts. Activated alumina and sodium feldspar catalysts addition provided a higher caloric value to the bio-oil. All the bio-oils were characterized by low oxygen content with a high H/C ratio. Comparison of the H/C ratios with conventional fuels indicates that the H/C ratio of the bio-oils is very similar to that of light petroleum products. Chemical class fraction and elemental analysis of the bio-oils showed that the oxygenated species decreased on using catalysts in the experiments. This is advantageous for their stability, handling and for upgrading purposes. All these factors favor the use of the obtained bio-oils as fuels. In conclusion, the sharp increase of the worldwide oil prices will play an important role in the realization of alternative, renewable energy systems such as bio-oil production. References
Antonakou, E., Lappas, A., Nilsen, M.H., Bouzga, A., Stcker, M., 2006. Evaluation of various types of A1-MCM-41 materials as catalysts in biomass pyrolysis for the production of bio-fuels and chemicals. Fuel 85, 22022212. Bridgwater, A.V., Bridge, S.A., 1991. Biomass pyrolysis liquids, upgrading and utilization. In: Bridgwater, A.V., Grassi, G. (Eds.). Elsevier, Amsterdam (Chapter 2). Bridgwater, A.V., 1996. Production of high grade fuels and chemicals from catalytic pyrolysis of biomass. Catal. Today 29, 285295. Chen, G., Andries, J., Spliethoff, H., 2003. Catalytic pyrolysis of biomass for hydrogen rich fuel gas production. Energ. Convers. Manage. 44, 22892296.
_ I. Demiral, S. Sensz / Bioresource Technology 99 (2008) 80028007 _ Demiral, I., Sensz, S., 2006a. Fixed-bed pyrolysis of hazelnut (Corylus Avellana L.) bagasse: Inuence of pyrolysis parameters on product yields. Energ. Source. Part A. 28, 11491158. _ Demiral, I., Sensz, S., 2006b. Structural analysis of pyrolysis products from pyrolysis of hazelnut (Corylus avellana L.) bagasse. Energ. Source. Part A 28, 11591168. Demirbas, A., 2001. Yields of hydrogen-rich gaseous products via pyrolysis from selected biomass samples. Fuel 80, 18851891. Demirbas, A., 2004. Hazelnut shell to hydrogen rich gaseous products via catalytic gasication process. Energ. Source. 26, 2533. Encinar, J.M., Beltran, F.J., Ramiro, A., Gonzalez, J.F., 1997. Catalyzed pyrolysis of grape and olive bagasse. Inuence of catalyst type and chemical treatment. Ind. Eng. Chem. Res. 36, 41764183. Encinar, J.M., Beltran, F.J., Ramiro, A., Gonzalez, J.F., 1998. Pyrolysis/gasication of agricultural residues by carbon dioxide in the presence of different additives: inuence of variables. Fuel Process. Technol. 55, 219233. Garcia, L., Salvador, M.L., Arauzo, J., Bilbao, R., 1998. Inuence of catalyst weight/biomass ow rate ratio on gas production in the catalytic pyrolysis of pine sawdust at low temperatures. Ind. Eng. Chem. Res. 37, 38123819. Gercel, H.F., 2002. Production and characterization of pyrolysis liquids from sunower-pressed bagasse. Bioresour. Technol. 85, 113117. Goyal, H.B., Seal, D., Saxena, R.C., 2008. Bio-fuels from thermochemical conversion of renewable resources: a review. Renew. Subst. Energ. Rev. 12, 504517. Hall, D.O., Barnard, G.W., Moss, P.A., 1982. Biomass for Energy in Developing Countries. Permagon Press, Oxford. Horne, P.A., Williams, P.T., 1994. Premium quality fuels and chemicals from the uidised bed pyrolysis of biomass with zeolite catalyst upgrading. Renew. Energy 5, 810812. Horne, P.A., Williams, P.T., 1996. Upgrading of biomass-derived pyrolytic vapours over zeolite ZSM-5 catalyst: effect of catalyst dilution on product yields. Fuel 75, 10431050. Islam, M.N., Islam, M.N., Beg, M.R.A., 2004. The fuel properties of pyrolysis liquid derived from urban solid wastes in Bangladesh. Bioresour. Technol. 92, 181 186. Jones, M.R., 1989. Biomass for energy (general). In: Kinati, O., Hall, C.W., Wagener, K., Tsuru, S., Suzuki, T., Sudo, S. (Eds.), Biomass Handbook. E Publishing Inc, Gordan and Breach Science Publisher, Amsterdam, pp. 97107. Lappas, A.A., Samolada, M.C., Iatridis, D.K., Voutetakis, S.S., Vasalos, I.A., 2002. Biomass pyrolysis in a circulating uid bed reactor for the production of fuels and chemicals. Fuel 81, 20872095. Liang, X.H., Kozinski, J.A., 2000. Numerical modeling of combustion and pyrolysis of cellulosic biomass in thermogravimetric systems. Fuel 79, 14771486.
8007
Mohan, D., Pittman, C.U., Steele, P.H., 2006. Pyrolysis of wood/biomass for bio-oil: A critical review. Energ. Fuel 20, 848889. Nokkosmaki, M.I., Krause, A.O.I., Leppamaki, E.A., Kuoppala, E.T., 1998a. A novel test method for catalysts in the treatment of biomass pyrolysis oil. Catal. Today 45, 405409. Nokkosmaki, M.I., Kuoppala, E.T., Leppamaki, E.A., Krause, A.O.I., 1998b. A novel test method for cracking catalysts. J. Anal. Appl. Pyrol. 44, 193204. Prasad, Y.S., Bakhshi, N.N., Mathews, J.F., Eager, R.L., 1986. Catalytic conversion of Canola oil to fuels and chemical feedstocks. Part I. Effect of process conditions on the performance of HZSM-5 catalyst. Can. J. Chem. Eng. 64, 278284. Samolada, M.C., Papafotica, A., Vasalos, I.A., 2000. Catalyst evaluation for catalytic biomass pyrolysis. Energ. Fuel 14, 11611167. Scurlock, J.M.O., Hall, D.O., House, J.I., 1993. Utilizing biomass crops as an energy sources:A European perspective. Water Air Soil Poll. 70, 499518. Srinivas, S.T., Dalai, A.K., Bakhshi, N.N., 2000. Thermal and catalytic upgrading of a biomass derived oil in a dual reactor system. Can. J. Chem. Eng. 78, 343354. Sutton, D., Kelleher, B., Ross, J.R.H., 2001. Review of literature on catalysts for biomass gasication. Fuel Process. Technol. 73, 155173. _ Sensz, S., Demiral, I., Gercel, H.F., 2006. Olive bagasse (Olea europea L.) pyrolysis. Bioresour. Technol. 97, 429436. TSE, 1971. Turkish Standards Institution. Oilseed residues, determination of oil content, TS 769. TSE, 1981a. Turkish Standards Institution. Methods of analysis of seed meals (cakes), determination of crude cellulose, TS 324, pp. 35. TSE, 1981b. Turkish Standards Institution. Methods of analysis of seed meals (cakes), determination of crude protein, TS 324, pp. 13. Turkish Statistical Institute, Turkstat. Available from: <http://www.turkstat.gov.tr>. Vitolo, S., Seggiani, M., Frediani, P., Ambrosini, G., Politi, L., 1999. Catalytic upgrading of pyrolytic oils to fuel over different zeolites. Fuel 78, 11471159. Williams, P.T., Brindle, A.J., 2002. Catalytic pyrolysis of tyres: inuence of catalyst temperature. Fuel 81, 24252434. Williams, P.T., Chishti, H.M., 2000. Two stage pyrolysis of oil shale using a zeolite catalyst. J. Anal. Appl. Pyrol. 55, 217234. Williams, P.T., Horne, P.A., 1995. The inuence of catalyst type on the composition of upgraded biomass pyrolysis oils. J. Anal. Appl. Pyrol. 31, 3961. Williams, P.T., Nugranad, N., 2000. Comparison of products from the pyrolysis and catalytic pyrolysis of rice husks. Energy 25, 493513. Yaman, S., Sahan, M., Haykr-Acma, H., Sesen, K., Kckbayrak, S., 2000. Production of fuel briquettes from olive refuse and paper mill waste. Fuel Process. Technol. 68, 2331. Zabaniotou, A.A., Kalogiannis, G., Kappas, E., Karabelas, A.J., 2000. Olive residues (cuttings and kernels) rapid pyrolysis product yields and kinetics. Biomass Bioenergy 18, 411420.
Вам также может понравиться
- Process Safety Day Presentations 2014pptxДокумент16 страницProcess Safety Day Presentations 2014pptximafishОценок пока нет
- GD Ex TypeДокумент2 страницыGD Ex TypeimafishОценок пока нет
- Risk Management Module 4 Summary PDFДокумент5 страницRisk Management Module 4 Summary PDFNdomaduОценок пока нет
- Barrier Management (PRS192a)Документ2 страницыBarrier Management (PRS192a)imafishОценок пока нет
- FQE Chemicals NORM Decontamination BrochureДокумент4 страницыFQE Chemicals NORM Decontamination BrochureimafishОценок пока нет
- Turnaround Management: Connected Excellence in All We DoДокумент8 страницTurnaround Management: Connected Excellence in All We Doimafish100% (1)
- Subsea Processing On Utube AddressДокумент1 страницаSubsea Processing On Utube AddressimafishОценок пока нет
- Creating Magic Summary - Lee CockerellДокумент6 страницCreating Magic Summary - Lee Cockerellimafish100% (2)
- Creating Magic Summary - Lee CockerellДокумент6 страницCreating Magic Summary - Lee Cockerellimafish100% (2)
- Coffee Roasting ManualДокумент20 страницCoffee Roasting ManualNaeem GherianyОценок пока нет
- General Principles of Freeze DryingДокумент12 страницGeneral Principles of Freeze DryingAdnan HuskicОценок пока нет
- TGA - PyrisДокумент4 страницыTGA - PyrisimafishОценок пока нет
- The Yellow House: A Memoir (2019 National Book Award Winner)От EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Рейтинг: 4 из 5 звезд4/5 (98)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeОт EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeРейтинг: 4 из 5 звезд4/5 (5794)
- The Little Book of Hygge: Danish Secrets to Happy LivingОт EverandThe Little Book of Hygge: Danish Secrets to Happy LivingРейтинг: 3.5 из 5 звезд3.5/5 (400)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureОт EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureРейтинг: 4.5 из 5 звезд4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryОт EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryРейтинг: 3.5 из 5 звезд3.5/5 (231)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceОт EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceРейтинг: 4 из 5 звезд4/5 (895)
- Team of Rivals: The Political Genius of Abraham LincolnОт EverandTeam of Rivals: The Political Genius of Abraham LincolnРейтинг: 4.5 из 5 звезд4.5/5 (234)
- Never Split the Difference: Negotiating As If Your Life Depended On ItОт EverandNever Split the Difference: Negotiating As If Your Life Depended On ItРейтинг: 4.5 из 5 звезд4.5/5 (838)
- The Emperor of All Maladies: A Biography of CancerОт EverandThe Emperor of All Maladies: A Biography of CancerРейтинг: 4.5 из 5 звезд4.5/5 (271)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaОт EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaРейтинг: 4.5 из 5 звезд4.5/5 (266)
- The Unwinding: An Inner History of the New AmericaОт EverandThe Unwinding: An Inner History of the New AmericaРейтинг: 4 из 5 звезд4/5 (45)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersОт EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersРейтинг: 4.5 из 5 звезд4.5/5 (345)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyОт EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyРейтинг: 3.5 из 5 звезд3.5/5 (2259)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreОт EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreРейтинг: 4 из 5 звезд4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)От EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Рейтинг: 4.5 из 5 звезд4.5/5 (121)
- Output Ratings: Diesel Generator Set Exclusively From Your Cat DealerДокумент4 страницыOutput Ratings: Diesel Generator Set Exclusively From Your Cat DealerMwai Janna67% (3)
- AviationДокумент3 страницыAviationTony JohnsonОценок пока нет
- Smart Car (Green Cars)Документ50 страницSmart Car (Green Cars)Radu_ISОценок пока нет
- Fisher Y291aДокумент20 страницFisher Y291aJavi MoralОценок пока нет
- Spec Sheet SS23-CPGKДокумент4 страницыSpec Sheet SS23-CPGKdifufnaОценок пока нет
- Asphalt Binder Basics Specifications, History and FutureДокумент40 страницAsphalt Binder Basics Specifications, History and FutureEdmundo Jaita CuellarОценок пока нет
- More User Manuals On: Ford - CaДокумент32 страницыMore User Manuals On: Ford - CaJORFREОценок пока нет
- Introduction Reciprocating CompressorДокумент6 страницIntroduction Reciprocating CompressorHirak BhattacharyaОценок пока нет
- Manual Estufas ColemanДокумент20 страницManual Estufas Colemankuizkuiz100% (1)
- Abnormal PressureДокумент41 страницаAbnormal PressuremitpgandhiОценок пока нет
- ASCO Engineering Guide 061108Документ34 страницыASCO Engineering Guide 061108Karuna gantiОценок пока нет
- Natural Building 2011-2Документ3 страницыNatural Building 2011-2Peter Paleon PatagonicoОценок пока нет
- Pratt & Whitney Canada: Maintenance Manual MANUAL PART NO. 3017042Документ40 страницPratt & Whitney Canada: Maintenance Manual MANUAL PART NO. 3017042Panca XpОценок пока нет
- Ripi - Light Naphtha IsomerizationДокумент4 страницыRipi - Light Naphtha IsomerizationAsifОценок пока нет
- Cat Sebp5922!00!01-All Mini Orugas PBДокумент377 страницCat Sebp5922!00!01-All Mini Orugas PBWilliam RicoОценок пока нет
- VTT Bio Oil Research + Bio Oil MarketДокумент84 страницыVTT Bio Oil Research + Bio Oil MarketMohammad Reza Anghaei100% (2)
- ch02 EngДокумент18 страницch02 Engapi-3695407Оценок пока нет
- BYCOДокумент2 страницыBYCOTarique AhmedОценок пока нет
- 3412C EMCP II For MUI Engines Electrical System: Tgc1-Up Djn1-Up 9EP1-2988 Tft1-Up BCW1-744 Lry1-Up Rty1-Up 4BZ1-6087Документ4 страницы3412C EMCP II For MUI Engines Electrical System: Tgc1-Up Djn1-Up 9EP1-2988 Tft1-Up BCW1-744 Lry1-Up Rty1-Up 4BZ1-6087Imtiaz AhmedОценок пока нет
- Biodiesel Production Through Waste Cooking OilДокумент15 страницBiodiesel Production Through Waste Cooking OilYan's Senora BescoroОценок пока нет
- MCL 1st Mock Board Exam PDFДокумент11 страницMCL 1st Mock Board Exam PDFAdrian Joshua BernagaОценок пока нет
- Basics of Gas Well DeliquificationДокумент139 страницBasics of Gas Well Deliquificationامسيديل رامو100% (1)
- Power Wave R500: Operator's ManualДокумент42 страницыPower Wave R500: Operator's ManualJohan ZraghozОценок пока нет
- Repari Manuaall PDFДокумент216 страницRepari Manuaall PDFaldy yasiОценок пока нет
- Aircraft Mooring DeviceДокумент32 страницыAircraft Mooring DevicePugal VenthanОценок пока нет
- Regal Raptor NAC Service Manual PDFДокумент55 страницRegal Raptor NAC Service Manual PDFKrzysztof Jakiś33% (6)
- Intra V20 Brochure LowДокумент2 страницыIntra V20 Brochure Lowalatberat sparepartОценок пока нет
- Fuel System D28Документ4 страницыFuel System D28Ian MuhammadОценок пока нет
- Barauni RefineryДокумент10 страницBarauni RefineryRajiv KalraОценок пока нет
- Handbook TabsДокумент1 страницаHandbook TabsABPОценок пока нет