Академический Документы
Профессиональный Документы
Культура Документы
Botulinum Toxin Blocks Muscle Contraction by Inhibiting Acetylcholine Release
Загружено:
Ardette De JesusИсходное описание:
Оригинальное название
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
Botulinum Toxin Blocks Muscle Contraction by Inhibiting Acetylcholine Release
Загружено:
Ardette De JesusАвторское право:
Доступные форматы
De Jesus, Ardette S. Biochemistry Section B1 CLINICAL CASE #1 BOTULISM 1. Discuss the functional unit of muscle and its myofibrils.
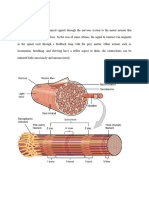
Answer: Each skeletal muscle fiber contains bundles of filaments, called myofibrils, running along the axis of the cell. It has a diameter of 1-2 um and consist of an end to end repetitive arrangement of sarcomeres. A myofibril can be subdivided longitudinally into SARCOMERE, the contractile unit in myofiber. Sarcomere is composed of myofilaments (contractile proteins) arranged in thick and thin filaments. The sarcomere is demarcated with two dark lines called Z lines and represents a repeating contractile unit in skeletal muscle. Light band (I band) contains thin filaments composed primarily of the protein actin. Dark band (A band) contains thick filaments composed primarily of the protein myosin. A light area present the in the center of the sarcomere is called the H band. A dark line called the M line is evident in the center of the sarcomere and includes proteins that appear to be critical for organization and alignment of the thick filaments in the sarcomere
2. What are the major proteins of muscle and their functions. Answer: a. ACTIN- consists of long filamentous polymers containing two strands of globular (G-actin) monomers, 5-6 nm in diameter, twisted around each other in a double helical formation. It is asymmetric and polymerize to produce a filament with polarity. It contains binding site for myosin. b. TROPONIN- consists of three subunits (Tt, Ti,Tc); it is present on each tropomyosin dimer and influences the position of the tropomyosin molecule on the actin filament and hence the ability of tropomyosin to inhibit binding of myosin to the actin filament. c. TROPOMYOSIN- dimer of the protein; extend over the entire actin filament and cover myosin binding sites on the actin molecule. d. MYOSIN- is large protein that consists of six different polypeptides with one pair of large heavy chains and two pairs of light chains. The head region extends away from the thick filament toward
the actin thin filament and is the portion of the molecule that can bind to actin. It is also able to hydrolyze ATP, and ATPase activity is located in the globular head as well. 3. What are the major biochemical events occurring during a muscle contraction and relaxation? Answer: A nerve impulse triggers the release of Ach from the synaptic knob into the synaptic cleft. Ach binds to Ach receptors in the motor end plate of the neuromuscular junction, initiating a muscle impulse in the sarcolemma of the muscle fiber. As the muscle impulse spreads quickly from the sarcolemma along T-tubules, calcium ions are released from terminal cisternae into the sarcoplasm. Calcium ions bind to troponin. Troponin changes shape, moving tropomyosin on the actin to expose active sites on actin molecules of thin filaments. Myosin heads of thick filaments attach to exposed active sites to form crossbridges. Myosin heads pivot, moving thin filaments toward the sarcomere center. ATP binds myosin heads and is broken down into ADP and P. Myosin heads detach from thin filaments and return to their pre-pivot position.
4. Discuss the synthesis, upgrade and degradation of acetylcholine in neuromuscular junction. Answer: ----In the axon terminal are many mitochondria that supply adenosine triphosphate (ATP), the energy source that is used for synthesis of an excitatory transmitter acetylcholine. The acetylcholine in turn excites the muscle fiber membrane. Acetylcholine is synthesized in the cytoplasm of the terminal, but it is absorbed rapidly into many small synaptic vesicles about 300,00 of which are normally in the terminals of a single end plate. In the synaptic space are large quantities of the enzyme acetylcholinesterase, which destroys acetylcholine a few milliseconds after it has been released from the synaptic vesicles. (Guyton, page 85). ---- Synthesis of acetylcholine is facilitated by the enzyme, choline acetyltransferase (CAT). This enzyme combines choline with acetate derived from acetyl coenzyme A (CoA). ACh in cholinergic nerve fibers is taken up into synaptic vesicles by an uptake process. Release of acetylcholine, like synaptic release at other junctions, is based on quantal release of vesicles containing preformed neurotransmitter molecules. Vesicular release depends on depolarization of the nerve terminal and the influx of calcium ion. Acetylcholine (ACh) is terminated by hydrolysis, which is greatly accelerated by one or more of the cholinesterase enzymes. Acetylcholinesterase (AChE) is present in high concentration in cholinergic synapses. (http://courses.washington.edu/chat543/cvans/sfp/acetylch.html)
5. Describe the structure of Botulinum toxin, and how it is activated. * The light chain (~50 kD - amino acids 1-448) acts as a zinc (Zn2+) endopeptidase similar to tetanus toxin with proteolytic activity located at the N-terminal end (see image below). The heavy chain (~100 kD - amino acids 449-1280) provides cholinergic specificity and is responsible for binding the toxin to presynaptic receptors; it also promotes light-chain translocation across the endosomal membrane. *A. BINDING TO RECEPTORS ON UNMYELINATED PRESYNAPTIC MEMBRANE (DOUBLE RECEPTOR) B. UPTAKE OF TOXIN INTO NERVE TERMINALS BY ENDOCYTOSIS C. TRANSLOCATION TO CYTOSOL D. INHIBITION OF TRANSMITTER EXOCYTOSIS FROM PRESYNAPTIC TERMINAL *BLOCK NT RELEASE AT PERIPHERAL CHOLINERGIC NERVE TERMINALS
SYMPATHETIC AND PARASYMPATHETIC * Botulinum toxin acts by binding presynaptically to high-affinity recognition sites on the cholinergic nerve terminals and decreasing the release of acetylcholine, causing a neuromuscular blocking effect.
6. How does Botulinum toxin cause FOOD poisoning? Discuss its main role in the pathophysiology of the symptoms shown in the case. Answer: 1. PASSAGE OF TOXIN FROM GI TRACT TO VASCULATURE 2. TOXIN PASSES OUT OF VASCULATURE TO PRESYNAPTIC REGIONS A. BINDING TO RECEPTORS ON UNMYELINATED PRESYNAPTIC MEMBRANE (DOUBLE RECEPTOR) B. UPTAKE OF TOXIN INTO NERVE TERMINALS BY ENDOCYTOSIS C. TRANSLOCATION TO CYTOSOL D. INHIBITION OF TRANSMITTER EXOCYTOSIS FROM PRESYNAPTIC TERMINAL * Clinical botulism results from the entry of botulinum toxin into the systemic circulation and subsequent inhibition of acetylcholine release from the presynaptic nerve terminal. The toxin enters the circulation through the mucosa (food-borne and inhalational) or via a break in the skin (wound and iatrogenic). Once absorbed into the bloodstream, the toxin is carried to the synapses of peripheral and cranial nerves. The heavy chain mediates binding of the toxin to presynaptic receptors, allowing for receptor-mediated endocytosis. Acetylcholine release at the neuromuscular junction is mediated by a synaptic-fusion complex. This complex consists of 3 soluble fusion attachment protein receptors (SNARE proteins). The toxin light chain inhibits vesicle release by cleaving peptide bonds of these SNARE proteins. As a result, stimulation of the presynaptic cell fails to produce transmitter release, resulting in motor paralysis or autonomic dysfunction when parasympathetic nerve terminals or autonomic ganglia are involved.
Вам также может понравиться
- Muscular SystemДокумент8 страницMuscular SystemJoannah Marie100% (2)
- Biology Lecture Notes: Sarcomere Structure and Muscle ContractionДокумент16 страницBiology Lecture Notes: Sarcomere Structure and Muscle ContractionAhmed KamelОценок пока нет
- Muscle Physiology: A Guide to Muscle Structure and Contraction MechanismsДокумент20 страницMuscle Physiology: A Guide to Muscle Structure and Contraction MechanismsPisiform90100% (1)
- Biochemistry of MuscleДокумент68 страницBiochemistry of MuscleWisnu KuncoroОценок пока нет
- Hypertensive Emergencies in The Emergency DepartmentДокумент13 страницHypertensive Emergencies in The Emergency DepartmentLuis Lopez RevelesОценок пока нет
- Principles and Practice of Single Implant and Restoration - Saunders 1 Edition (March 26, 2013)Документ204 страницыPrinciples and Practice of Single Implant and Restoration - Saunders 1 Edition (March 26, 2013)Sergiu Pinte100% (1)
- MCQs blood & cell physiology blogДокумент8 страницMCQs blood & cell physiology bloglubna malikОценок пока нет
- The Circulatory System - Lecture STEMДокумент50 страницThe Circulatory System - Lecture STEMMargeory Calatan100% (1)
- Muscle Contraction MechanismДокумент14 страницMuscle Contraction MechanismAlexОценок пока нет
- Family Case Study For HydrocephalusДокумент9 страницFamily Case Study For HydrocephalusjaegergranОценок пока нет
- Chapter 12 Muscle Physiology - Important Questions: 6. Mechanism of Muscle ContractionДокумент7 страницChapter 12 Muscle Physiology - Important Questions: 6. Mechanism of Muscle ContractionIrum RafeeqОценок пока нет
- Conference 1 - BiochemistryДокумент12 страницConference 1 - BiochemistryJoicey CatamioОценок пока нет
- Aapi Ebook June 19 2017Документ621 страницаAapi Ebook June 19 2017AAPIUSAОценок пока нет
- Chapte 3 MuscleДокумент128 страницChapte 3 MuscleErmias HadguОценок пока нет
- NEUROMUSCULAR TRANSMISSION: ANATOMY AND PHYSIOLOGYДокумент45 страницNEUROMUSCULAR TRANSMISSION: ANATOMY AND PHYSIOLOGYparuОценок пока нет
- Skeletal Muscle Physiology LectureДокумент65 страницSkeletal Muscle Physiology Lectureanon_737973908Оценок пока нет
- Excitation Contraction Coupling: Nandini GoyalДокумент32 страницыExcitation Contraction Coupling: Nandini GoyalNandini GoyalОценок пока нет
- MUSCLE PHYSIOLOGY: AN OVERVIEWДокумент50 страницMUSCLE PHYSIOLOGY: AN OVERVIEWJoyce Adjei-boateng100% (1)
- NASAL SEPTUM DEVIATION: CAUSES, SYMPTOMS AND SURGICAL CORRECTIONДокумент105 страницNASAL SEPTUM DEVIATION: CAUSES, SYMPTOMS AND SURGICAL CORRECTIONNguyễn ThànhОценок пока нет
- Asthma Lesson PlanДокумент26 страницAsthma Lesson PlanBharat Singh BanshiwalОценок пока нет
- Zbornik Limes Vol 2Документ354 страницыZbornik Limes Vol 2Morrigan100% (1)
- Energy For Muscle ContractionДокумент32 страницыEnergy For Muscle ContractionAndy SkatePunkОценок пока нет
- Tell Them What You're Going To Tell Them. Tell Them. Tell Them What You Just Told Them. AristotleДокумент17 страницTell Them What You're Going To Tell Them. Tell Them. Tell Them What You Just Told Them. AristotleSafiya JamesОценок пока нет
- Myosin, Actin & Contraction NotesДокумент4 страницыMyosin, Actin & Contraction NotesGhazali BalochОценок пока нет
- Skeletal Muscle Physiology ExplainedДокумент26 страницSkeletal Muscle Physiology ExplainedUSAMA AHMEDОценок пока нет
- Contractile Tissues NotesДокумент89 страницContractile Tissues NotesteeОценок пока нет
- UG S5 Cell Bio & Mol Bio Scheme Jan 2023Документ13 страницUG S5 Cell Bio & Mol Bio Scheme Jan 2023beatriceelistenОценок пока нет
- Skeletal MusclesДокумент7 страницSkeletal MusclesAhmed OudahОценок пока нет
- Physiology of Skeletal & Smooth MusclesДокумент10 страницPhysiology of Skeletal & Smooth MusclesAli AlhamadОценок пока нет
- Myosin Powered Movement and Contraction in Muscle and Non-Muscle CellsДокумент8 страницMyosin Powered Movement and Contraction in Muscle and Non-Muscle CellsIndranil BanerjeeОценок пока нет
- Cytoskeletal NetworkДокумент51 страницаCytoskeletal Networkconan128Оценок пока нет
- Neuromuscular PhysiologyДокумент6 страницNeuromuscular PhysiologySuresh KumarОценок пока нет
- Muscular System OverviewДокумент10 страницMuscular System OverviewOliver MianaОценок пока нет
- Fisiologi MusculoskeletalДокумент25 страницFisiologi MusculoskeletalSuardimanAchoОценок пока нет
- Lecture 4Документ6 страницLecture 4Mahreen NoorОценок пока нет
- Muscle Contraction MechanismsДокумент44 страницыMuscle Contraction MechanismsJon SteinerОценок пока нет
- Muscular SystemДокумент12 страницMuscular SystemChrisjohn Aster OsioОценок пока нет
- Physiology of Excitable Tissue and Action PotentialsДокумент12 страницPhysiology of Excitable Tissue and Action PotentialsTamar PertiaОценок пока нет
- Essay On Physiology (Excitable)Документ33 страницыEssay On Physiology (Excitable)Emmanuel IshiomaОценок пока нет
- PMUS1-11 Biology of Muscles IДокумент68 страницPMUS1-11 Biology of Muscles IVictor ChanОценок пока нет
- Cell Structure Lecture OverviewДокумент5 страницCell Structure Lecture OverviewDan ChoiОценок пока нет
- Contractia MuscularaДокумент33 страницыContractia MuscularaIulia JalbaОценок пока нет
- Muscle Contraction ExplainedДокумент11 страницMuscle Contraction ExplainedRokunuz Jahan RudroОценок пока нет
- Muscle System 2019Документ32 страницыMuscle System 2019ZNA EntertainmentОценок пока нет
- Muscle Tissue Lesson 1Документ5 страницMuscle Tissue Lesson 1Steves LeeboyОценок пока нет
- Muscle PhysiologyДокумент35 страницMuscle Physiologyzahra nabilaОценок пока нет
- Muscular TissueДокумент65 страницMuscular TissueMitzel SapaloОценок пока нет
- Neuromuscular JunctionДокумент11 страницNeuromuscular JunctionIdenyi Daniel Ewa EdeОценок пока нет
- Neuromuscular JunctionДокумент16 страницNeuromuscular JunctionSamadshahirОценок пока нет
- Sliding Filament Theory of Muscle Contraction: Muscular TissueДокумент8 страницSliding Filament Theory of Muscle Contraction: Muscular TissueMaryam Nur Azizah RahmahОценок пока нет
- Neuromuscular PsysioloyДокумент59 страницNeuromuscular Psysioloypuppy2207100% (1)
- Biochem Report Muscle and CytoskeletonДокумент87 страницBiochem Report Muscle and CytoskeletonKate Lynne CamonayanОценок пока нет
- Sliding Filament Theory of Muscle ContractionДокумент5 страницSliding Filament Theory of Muscle ContractionRica NorcioОценок пока нет
- Muscle Physiology Lecture on Neuromuscular Junction and Contraction MechanismsДокумент20 страницMuscle Physiology Lecture on Neuromuscular Junction and Contraction MechanismsSandy MonirОценок пока нет
- Muscle Tissue 2012Документ13 страницMuscle Tissue 2012ST. Fatimah KadirОценок пока нет
- Solution Manual For Holes Essentials of Human Anatomy Physiology 14th Edition Charles WelshДокумент8 страницSolution Manual For Holes Essentials of Human Anatomy Physiology 14th Edition Charles WelshJamesThomasngec100% (39)
- MuscleДокумент5 страницMuscleAbdulrahman AqraОценок пока нет
- Daftar Pustaka (Mini Projek)Документ18 страницDaftar Pustaka (Mini Projek)herfikaОценок пока нет
- It Removes A Phosphate Directly From A Substrate and Transfers It To ADPДокумент8 страницIt Removes A Phosphate Directly From A Substrate and Transfers It To ADPseussyОценок пока нет
- Muscle RelaxantsДокумент49 страницMuscle RelaxantsApriani MargastutieОценок пока нет
- Locomotion and MovementДокумент7 страницLocomotion and MovementAnonymous -Оценок пока нет
- Control of Skeletal Muscle Contractions ExplainedДокумент3 страницыControl of Skeletal Muscle Contractions ExplainedTofik MohammedОценок пока нет
- Inter Grated Human PhysiologyДокумент26 страницInter Grated Human PhysiologyMichelle BurkeОценок пока нет
- Musculoskeletal PhysiologyДокумент5 страницMusculoskeletal PhysiologyKhairul IkhwanОценок пока нет
- Chapter 2 NeuronДокумент49 страницChapter 2 NeuronNithinОценок пока нет
- Module 10 Kin 267 Muscle TissueДокумент4 страницыModule 10 Kin 267 Muscle TissueJ aОценок пока нет
- Postmortem Biochemistry of Meat and FishДокумент10 страницPostmortem Biochemistry of Meat and FishDaphne Janine Perez GuzmanОценок пока нет
- Histology QuestionДокумент4 страницыHistology QuestionLeng BunthaiОценок пока нет
- Full Text of The Official Result of December 2011 Nurse Licensure ExaminationДокумент19 страницFull Text of The Official Result of December 2011 Nurse Licensure ExaminationRay Andrew del RosarioОценок пока нет
- Dimensional AnalysisДокумент13 страницDimensional AnalysisArdette De JesusОценок пока нет
- Nurses' Application FormДокумент2 страницыNurses' Application FormTricia GervacioОценок пока нет
- Full Text of The Official Result of December 2011 Nurse Licensure ExaminationДокумент19 страницFull Text of The Official Result of December 2011 Nurse Licensure ExaminationRay Andrew del RosarioОценок пока нет
- 1 OrthoTraumaДокумент8 страниц1 OrthoTraumaArdette De JesusОценок пока нет
- Nursing attendance sheet for subjectДокумент1 страницаNursing attendance sheet for subjectArdette De JesusОценок пока нет
- Future PerfectДокумент3 страницыFuture PerfectArdette De JesusОценок пока нет
- Lesson 1: Health and Skill Related FitnessДокумент2 страницыLesson 1: Health and Skill Related FitnessCrhystal Joy ReginioОценок пока нет
- Health Promotion and Disease Prevention in the ElderlyДокумент1 страницаHealth Promotion and Disease Prevention in the ElderlyBeverly PagcaliwaganОценок пока нет
- To Remember The Four Causes of Cell InjuryДокумент43 страницыTo Remember The Four Causes of Cell Injuryapi-3825096Оценок пока нет
- Emergency Ultrasound教學 (8) 急診超音波在深部靜脈栓塞之應用Документ40 страницEmergency Ultrasound教學 (8) 急診超音波在深部靜脈栓塞之應用juice119Оценок пока нет
- Gastrointestinal Tract Infections: General ConsiderationsДокумент17 страницGastrointestinal Tract Infections: General ConsiderationsDarpan GelalОценок пока нет
- Yes, aside from the educational SMS intervention, the groups were treated equally as they both received routine trainingДокумент54 страницыYes, aside from the educational SMS intervention, the groups were treated equally as they both received routine trainingYogiОценок пока нет
- Standards of Care in Child Care InstitutionsДокумент36 страницStandards of Care in Child Care InstitutionsVaishnavi JayakumarОценок пока нет
- BSДокумент6 страницBSBeda MalecdanОценок пока нет
- Physiology of Sleep: Michael Schupp MD FRCA Christopher D Hanning MD FRCAДокумент6 страницPhysiology of Sleep: Michael Schupp MD FRCA Christopher D Hanning MD FRCArichie_ciandraОценок пока нет
- RT MapehДокумент2 страницыRT MapehRowan ImperialОценок пока нет
- Atc DDD OlukaДокумент65 страницAtc DDD Olukarini setyawatiОценок пока нет
- Turkish Angora CatДокумент6 страницTurkish Angora CatprosvetiteljОценок пока нет
- Daftar Pustaka HipertensiДокумент43 страницыDaftar Pustaka HipertensiFeni Nurmia PutriОценок пока нет
- Salivation PDFДокумент5 страницSalivation PDFmehdi mafakheri100% (1)
- Basic Life Support Field GuideДокумент56 страницBasic Life Support Field GuidelmaoheartsОценок пока нет
- E Book Coffee Leaf TeaДокумент18 страницE Book Coffee Leaf TeaAsun ArceОценок пока нет
- WORKSHEET 3 Lymphocyte ActivationДокумент5 страницWORKSHEET 3 Lymphocyte ActivationNeha ChoudharyОценок пока нет
- Wound Care InstructionsДокумент3 страницыWound Care InstructionsKat TaasinОценок пока нет
- Cement CSRДокумент35 страницCement CSRKasak Gupta100% (1)
- A Novel Technique For Pudendal Nerve BlockДокумент4 страницыA Novel Technique For Pudendal Nerve Blockmohs2007100% (1)