Академический Документы
Профессиональный Документы
Культура Документы
Crude Oil
Загружено:
Muneer Basha BashaИсходное описание:
Оригинальное название
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
Crude Oil
Загружено:
Muneer Basha BashaАвторское право:
Доступные форматы
Crude oil Petroleum or crude oil is a naturally occurring, toxic, flammable liquid consisting of a complex mixture of hydrocarbons of various
molecular weights, and other organic compounds, that are found in geologic formations beneath the Earth's surface. Introduction In its strictest sense, petroleum includes only crude oil,but in common usage it includes both crude oil and natural gas. Both crude oil and natural gas are predominantly a mixture of hydrocarbons. Under surface pressure and temperature conditions, the lighter hydrocarbons methane, ethane, propane and butane occur as gases, while the heavier ones from pentane and up are in the form of liquids or solids. However, in the underground oil reservoir the proportion which is gas or liquid varies depending on the subsurface conditions, and on the phase diagram of the petroleum mixture. An oil well produces predominantly crude oil, with some natural gas dissolved in it. Because the pressure is lower at the surface than underground, some of the gas will come out of solution and be recovered (or burned) as associated gas or solution gas. A gas well produces predominately natural gas. However, because the underground temperature and pressure are higher than at the surface, the gas may contain heavier hydrocarbons such as pentane, hexane, and heptane in the gaseous state. Under surface conditions these will condense out of the gas and form natural gas condensate, often shortened to condensate. Condensate resembles gasoline in appearance and is similar in composition to some volatile light crude oils. Three conditions must be present for oil reservoirs to form: a source rock rich in hydrocarbon material buried deep enough for subterranean heat to cook it into oil; a porous and permeable
reservoir rock for it to accumulate in; and a cap rock (seal) or other mechanism that prevents it from escaping to the surface. Within these reservoirs, fluids will typically organize themselves like a three-layer cake with a layer of water below the oil layer and a layer of gas above it, although the different layers vary in size between reservoirs. Because most hydrocarbons are lighter than rock or water, they often migrate upward through adjacent rock layers until either reaching the surface or becoming trapped within porous rocks (known as reservoirs) by impermeable rocks above. However, the process is influenced by underground water flows, causing oil to migrate hundreds of kilometres horizontally or even short distances downward before becoming trapped in a reservoir. When hydrocarbons are concentrated in a trap, an oil field forms, from which the liquid can be extracted by drilling and pumping. Refining of Crude Oil Fractional distillation of crude oil is the first step in the production of many of the materials we have come to rely on in modern life. All our fossil fuels, virtually all our plastics, detergents and commercial alcohols are made from products of this process. Oil Field to the Refinery The first step is the transport of the crude oil from its natural location to the refinery. Oil drilling occurs both at sea and on land, depending on the size and profitability of the oil deposits located. Fossil fuel formation is the result of environmental conditions in the distant past and the geological processes that have occurred since the laying down of the original organic matter from which the oil is formed. Once obtained from the ground, the oil is transported by ship, truck or pipeline to the refinery. At the Refinery Once the oil reaches the refinery the work to separate it into useful products begins. Oil refineries are enormously
complex and each part of the distilled oil goes through several stages of processing. However, the very first step is to break up the crude oil. The crude oil is a mixture of many different chemicals. The majority of these are hydrocarbons, which are molecules made only from the element Carbon and the element Hydrogen. The mixture of hydrocarbons contains both alkane and alkene molecules and the length of the chains vary wildly, from five Carbon atoms long to 60 Carbons or more. Since fuels need to be very specific in terms of the length of the Carbon chain, the different lengths need to be separated. These different length chains are called FRACTIONS. The boiling point of a Hydrocarbon fraction, which is the temperature at which it evaporates, is dependent on the length of the Carbon chain. Those fractions with shorter chains evaporate more easily than those with longer chains. This explains why petrol, which is mainly made of the 8-Carbon molecule octane, evaporates more easily than engine oil which has carbon chains in the range of 20 or more. Fractional distillation of Crude Oil In order to separate the different length chains in the crude mix, it is heated to a very high temperature. The temperature is set so that all those fractions with a Carbon chain length of 20 and below are evaporated from the crude mix. The temperature cannot be set higher than this as there is a risk that the lighter fractions will ignite. The remaining liquid, which is composed of only the heavier fractions, passes to a second location where it is heated to a similar temperature, but at lower pressure. This has the effect of making the heavy Hydrocarbon fractions more likely to evaporate. The way the Distillation Tower works is by becoming progressively cooler from the base to the top. All the Hydrocarbon fractions start off in gas form, as they have been heated to that point. The gases then rise up the tower. The gas mixture then encounters a barrier
through which there are only openings into the bubble caps. The gas mixture is then forced to go through a liquid before continuing upwards. The liquid in the first tray is at a cool enough temperature to get the heaviest gas fractions to condense into liquid form, while the lighter fractions stay gaseous. Working of the Distillation Tower In this way the heaviest hydrocarbon fractions are separated out from the mixed gas. The remaining gas continues its journey up the tower until it reaches another barrier. Here the bubble cap process is repeated but at a lower temperature than before, which then filters out the next lightest set of fractions. This process continues until only the very lightest fractions, those of 1 to 4 Carbon atoms, are left. These stay in gas form and are collected at the top of the tower. The separation of the heavier elements in the second tower follows exactly the same process but at lower pressure A large part of refining involves separating the different fractions of crude oil and other intermediate streams. While water boils at a single temperature, crude oil boils over a wide range of temperatures. By controlling the temperature using very tall distillation columns, different fractions of the crude oil can be separated. At the top of the column, fuel gas and liquefied petroleum gas (or LPG) is produced. A little lower down the column, gasoline (or, more precisely, one of the blending stocks for gasoline called naphtha) is withdrawn. Still lower down the column, jet fuel and diesel fuel are produced. At the very bottom of the column, asphalt is the "bottom of the barrel". Asphalt can either be sold to make roads and roofing materials, or it can be thermally cracked in a coker, where under
high temperatures, the asphalt breaks down into a synthetic crude oil, producing the same products as crude oil as well as petroleum coke. Similar columns are found at all the cokers. Between asphalt and diesel fuel is a substance called gas oil. In some refineries, this stream can be further processed into lubricating oil. However, the crude oil that FHR processes doesn't make very good lubricating oil, so like asphalt it is catalytically cracked into another synthetic crude oil. Again, similar columns are found in the catalytic cracking unit.
Another significant portion of refining is converting individual molecules into different sizes and shapes. There are three main conversion processes in the refinery: Cracking Combining Reforming Cracking takes place in two units: the cokers and the fluid catalytic cracking units (or FCCUs). In the coker, asphalt is heated to 900 to 1000 F in large drums. The asphalt is allowed to cook for 16 to 24 hours. While cooking, the asphalt turns into a synthetic crude oil which is separated into the different fractions. What remains is a black solid, similar in appearance to coal, called petroleum coke. Petroleum coke is used the same way that coal is, primarily to produce electricity in coal fired power plants. Gasoil, a fraction of crude oil between asphalt and diesel fuel, is cracked in the FCCU. Gasoil is contacted by very hot (> 1300 F), very small (like flour) particles of catalyst, which cracks it into a synthetic crude oil which is again separated into different fractions. In the process, some of the gasoil turns into coke, which attaches to the catalyst particles. This coke must be burned off in the FCCU regenerator, which also reheats the catalyst for another ride around the unit. Eventually, some of the catalyst wears down and is too small to be captured by the internal capture mechanisms, and escapes from the regenerator. An electrostatic precipitator (like a 14 story electronic
dust cleaner on your furnace at home) removes most of these fine pieces of catalyst before they escape into the air. Combining takes place in three units: the alkylation (or Alky) unit, the dimersol and the polymerization (or Polly) unit. In all three of these units, small LPG molecules are combined into larger, gasoline molecules. FHR uses sulfuric acid, nickel and phosphoric acid to combine different combinations of LPG into gasoline. Reforming takes place in three units as well: the platformer, the powerformer and the isomerization (or isom) unit. Reforming changes the shapes of molecules so that they have more desirable characteristics. For example, the gasoline which comes directly from the separation of crude oil has too low an octane rating to be used directly in automobiles. By reforming this gasoline with the help of a platinum catalyst, the octane can be raised significantly. FHR uses platinum and other rare metals to rearrange gasoline molecules to improve their octane and other properties. Making clean products out of dirty raw materials FHR processes a type of crude oil known as "heavy, sour" crude oil - heavy, because it contains more asphalt than most crudes, and sour because it contains a lot of sulfur. In order to meet state and federal standards for clean burning transportation fuels, FHR must treat the oil to remove sulfur and other contaminants. The treating process starts with the desalters. Crude oil contains a small percentage of salt water (which is present in the rock formations from which it is pumped) and a small amount of solids, which must be removed so that they do not plug up or corrode the processing equipment. The water and solids do not form separate
layers, but are mixed in with the oil in an emulsion. To break the emulsion, chemicals are added to the crude oil, and the oil passes through an electrically charged grid, which separates the oil from the water and solids. The oil is washed with additional water to be sure all the salt and solids are removed, and the water, salts and solids are sent to the wastewater treatment plant where oil and solids are recovered. The oil and solids are returned to the process to make more products.
Paraffins
Naphthenes
Aromatics
Every refinery begins with the separation of crude oil into different fractions by distillation. The fractions are further treated to convert
them into mixtures of more useful saleable products by various methods such as cracking, reforming, alkylation, polymerisation and isomerisation. These mixtures of new compounds are then separated using methods such as fractionation and solvent extraction. Impurities are removed by various methods, e.g. dehydration, desalting, sulphur removal and hydrotreating. Refinery processes have developed in response to changing market demands for certain products. With the advent of the internal combustion engine the main task of refineries became the production of petrol. The quantities of petrol available from distillation alone was insufficient to satisfy consumer demand. Refineries began to look for ways to produce more and better quality petrol. Two types of processes have been developed: breaking down large, heavy hydrocarbon molecules reshaping or rebuilding hydrocarbon molecules.
Cracking processes break down heavier hydrocarbon molecules (high boiling point oils) into lighter products such as petrol and diesel. These processes include catalytic cracking, thermal cracking and hydrocracking. e.g. A typical reaction: C16H34 -> C8H18 + C8H16 Catalytic cracking is used to convert heavy hydrocarbon fractions obtained by vacuum distillation into a mixture of more useful products such as petrol and light fuel oil. In this process, the
feedstock undergoes a chemical breakdown, under controlled heat (450 - 500oC) and pressure, in the presence of a catalyst - a substance which promotes the reaction without itself being chemically changed. Small pellets of silica - alumina or silica magnesia have proved to be the most effective catalysts. The cracking reaction yields petrol, LPG, unsaturated olefin compounds, cracked gas oils, a liquid residue called cycle oil, light gases and a solid coke residue. Cycle oil is recycled to cause further breakdown and the coke, which forms a layer on the catalyst, is removed by burning. The other products are passed through a fractionator to be separated and separately processed. Thermal cracking uses heat to break down the residue from vacuum distillation. The lighter elements produced from this process can be made into distillate fuels and petrol. Cracked gases are converted to petrol blending components by alkylation or polymerisation. Naphtha is upgraded to high quality petrol by reforming. Gas oil can be used as diesel fuel or can be converted to petrol by hydrocracking. The heavy residue is converted into residual oil or coke which is used in the manufacture of electrodes, graphite and carbides.
Reforming is a process which uses heat, pressure and a catalyst (usually containing platinum) to bring about chemical reactions which upgrade naphthas into high octane petrol and petrochemical feedstock. The naphthas are hydrocarbon mixtures containing many paraffins and naphthenes. In Australia, this naphtha feedstock comes from the crudes oil distillation or catalytic cracking processes, but overseas it also comes from thermal cracking and hydrocracking processes. Reforming converts a portion of these compounds to isoparaffins and aromatics, which are used to blend higher octane petrol. paraffins are converted to isoparaffins
paraffins are converted to naphthenes naphthenes are converted to aromatics e.g. Catalyst heptane -> toluene + hydrogen C7H16 -> C7H8 + 4H2 Catalyst cyclohexane -> benzene + hydrogen C6H12 -> C6H6 + 3H2 Air, water and land can all be affected by refinery operations. Refineries are well aware of their responsibility to the community mand employ a variety of processes to safeguard the environment. The processes described below are those used by the Shell refinery at Geelong in Victoria, but all refineries employ similar techniques in managing the environmental aspects of refining. Preserving air quality around a refinery involves controlling the following emissions: sulphur oxides Hydrocarbon vapors Smoke Smells Sulphur enters the refinery in crude oil feed. Gippsland and most other Australian crude oils have a low sulphur content but other crude's may contain up to 5 per cent sulphur. To deal with this refineries incorporate a sulphur recovery unit which operates on the principles described above. Many of the products used in a refinery produce hydrocarbon vapours. The escape of vapours to atmosphere are prevented by
various means. Floating roofs are installed in tanks to prevent evaporation and so that there is no space for vapour to gather in the tanks. Where floating roofs cannot be used, the vapours from the tanks are collected in a vapour recovery system and absorbed back into the product stream. In addition, pumps and valves are routinely checked for vapour emissions and repaired if a leakage is found. Smoke is formed when the burning mixture contains insufficient oxygen or is not sufficiently mixed. Modern furnace control systems prevent this from happening during normal operation. Smells are the most difficult emission to control and the easiest to detect. Refinery smells are generally associated with compounds containing sulphur, where even tiny losses are sufficient to cause a noticeable odour. Aqueous effluent's consist of cooling water, surface water and process water. The majority of the water discharged from the refinery has been used for cooling the various process streams. The cooling water does not actually come into contact with the process material and so has very little contamination. The cooling water passes through large "interceptors" which separate any oil from minute leaks etc., prior to discharge. The cooling water system at Geelong Refinery is a once-through system with no recirculation. Rainwater falling on the refinery site must be treated before discharge to ensure no oily material washed off process equipment leaves the refinery. This is done first by passing the water through smaller "plant oil catchers", which each treat rainwater from separate areas on the site, and then all the streams pass to large
"interceptors" similar to those used for cooling water. The rainwater from the production areas is further treated in a Dissolved Air Flotation (DAF) unit. This unit cleans the water by using a flocculation agent to collect any remaining particles or oil droplets and floating the resulting flock to the surface with millions of tiny air bubbles. At the surface the flock is skimmed off and the clean water discharged. Process water has actually come into contact with the process streams and so can contain significant contamination. This water is treated in the "sour water treater" where the contaminants (mostly ammonia and hydrogen sulphide) are removed and then recovered or destroyed in a downstream plant. The process water, when treated in this way, can be reused in parts of the refinery and discharged through the process area rainwater treatment system and the DAF unit. Any treated process water that is not reused is discharged as Trade Waste to the sewerage system. This trade waste also includes the effluent from the refinery sewage treatment plant and a portion of treated water from the DAF unit. As most refineries import and export many feed materials and products by ship, the refinery and harbour authorities are prepared for spillage from the ship or pier. In the event of such a spill, equipment is always on standby at the refinery and it is supported by the facilities of the Australian Marine Oil Spill Centre at Geelong, Victoria. The refinery safeguards the land environment by ensuring the appropriate disposal of all wastes.
Within the refinery, all hydrocarbon wastes are recycled through the refinery slops system. This system consists of a network of collection pipes and a series of dewatering tanks. The recovered hydrocarbon is reprocessed through the distillation units. Wastes that cannot be reprocessed are either recycled to manufacturers (e.g. some spent catalysts can be reprocessed), disposed of in EPA-approved facilities off-site, or chemically treated on-site to form inert materials which can be disposed to land-fill within the refinery. Waste movements within the refinery require a "Process liquid, Sludge and Solid waste disposal permit". Wastes that go off-site must have an EPA "Waste Transport Permit".
The mixture can be split into simpler fractions by fractional distillation (animation)
Liquified petroleum gas (LPG) Gasoline (also known as petrol) Naphtha Kerosene and related jet aircraft fuels Diesel fuel Fuel oils Lubricating oils Paraffin wax Asphalt and tar Petroleum coke
Вам также может понравиться
- The Yellow House: A Memoir (2019 National Book Award Winner)От EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Рейтинг: 4 из 5 звезд4/5 (98)
- Lending Kart LAI-100986742Документ17 страницLending Kart LAI-100986742Muneer Basha BashaОценок пока нет
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceОт EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceРейтинг: 4 из 5 звезд4/5 (895)
- TNHB AllotmentДокумент3 страницыTNHB AllotmentMuneer Basha BashaОценок пока нет
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeОт EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeРейтинг: 4 из 5 звезд4/5 (5794)
- Crude Oil 32Документ11 страницCrude Oil 32Muneer Basha BashaОценок пока нет
- The Little Book of Hygge: Danish Secrets to Happy LivingОт EverandThe Little Book of Hygge: Danish Secrets to Happy LivingРейтинг: 3.5 из 5 звезд3.5/5 (399)
- Crude Oil 32Документ11 страницCrude Oil 32Muneer Basha BashaОценок пока нет
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaОт EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaРейтинг: 4.5 из 5 звезд4.5/5 (266)
- Focus Presentation On Indian Transport ScenarioДокумент15 страницFocus Presentation On Indian Transport ScenarioMuneer Basha BashaОценок пока нет
- 1.ENERGY SCENARIO-merged PDFДокумент188 страниц1.ENERGY SCENARIO-merged PDFraj walkeОценок пока нет
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureОт EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureРейтинг: 4.5 из 5 звезд4.5/5 (474)
- Rules of Thumb For Process EngineerДокумент105 страницRules of Thumb For Process EngineerHary100% (6)
- Never Split the Difference: Negotiating As If Your Life Depended On ItОт EverandNever Split the Difference: Negotiating As If Your Life Depended On ItРейтинг: 4.5 из 5 звезд4.5/5 (838)
- k-441 Gas Ratio K-441 PDFДокумент17 страницk-441 Gas Ratio K-441 PDFAhmedОценок пока нет
- PDF Document 4Документ2 страницыPDF Document 4miriam harriottОценок пока нет
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryОт EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryРейтинг: 3.5 из 5 звезд3.5/5 (231)
- Plasma Hexane 2014 IEEE PDFДокумент11 страницPlasma Hexane 2014 IEEE PDFCAMILA COBOS MOLANOОценок пока нет
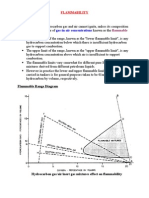
- FLAMMABILITYДокумент2 страницыFLAMMABILITYBharatiyulamОценок пока нет
- The Emperor of All Maladies: A Biography of CancerОт EverandThe Emperor of All Maladies: A Biography of CancerРейтинг: 4.5 из 5 звезд4.5/5 (271)
- CompotecLine Hoses OIL MIC CompressedДокумент2 страницыCompotecLine Hoses OIL MIC CompressedAndrian HadianaОценок пока нет
- Matriculation Chemistry (Hydrocarbon) Part 2 AlkaneДокумент30 страницMatriculation Chemistry (Hydrocarbon) Part 2 AlkaneridwanОценок пока нет
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyОт EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyРейтинг: 3.5 из 5 звезд3.5/5 (2259)
- Module 4: Alkenes: Nomenclature, Preparation and ReactionДокумент2 страницыModule 4: Alkenes: Nomenclature, Preparation and ReactionPeña, RodolfoОценок пока нет
- Fundamental Reservoir Fluid BehaviourДокумент20 страницFundamental Reservoir Fluid BehaviourBrian CbtngnОценок пока нет
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersОт EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersРейтинг: 4.5 из 5 звезд4.5/5 (344)
- ShilpaДокумент11 страницShilpashilpakprОценок пока нет
- Discontinued ASTM MethodsДокумент3 страницыDiscontinued ASTM MethodsNoorshalehahОценок пока нет
- Team of Rivals: The Political Genius of Abraham LincolnОт EverandTeam of Rivals: The Political Genius of Abraham LincolnРейтинг: 4.5 из 5 звезд4.5/5 (234)
- The Effects of Neat Biodiesel and Biodiesel and HVO Blends in Diesel Fuel On Exhaust Emissions From A Light Duty Vehicle With Diesel Engine PDFДокумент10 страницThe Effects of Neat Biodiesel and Biodiesel and HVO Blends in Diesel Fuel On Exhaust Emissions From A Light Duty Vehicle With Diesel Engine PDFpatikavalcoОценок пока нет
- CBSE Class 11 Chemistry Quick Revision Notes Hydrocarbons: Material Downloaded From - 1 / 2Документ2 страницыCBSE Class 11 Chemistry Quick Revision Notes Hydrocarbons: Material Downloaded From - 1 / 2Bibha KumariОценок пока нет
- 12 U Orgo - 1 - Hydrocarbon Nomenclature WorksheetДокумент4 страницы12 U Orgo - 1 - Hydrocarbon Nomenclature WorksheetZia Rathore100% (1)
- The Unwinding: An Inner History of the New AmericaОт EverandThe Unwinding: An Inner History of the New AmericaРейтинг: 4 из 5 звезд4/5 (45)
- Smoke PointДокумент10 страницSmoke PointMUHAMMAD AKRAMОценок пока нет
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreОт EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreРейтинг: 4 из 5 звезд4/5 (1090)
- Silabo - 17201Документ7 страницSilabo - 17201Enrique Rojas BermudoОценок пока нет
- So Lucio Nario Bailey Chapter 2Документ37 страницSo Lucio Nario Bailey Chapter 2Miguel Angel Rozo ArangoОценок пока нет
- Chemistry Form 6 Organic Chemistry: Chapter 2: HydrocarbonДокумент51 страницаChemistry Form 6 Organic Chemistry: Chapter 2: HydrocarbonNurul FarhanaОценок пока нет
- LEC M4 Post Task 2 Organic ChemistryДокумент3 страницыLEC M4 Post Task 2 Organic Chemistryhenry bernardОценок пока нет
- Noo Xii Ch10 Haloalkanes and HaloarenesДокумент64 страницыNoo Xii Ch10 Haloalkanes and HaloarenesG boi100% (3)
- ALKANES ORGANIC CHEMISTRY Chapter - 1Документ39 страницALKANES ORGANIC CHEMISTRY Chapter - 1rozОценок пока нет
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)От EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Рейтинг: 4.5 из 5 звезд4.5/5 (121)
- Types of Chemical ReactionsДокумент4 страницыTypes of Chemical ReactionsVõ Thùy DươngОценок пока нет
- Crude Oil Spiled Water TreatmentДокумент9 страницCrude Oil Spiled Water TreatmentDicky Andre SaputraОценок пока нет
- Testo-Flue Gas in Industry 3-27-2008Документ149 страницTesto-Flue Gas in Industry 3-27-2008leruaitesОценок пока нет
- The Quality of CandleДокумент32 страницыThe Quality of Candlebellesuper100% (1)
- Lesson-Guide-G9 - Q2 M3 Chemistry On TemplateДокумент32 страницыLesson-Guide-G9 - Q2 M3 Chemistry On TemplateEvelyn AndosonОценок пока нет
- Aniline Point & Diesel IndexДокумент1 страницаAniline Point & Diesel IndexSerena Serena0% (1)
- X Science Full Test 1301Документ4 страницыX Science Full Test 1301Aakash DwivediОценок пока нет
- Chemistry Sample Lab ReportДокумент23 страницыChemistry Sample Lab ReportUltramix100% (2)