Академический Документы
Профессиональный Документы
Культура Документы
Veterinary Pathology Online: Coronary Arteriosclerosis With Myocardial Atrophy in A 13-Year-Old Dog
Загружено:
Rais YunarkoИсходное описание:
Оригинальное название
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
Veterinary Pathology Online: Coronary Arteriosclerosis With Myocardial Atrophy in A 13-Year-Old Dog
Загружено:
Rais YunarkoАвторское право:
Доступные форматы
Veterinary Pathology Online
http://vet.sagepub.com/ Coronary Arteriosclerosis with Myocardial Atrophy in a 13-year-old Dog
K. J. Williams Vet Pathol 2003 40: 695 DOI: 10.1354/vp.40-6-695 The online version of this article can be found at: http://vet.sagepub.com/content/40/6/695
Published by:
http://www.sagepublications.com
On behalf of:
American College of Veterinary Pathologists, European College of Veterinary Pathologists, & the Japanese College of Veterinary Pathologists.
Additional services and information for Veterinary Pathology Online can be found at: Email Alerts: http://vet.sagepub.com/cgi/alerts Subscriptions: http://vet.sagepub.com/subscriptions Reprints: http://www.sagepub.com/journalsReprints.nav Permissions: http://www.sagepub.com/journalsPermissions.nav
>> Version of Record - Nov 1, 2003 What is This?
Downloaded from vet.sagepub.com by guest on June 13, 2012
Vet Pathol 40:6, 2003
Brief Communications and Case Reports
695
Vet Pathol 40:695697 (2003)
Coronary Arteriosclerosis with Myocardial Atrophy in a 13-year-old Dog
K. J. WILLIAMS
Abstract. Myocardial ischemia, an uncommon cause of sudden death in dogs, usually results in infarction and brosis of the myocardium. Necropsy examination of a 13-year-old German Shepherd dog that died suddenly demonstrated multifocal myocardial thinning and loss in the left and right ventricular free wall and right atrium. Histopathologic examination conrmed the myocardial thinning to be sites of myocyte atrophy and loss, with loose reticulin-positive brovascular tissue and adipocytes and little brosis. Many intramural coronary arteries were irregularly thickened and partially occluded by segmental intimal and medial deposits of periodic acidSchiff-positive, Congo rednegative amorphous extracellular material. This nding is consistent with hyaline arteriosclerosis. These vascular lesions likely lead to insufcient perfusion of the affected myocardium and gradual loss of myobers without the acute necrosis and brosis characteristic of infarction. Key words: Atrophy; dogs; hyaline arteriosclerosis; myocardium. hematoxylin and eosin, periodic acidSchiff (PAS), Masson trichrome, reticulin stain, or Congo red. At histopathologic examination, the affected regions of the heart were devoid of cardiac myocytes but contained condensed and collapsed stroma, with sparse mature collagen bundles and adipocytes (Fig. 3). The affected regions of the right ventricle were transmural, with atrophy of the myocytes. In the left ventricle the myober loss involved primarily the subendocardial myocardium but did not extend to the epicardial surface. Reticulin and Masson trichrome staining of the stroma revealed an abundance of loose reticulin bers in the regions of myocardial atrophy (Fig. 3). At the periphery of the myocardial thinning, there was a prominent band of inltrating lymphocytes and plasma cells with smaller numbers of macrophages (Fig. 3), consistent with nonsuppurative inammation. The myocytes interspersed with these cells were atrophied and often degenerate (Fig. 4). The walls of multiple intramural coronary arteries, both within and adjacent to the myocardial atrophy, were markedly segmentally thickened (Fig. 5). The intima and media were expanded and disrupted by irregular deposits of pale, eosinophilic, amorphous hyaline material (Fig. 5). This material stained positively with PAS and Masson trichrome and did not stain with Congo red. These hyaline deposits elevated the endothelium, and the lumen of many of the vessels was markedly diminished. Myocardial atrophy, without evidence of other sites of infarction, is an unusual manifestation of arteriosclerosis. The development of infarction in the myocardium as the result of primary coronary arteriosclerosis is well described in humans, dogs, and experimental models of cardiovascular disease.2,3,5,6 The sequence of events in myocardial infarction has been documented in humans and experimental models of infarction after coronary arterial occlusion in rabbits.5,6 In both, the chronic manifestations are characterized by myocyte replacement with granulation tissue followed by dense collagen deposition.5,6 This process leads to grossly discernable, pale, shrunken foci of scarring after the initial ischemic event. Neither the necropsy nor the histopathologic features
Arteriosclerosis is a nonspecic term for changes in arteries that result in a loss of elasticity in the vessel wall.6 Atherosclerosis, the most common form of arteriosclerosis in humans, is the result of lipid and inammatory cell accumulations (plaques) within the arterial intima.6 Atherosclerosis in dogs is usually associated with hypercholesterolemia and hyperlipidemia coincident with hypothyroidism; myocardial infarction is reported to be common in hypothyroid dogs.3 Arteriolosclerosis, a subtype of arteriosclerosis specically involving small arteries, has been found to be an important cause of ischemic heart disease in the dog, usually resulting in myocardial infarction.2 This case involves intramural hyaline coronary arteriolosclerosis associated with atrophy and loss of the myocardium without evidence of myocardial infarction. A 13-year-old German Shepherd dog had been considered healthy until the owners reported a 24-hour period of lethargy and recumbency before death. The animal was subsequently submitted to the College of Veterinary Medicine at Michigan State University for necropsy. At necropsy, approximately 100150 ml of serosanguineous uid was present in the thoracic cavity, and the lungs contained diffuse moderate edema. Scattered within the free wall of both the right atrium and the right ventricle, there were multifocal discrete foci of myocardial thinning (Fig. 1). The myocardial thinning formed depressions within the wall of both the atrium and the ventricle; however, there was preservation of the normal epicardial vasculature overlying these sites (Fig. 1). The periphery of the myocardial lesions were demarcated by a thin (12 mm), pale rim; centrally, the myocardium was thin and translucent (Fig. 2). Depressions in the myocardium were not present on the epicardial or endocardial surfaces of the left ventricle; however, the cut section of the free wall of the left ventricle contained several depressions within the myocardium (Fig. 2). Tissues collected at the time of necropsy were immersionxed in 10% neutral buffered formalin and embedded in parafn for histopathologic examination. Separate, successive 6- m sections of the myocardium were stained using
Downloaded from vet.sagepub.com by guest on June 13, 2012
696
Brief Communications and Case Reports
Vet Pathol 40:6, 2003
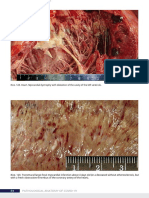
Fig. 1. Heart; dog. Multifocal myocardial atrophy (arrows), right ventricle characterized by depressions in the free wall, with preservation of the epicardial vasculature.
Fig. 2. Heart; dog. Multifocal myocardial atrophy (arrows), left and right ventricle. Note the accumulation of inammatory cells at the periphery of the foci of right atrial atrophy (arrowheads).
Fig. 3. Myocardium; dog. Myocardial atrophy with myocyte loss and replacement with loose reticulin-positive stroma. Note the adjacent myocardium (M) with band of lymphoplasmacytic inammation (arrows). Inset: High magnication of reticulin stroma. Snooks reticulin stain. Bar, low magnication 400 m; bar, inset 50 m.
Fig. 4. Lymphoplasmacytic myocarditis with myocyte degeneration. HE. Bar 50 m. Fig. 5. Hyaline arteriolosclerosis with partial lumen occlusion, intramural coronary artery, left ventricle. HE. Bar 50 m.
Downloaded from vet.sagepub.com by guest on June 13, 2012
Vet Pathol 40:6, 2003
Brief Communications and Case Reports
697
of this heart are consistent with prior infarction underlying the lesions in this animal. Decreased blood supply is a common cause of atrophy, irrespective of tissue type.1 Apoptosis of cardiomyocytes, a known consequence of hypoxia, may have been a factor in the development of myocardial atrophy in this animal.4 Experimental myocardial infarction and chronic heart failure in dogs, induced through intracoronary microembolization, resulted in cardiocyte apoptosis adjacent to the foci of infarction.7,8 A similar process may occur in hypoxic but not ischemic myocardium when there is insufcient myocardial perfusion because of arteriolosclerosis. Falk and Jonsson de scribed 65 cases of ischemic heart disease in dogs with histopathologically conrmed arteriosclerosis.2 Myocardial infarction with brosis was found in 51 of the 65 dogs with arteriolosclerosis, but there is no mention of focal myocardial atrophy as was seen in the dog in this study.2 The mononuclear inammation in the myocardium was restricted to the peripheral regions of myocardial degeneration. Lymphocytic inammation occurs after myocardial ischemia, typically interspersed with the granulation tissue and macrophage inltrates common to early infarction, but these ndings were absent in the myocardium of the dog in this study.5 The pathogenesis of hyaline coronary arteriolar lesions in the dog is not well understood. Arteriolosclerosis can be subdivided into hyaline and hyperplastic subtypes.6 Hyperplastic arteriolosclerosis is characteristic of hypertensive states, particularly acute, severe elevations in blood pressure.6 The vascular wall in such patients is characterized by concentric, laminar proliferations of smooth muscle cells and basement membrane, with the lesions most common in the kidney.6 In contrast, hyaline arteriolosclerosis may be seen in normotensive individuals and is considered a common nding in elderly people. When it occurs in hypertensive people, hyaline arteriolosclerosis is generalized and more severe.6 Fibromuscular intimal thickening and hyalinosis were reported in the coronary arteries of the 65 dogs with ischemic heart disease, with no disease reported in organs other than the heart.2 The lesions in the present animal were also restricted to the heart, and there was no histopathologic evidence of generalized hypertension or additional disease processes in other organs. Pure hyaline arteriolosclerosis is assumed to be the result of earlier endothelial injury, allowing leakage of plasma proteins into the subendothelial space and deposition of smooth musclederived extracellular proteins.2,6
Myocardial atrophy, without coexistent infarction, is an unusual manifestation of arteriolosclerosis in dogs. The diagnosis of atrophy can be made by documenting the lack of brosis in the regions of myocardial ber loss with concomitant condensation of the reticulin stroma. This myocardial change may be associated with rapid clinical deterioration and death in dogs.
References
1 Cotran RS, Kumar V, Collins TC: Cellular pathlogy I: cell injury and cell death. In: Robbins Pathologic Basis of Disease, ed. Cotran RS, Kumar V, and Collins TC, 6th ed., pp. 129. WB Saunders, Philadelphia, PA, 1999 2 Falk T, Jonsson L: Ischaemic heart disease in the dog: a review of 65 cases. J Small Anim Pract 41:97103, 2000 3 Liu SK, Tilley LP, Fox PR: Clinical and pathologic ndings in dogs with atherosclerosis: 21 cases (19701983). J Am Vet Med Assoc 189(2):227232, 1986 4 Matsuoka R, Ogawa K, Yaoita H, Naganuma W, Maehara K, Maruyama Y: Characteristics of death of neonatal rat cardiomyocytes following hypoxia or hypoxiareoxygenation: the association of apoptosis and cell membrane disintegrity. Heart Vessels 16(6):241248, 2002 5 Morales C, Gonzalez GE, Rodriguez M, Bertolasi CA, Gelpi RJ: Histopathologic time course of myocardial infarct in rabbit hearts. Cardiovasc Pathol 11(6):339345, 2002 6 Schoen FJ, Cotran RS: Blood vessels. In: Robbins Pathologic Basis of Disease, ed. Cotran RS, Kumar V, and Collins TC, 6th ed., pp. 493541. WB Saunders, Philadelphia, PA, 1999 7 Sharov VG, Sabbah HN, Ali AS, Shimoyama H, Lesch M, Goldstein S: Abnormalities of cardiocytes in regions bordering brous scars of dogs with heart failure. Int J Cardiol 60(3):273279, 1997 8 Sharov VG, Sabbah HN, Shimoyama H, Goussev AV, Lesch M, Goldstein S: Evidence of cardiocyte apoptosis in myocardium of dogs with chronic heart failure. Am J Pathol. 148(1):141149, 1996 Request reprints from Dr. Kurt J. Williams, Department of Pathobiology and Diagnostic Investigation, College of Veterinary Medicine, 210 Food Safety and Toxicology Building, Michigan State University, East Lansing, MI 48824 (USA). E-mail: williamsk@dcpah.msu.edu.
Downloaded from vet.sagepub.com by guest on June 13, 2012
Вам также может понравиться
- Death Due To Myocardium Infarct (A Case Report) : Ahmad Yudianto, H.MutahalДокумент31 страницаDeath Due To Myocardium Infarct (A Case Report) : Ahmad Yudianto, H.MutahalLinda RatusehakaОценок пока нет
- Pathology Case Study - EvangelistaДокумент17 страницPathology Case Study - EvangelistaAubrey Unique EvangelistaОценок пока нет
- Pathology of Acute Myocardial InfarctionДокумент13 страницPathology of Acute Myocardial InfarctionNaily Nuzulur RohmahОценок пока нет
- Cardiovascular2006 Lecture NotesДокумент31 страницаCardiovascular2006 Lecture NotescystanarisaОценок пока нет
- Acute StrokeДокумент13 страницAcute StrokeJoel CanenciaОценок пока нет
- Microcirculation in Cardiovascular DiseasesОт EverandMicrocirculation in Cardiovascular DiseasesEnrico Agabiti-RoseiОценок пока нет
- Kelainan Pada Struktur Histologis Pembuluh DarahДокумент5 страницKelainan Pada Struktur Histologis Pembuluh DarahAwatif Al MakiyahОценок пока нет
- 1.old Posterior-WPS OfficeДокумент6 страниц1.old Posterior-WPS Officeashokyd1411Оценок пока нет
- Acute Myocardial InfarctionДокумент14 страницAcute Myocardial InfarctionJardee DatsimaОценок пока нет
- Jurnal EcgДокумент7 страницJurnal EcgrahmedОценок пока нет
- Pathology LectureДокумент3 страницыPathology LectureAbdullahayad farouqОценок пока нет
- Kardo S 1998Документ3 страницыKardo S 1998Sarly FebrianaОценок пока нет
- Pathology EssayДокумент19 страницPathology EssayARUNSKОценок пока нет
- Pathophysiology and Treatment of Hypertrophic CardiomyopathyДокумент29 страницPathophysiology and Treatment of Hypertrophic CardiomyopathyAdrin Ma'rufОценок пока нет
- Cardiovascular DentДокумент92 страницыCardiovascular DentVincent De AsisОценок пока нет
- Degeneración y Necrosis Del MiocardioДокумент7 страницDegeneración y Necrosis Del MiocardiosebastianОценок пока нет
- Pharmacokinetics and PharmacodynamicsДокумент166 страницPharmacokinetics and PharmacodynamicsEvgeny BadmaevОценок пока нет
- Ischaemic Heart Disease (IHD) Is Defined As Acute or Chronic Form of Cardiac Disability Arising FromДокумент5 страницIschaemic Heart Disease (IHD) Is Defined As Acute or Chronic Form of Cardiac Disability Arising FromIsak ShatikaОценок пока нет
- Chapter 010-Cardiovascular DXДокумент15 страницChapter 010-Cardiovascular DXjaniceli0207Оценок пока нет
- Ischemic Heart DiseaseДокумент6 страницIschemic Heart DiseaseApril Joy Villacorta PonceОценок пока нет
- StrokeДокумент17 страницStrokeJo CanensОценок пока нет
- Heart Pathology.Документ165 страницHeart Pathology.Mișulică CerlatОценок пока нет
- Reconocimiento Del Acv CLINISC 2012Документ21 страницаReconocimiento Del Acv CLINISC 2012Camilo GomezОценок пока нет
- Literature ReviewДокумент28 страницLiterature ReviewNyo LayОценок пока нет
- Pathophysiology of StrokeДокумент9 страницPathophysiology of StrokeAnj MandeoyaОценок пока нет
- Ischemic Heart DiseaseДокумент67 страницIschemic Heart Diseasealfaz lakhani80% (5)
- Brainsci 10 00538Документ12 страницBrainsci 10 00538Charles MorrisonОценок пока нет
- Cardiovascular Systems - 2 (Blood and Lymphatic Vessel)Документ37 страницCardiovascular Systems - 2 (Blood and Lymphatic Vessel)RezaОценок пока нет
- Pathophysiology Worksheet MIДокумент2 страницыPathophysiology Worksheet MIpjbedelОценок пока нет
- Feline Arterial Thromboembolism - (PDF) - in EnglishДокумент7 страницFeline Arterial Thromboembolism - (PDF) - in EnglishjedicitoОценок пока нет
- Cardiovascular SystemДокумент22 страницыCardiovascular SystemWadabiОценок пока нет
- COMPASSIONATE Cardiovascular Pathology Cases PDFДокумент47 страницCOMPASSIONATE Cardiovascular Pathology Cases PDFAdrian CaballesОценок пока нет
- 2 Embolism, Infarction and ShockДокумент57 страниц2 Embolism, Infarction and ShockSuman MahmoodОценок пока нет
- Vascular Pathology Atherosclerosis, Hypertension, Vasculitis - 0Документ151 страницаVascular Pathology Atherosclerosis, Hypertension, Vasculitis - 0Mișulică CerlatОценок пока нет
- Arteriosclerosis & AtherosclerosisДокумент29 страницArteriosclerosis & AtherosclerosisblossomkdcОценок пока нет
- Atherosclerosis: Atherosclerosis (Also Known As Arteriosclerotic Vas-Cular Disease or ASVD) Is A Specific Form ofДокумент15 страницAtherosclerosis: Atherosclerosis (Also Known As Arteriosclerotic Vas-Cular Disease or ASVD) Is A Specific Form ofZiedTrikiОценок пока нет
- Acute Limb Ischemia: Clinical PracticeДокумент9 страницAcute Limb Ischemia: Clinical PracticeIndah MaulidawatiОценок пока нет
- Inbound 7217984276738636566Документ12 страницInbound 7217984276738636566melshouhomyОценок пока нет
- Class 17Документ46 страницClass 17Nishani SatiyaseelanОценок пока нет
- Endocarditis in Six HorsesДокумент5 страницEndocarditis in Six HorsesCan HeladoОценок пока нет
- Atheroma: 2 History of ResearchДокумент7 страницAtheroma: 2 History of ResearchZiedTrikiОценок пока нет
- Alfonso Lopez CardiovascularДокумент15 страницAlfonso Lopez Cardiovascularjaniceli0207Оценок пока нет
- Acute Stroke ManagementДокумент51 страницаAcute Stroke Managementmae sarohОценок пока нет
- Patologicheskaya Anatomia COVID 19 Atlas 86 98.ru - enДокумент13 страницPatologicheskaya Anatomia COVID 19 Atlas 86 98.ru - enkapil pancholiОценок пока нет
- Types of Strokes - Joao Gomes and Ari Marc WachsmanДокумент18 страницTypes of Strokes - Joao Gomes and Ari Marc WachsmantomazlmОценок пока нет
- Pathology of Stroke: Dr. Isnaniah, Sp. SДокумент51 страницаPathology of Stroke: Dr. Isnaniah, Sp. SFiladelfia sariОценок пока нет
- ACS HandoutДокумент19 страницACS HandoutGloriana Julia TeopeОценок пока нет
- Coronary Artery DiseaseДокумент13 страницCoronary Artery DiseaseChristianHanjokarОценок пока нет
- Atherosclerosis (Also Known As Arteriosclerotic Vascular Disease or ASVD) Is AДокумент27 страницAtherosclerosis (Also Known As Arteriosclerotic Vascular Disease or ASVD) Is AChristine WidjajaОценок пока нет
- Silent Myocardial IschaemiaДокумент7 страницSilent Myocardial IschaemiaSmitha ShettyОценок пока нет
- LympangiosarДокумент4 страницыLympangiosarKreshna MedhaОценок пока нет
- Surgery Reviosn NotesДокумент41 страницаSurgery Reviosn Noteshy7tn100% (1)
- 1964 30 611-618 Robert S. Eliot, Vladimir I. Kanjuh and Jesse E. EdwardsДокумент9 страниц1964 30 611-618 Robert S. Eliot, Vladimir I. Kanjuh and Jesse E. EdwardsLuis Marcas VilaОценок пока нет
- نسخة HeartДокумент29 страницنسخة HeartKyunaОценок пока нет
- Acute Limb Ischemia: Clinical PracticeДокумент9 страницAcute Limb Ischemia: Clinical PracticeKezia TambunanОценок пока нет
- Acute Arterial OcclusionДокумент49 страницAcute Arterial OcclusiondrvsvasuОценок пока нет
- Ischemic Heart Disease (IHD)Документ37 страницIschemic Heart Disease (IHD)Yowan SusantiОценок пока нет
- Microcirculation: From Bench to BedsideОт EverandMicrocirculation: From Bench to BedsideMaria DorobantuОценок пока нет
- Basic Life SupportДокумент14 страницBasic Life SupportKristine A. CAYABANОценок пока нет
- Circulatory System WorksheetДокумент2 страницыCirculatory System Worksheetrubixcube4550% (12)
- Protein Losing EnteropathyДокумент9 страницProtein Losing EnteropathyrajibОценок пока нет
- Circulatory System ActivityДокумент7 страницCirculatory System ActivityReginald MalibiranОценок пока нет
- Task 2: Physiological Exercise and Physical Activity: To Transport Nutrients, Gases and Waste Products Around The BodyДокумент2 страницыTask 2: Physiological Exercise and Physical Activity: To Transport Nutrients, Gases and Waste Products Around The Bodyjohn paul PatronОценок пока нет
- Vascular Risk Assessment ProgramДокумент15 страницVascular Risk Assessment Programheenamodi100% (1)
- Desmids of The United StatesДокумент446 страницDesmids of The United Statessofia100% (1)
- Functional Relationship of The Different Organ Systems in Ensuring Animal SurvivalДокумент22 страницыFunctional Relationship of The Different Organ Systems in Ensuring Animal SurvivalChristopher VillanuevaОценок пока нет
- Aortic RegurgitationДокумент134 страницыAortic RegurgitationAbnet WondimuОценок пока нет
- Gas Exchange Transport and CirculationДокумент34 страницыGas Exchange Transport and CirculationRaineir PabiranОценок пока нет
- ThrombosisДокумент11 страницThrombosisNobby Onist JuniorОценок пока нет
- Cardio MechДокумент84 страницыCardio MechTim LeeОценок пока нет
- History of Coronary ScoringДокумент32 страницыHistory of Coronary ScoringAfiani JannahОценок пока нет
- Placenta and Fetal Membrane-6614Документ45 страницPlacenta and Fetal Membrane-6614Incredible DivineОценок пока нет
- Ecg ReadingДокумент6 страницEcg ReadingMarianette CainongОценок пока нет
- Why Animals Don't Get Heart Attacks - But - Rath, Matthias, M.D PDF (Pauling-Rath Therapy Protocol)Документ292 страницыWhy Animals Don't Get Heart Attacks - But - Rath, Matthias, M.D PDF (Pauling-Rath Therapy Protocol)Anonymous Jap77xvqPKОценок пока нет
- Parameters of Fluid Responsiveness Curr Op Crit Care Shi2020Документ8 страницParameters of Fluid Responsiveness Curr Op Crit Care Shi2020Tadeo PradoОценок пока нет
- Chapter 28: Management of Patients With Structural, Infectious, and Inflammatory Cardiac DisordersДокумент19 страницChapter 28: Management of Patients With Structural, Infectious, and Inflammatory Cardiac DisordersBrian BileckyОценок пока нет
- PRE - ASSESSMENT PAR-Q (Physical Activity Readiness Questionnaire)Документ5 страницPRE - ASSESSMENT PAR-Q (Physical Activity Readiness Questionnaire)Sarah DecenaОценок пока нет
- ECG Discussion: Wellens Syndrome: A Subtle ECG FindingДокумент1 страницаECG Discussion: Wellens Syndrome: A Subtle ECG FindingEINSTEIN2DОценок пока нет
- June 2018 (IAL) QP - Unit 1 Edexcel Biology A-Level PDFДокумент24 страницыJune 2018 (IAL) QP - Unit 1 Edexcel Biology A-Level PDFMoataz NassefОценок пока нет
- IMD - Step-Up To USMLE Step 3 - Chapter 1 - CardiologyДокумент124 страницыIMD - Step-Up To USMLE Step 3 - Chapter 1 - CardiologyAly SherifОценок пока нет
- Transportation in AnimalsДокумент6 страницTransportation in AnimalskrovrainkОценок пока нет
- Cardiac SystemДокумент7 страницCardiac Systemsccctutor100% (3)
- Cardio OrientationДокумент6 страницCardio OrientationBandana RajpootОценок пока нет
- Kingdom AnimaliaДокумент3 страницыKingdom AnimaliaLloydDagsil100% (1)
- Pregnancy Induced Hypertension (Pih)Документ56 страницPregnancy Induced Hypertension (Pih)shandi23100% (5)
- P 6-ScienceДокумент8 страницP 6-ScienceMonydit santinoОценок пока нет
- Cardiogenic Shock HandoutsДокумент2 страницыCardiogenic Shock HandoutsAileenОценок пока нет
- Immune System by Asif PresentationДокумент35 страницImmune System by Asif PresentationHassan AsifОценок пока нет