Академический Документы
Профессиональный Документы
Культура Документы
Thyroid
Загружено:
Bharti NawalpuriИсходное описание:
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
Thyroid
Загружено:
Bharti NawalpuriАвторское право:
Доступные форматы
hyroid hormones affect three fundamental physiologic processes: cellular differentiation, growth, and metabolism.
If you think about that statement for a minute, you might legitimately ask "So what else is there?" which emphasizes just how much of physiology is affected by thyroid hormones. Not many hormones can claim as diverse a set of target cells. The thyroid gland also produces another hormone called calcitonin, and the parathyroid glands secrete parathyroid hormone. Parathyroid hormone and calcitonin participate in control of calcium and phosphorus homeostasis and have significant effects on bone physiology. Thyroid glands are located in the neck, in close approximation to the first part of the trachea. In humans, the thyroid gland has a "butterfly" shape, with two lateral lobes that are connected by a narrow section called the isthmus. Most animals, however, have two separate glands on either side of the trachea. Thyroid glands are brownish-red in color. Close examination of a thyroid gland will reveal one or more small, light-colored nodules on or protruding from its surface - these are parathyroid glands (meaning "beside the thyroid"). The image to the right shows a canine thyroid gland and one attached parathyroid gland. The microscopic structure of the thyroid is quite distinctive. Thyroid epithelial cells - the cells responsible for synthesis of thyroid hormones - are arranged in spheres called thyroid follicles. Follicles are filled with colloid, a proteinaceous depot of thyroid hormone precursor. In the low (left) and high-magnification (right) images of a cat thyroid below, follicles are cut in cross section at different levels, appearing as roughly circular forms of varying size. In standard histologic preparations such as these, colloid stains pink.
In addition to thyroid epithelial cells, the thyroid gland houses one other important endocrine cell. Nestled in spaces between thyroid follicles are parafollicular or C cells, which secrete the hormone calcitonin. The structure of a parathyroid gland is distinctly different from a thyroid gland. The cells that synthesize and secrete parathyroid hormone are arranged in rather dense cords or nests around abundant capillaries. The image below shows a section of a feline parathyroid gland on the left, associated with thyroid gland (note the follicles) on the right.
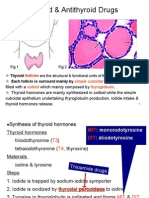
hyroid hormones are synthesized by mechanisms fundamentally different from what is seen in other endocrine systems. Thyroid follicles serve as both factory and warehouse for production of thyroid hormones.
Constructing Thyroid Hormones
The entire synthetic process occurs in three major steps, which are, at least in some ways, analagous to those used in the manufacture of integrated circuits (ICs):
Production and accumulation of the raw materials (in the case of ICs, a large wafer of doped silicon) Fabrication or synthesis of the hormones on a backbone or scaffold of precursor (etching several ICs on the silicon wafer) Release of the free hormones from the scaffold and secretion into blood (cutting individual ICs out of the larger wafer and distributing them)
The recipe for making thyroid hormones calls for two principle raw materials:
Tyrosines are provided from a large glycoprotein scaffold called thyroglobulin, which is synthesized by thyroid epithelial cells and secreted into the lumen of the follicle colloid is essentially a pool of thyroglobulin. A molecule of thyroglobulin contains 134 tyrosines, although only a handful of these are actually used to synthesize T4 and T3. Iodine, or more accurately iodide (I-), is avidly taken up from blood by thyroid epithelial cells, which have on their outer plasma membrane a sodium-iodide symporter or "iodine trap". Once inside the cell, iodide is transported into the lumen of the follicle along with thyroglobulin.
Fabrication of thyroid hormones is conducted by the enzyme thyroid peroxidase, an integral membrane protein present in the apical (colloid-facing) plasma membrane of thyroid epithelial cells. Thyroid peroxidase catalyzes two sequential reactions:
1. Iodination of tyrosines on thyroglobulin (also known as "organification of iodide"). 2. Synthesis of thyroxine or triiodothyronine from two iodotyrosines.
Through the action of thyroid peroxidase, thyroid hormones accumulate in colloid, on the surface of thyroid epithelial cells. Remember that hormone is still tied up in molecules of thyroglobulin - the task remaining is to liberate it from the scaffold and secrete free hormone into blood. Thyroid hormones are excised from their thyroglobulin scaffold by digestion in lysosomes of thyroid epithelial cells. This final act in thyroid hormone synthesis proceeds in the following steps:
Thyroid epithelial cells ingest colloid by endocytosis from their apical borders - that colloid contains thyroglobulin decorated with thyroid hormone. Colloid-laden endosomes fuse with lysosomes, which contain hydrolytic enzymes that digest thyroglobluin, thereby liberating free thyroid hormones. Finally, free thyroid hormones apparently diffuse out of
lysosomes, through the basal plasma membrane of the cell, and into blood where they quickly bind to carrier proteins for transport to target cells.
Control of Thyroid Hormone Synthesis and Secretion
Each of the processes described above appears to be stimulated by thyroid-stimulating hormone from the anterior pituitary gland. Binding of TSH to its receptors on thyroid epithelial cells stimulates synthesis of the iodine transporter, thyroid peroxidase and thyroglobulin. The magnitude of the TSH signal also sets the rate of endocytosis of colloid - high concentrations of TSH lead to faster rates of endocytosis, and hence, thyroid hormone release into the circulation. Conversely, when TSH levels are low, rates of thyroid hormone synthesis and release diminish.
hyroid Hormone Receptors and Mechanism of Action
Receptors for thyroid hormones are intracellular DNA-binding proteins that function as hormone-responsive transcription factors, very similar conceptually to the receptors for steroid hormones. Thyroid hormones enter cells through membrane transporter proteins. A number of plasma membrane transporters have been identified, some of which require ATP hydrolysis; the relative importance of different carrier systems is not yet clear and may differ among tissues. Once inside the nucleus, the hormone binds its receptor, and the hormone-receptor complex interacts with specific sequences of DNA in the promoters of responsive genes. The effect of the hormone-receptor complex binding to DNA is to modulate gene expression, either by stimulating or inhibiting transcription of specific genes. For the purpose of illustration, consider one mechanism by which thyroid hormones increase the strength of contraction of the heart. Cardiac contractility depends, in part, on the relative ratio of different types of myosin proteins in cardiac muscle. Transcription of some myosin genes is stimulated by thyroid hormones, while transcription of others in inhibited. The net effect is to alter the ratio toward increased contractility. For additional details on mechanism of action and how these receptors interact with other transcription factors, examine the section Thyroid Hormone Receptors.
Physiologic Effects of Thyroid Hormones
It is likely that all cells in the body are targets for thyroid hormones. While not strictly necessary for life, thyroid hormones have profound effects on many "big time" physiologic processes, such as development, growth and metabolism, and deficiency in thyroid hormones is not compatible with normal health. Additionally, many of the effects of thyroid hormone have been delineated by study of deficiency and excess states, as discussed briefly below. Metabolism: Thyroid hormones stimulate diverse metabolic activities most tissues, leading to an increase in basal metabolic rate. One consequence of this activity is to increase body heat production, which seems to result, at least in part, from increased oxygen consumption and rates of ATP hydrolysis. By way of analogy, the action of thyroid hormones is akin to blowing on a smouldering fire. A few examples of specific metabolic effects of thyroid hormones include:
Lipid metabolism: Increased thyroid hormone levels stimulate fat mobilization, leading to increased concentrations of fatty acids in plasma. They also enhance oxidation of fatty acids in many tissues. Finally, plasma concentrations of cholesterol and triglycerides are inversely correlated with thyroid hormone levels - one diagnostic indiction of hypothyroidism is increased blood cholesterol
concentration. Carbohydrate metabolism: Thyroid hormones stimulate almost all aspects of carbohydrate metabolism, including enhancement of insulin-dependent entry of glucose into cells and increased gluconeogenesis and glycogenolysis to generate free glucose.
Growth: Thyroid hormones are clearly necessary for normal growth in children and young animals, as evidenced by the growth-retardation observed in thyroid deficiency. Not surprisingly, the growth-promoting effect of thyroid hormones is intimately intertwined with that of growth hormone, a clear indiction that complex physiologic processes like growth depend upon multiple endocrine controls. Development: A classical experiment in endocrinology was the demonstration that tadpoles deprived of thyroid hormone failed to undergo metamorphosis into frogs. Of critical importance in mammals is the fact that normal levels of thyroid hormone are essential to the development of the fetal and neonatal brain. Other Effects: As mentioned above, there do not seem to be organs and tissues that are not affected by thyroid hormones. A few additional, well-documented effects of thyroid hormones include:
Cardiovascular system: Thyroid hormones increases heart rate, cardiac contractility and cardiac output. They also promote vasodilation, which leads to enhanced blood flow to many organs. Central nervous system: Both decreased and increased concentrations of thyroid hormones lead to alterations in mental state. Too little thyroid hormone, and the individual tends to feel mentally sluggish, while too much induces anxiety and nervousness. Reproductive system: Normal reproductive behavior and physiology is dependent on having essentially normal levels of thyroid hormone. Hypothyroidism in particular is commonly associated with infertility.
Thyroid Disease States
Disease is associated with both inadequate production and overproduction of thyroid hormones. Both types of disease are relatively common afflictions of man and animals. Hypothyroidism is the result from any condition that results in thyroid hormone deficiency. Two well-known examples include:
Iodine deficiency: Iodide is absolutely necessary for production of thyroid hormones; without adequate iodine intake, thyroid hormones cannot be synthesized. Historically, this problem was seen particularly in areas with iodine-deficient soils, and frank iodine deficiency has
been virtually eliminated by iodine supplementation of salt. Primary thyroid disease: Inflammatory diseases of the thyroid that destroy parts of the gland are clearly an important cause of hypothyroidism.
Common symptoms of hypothyroidism arising after early childhood include lethargy, fatigue, cold-intolerance, weakness, hair loss and reproductive failure. If these signs are severe, the clinical condition is called myxedema. In the case of iodide deficiency, the thyroid becomes inordinantly large and is called a goiter. The most severe and devestating form of hypothyroidism is seen in young children with congenital thyroid deficiency. If that condition is not corrected by supplemental therapy soon after birth, the child will suffer from cretinism, a form of irreversible growth and mental retardation. Most cases of hypothyroidism are readily treated by oral administration of synthetic thyroid hormone. In times past, consumption of dessicated animal thyroid gland was used for the same purpose. Hyperthyroidism results from secretion of thyroid hormones. In most species, this condition is less common than hypothyroidism. In humans the most common form of hyperthyroidism is Graves disease, an immune disease in which autoantibodies bind to and activate the thyroid-stimulating hormone receptor, leading to continual stimulation of thyroid hormone synthesis. Another interesting, but rare cause of hyperthyroidism is so-called hamburger thyrotoxicosis. Common signs of hyperthyroidism are basically the opposite of those seen in hypothyroidism, and include nervousness, insomnia, high heart rate, eye disease and anxiety. Graves disease is commonly treated with anti-thyroid drugs (e.g. propylthiourea, methimazole), which suppress synthesis of thyroid hormones primarily by interfering with iodination of thyroglobulin by thyroid peroxidase. The chief stimulator of thyroid hormone synthesis is thyroid-stimulating hormone from the anterior pituitary. Binding of TSH to receptors on thyroid epithelial cells seems to enhance all of the processes necessary for synthesis of thyroid hormones, including synthesis of the iodide transporter, thyroid peroxidase and thyroglobulin. The magnitude of the TSH signal also sets the rate of endocytosis of colloid - high concentrations of TSH lead to faster rates of endocytosis, and hence, thyroid hormone release into the circulation. Conversely, when TSH levels are low, rates of thyroid hormone synthesis and release diminish. The thyroid gland is part of the hypothalamic-pituitary-thyroid axis, and control of thyroid hormone secretion is exerted by classical negative feedback, as depicted in the diagram. Thyroid-releasing hormone (TRH) from the hypothalamus stimulates TSH from the pituitary, which stimulates thyroid hormone release. As blood concentrations of
thyroid hormones increase, they inhibit both TSH and TRH, leading to "shutdown" of thyroid epithelial cells. Later, when blood levels of thyroid hormone have decayed, the negative feedback signal fades, and the system wakes up again. A number of other factors have been shown to influence thyroid hormone secretion. In rodents and young children, exposure to a cold environment triggers TRH secretion, leading to enhanced thyroid hormone release. This makes sense considering the known ability of thyroid hormones to spark body heat production.
Receptors for thyroid hormones are members of a large family of nuclear receptors that include those of the steroid hormones. They function as hormone-activated transcription factors and thereby act by modulating gene expression. In contrast to steroid hormone receptors, thyroid hormone receptors bind DNA in the absence of hormone, usually leading to transcriptional repression. Hormone binding is associated with a conformational change in the receptor that causes it to function as a transcriptional activator.
Receptor Structure
Mammalian thyroid hormone receptors are encoded by two genes, designated alpha and beta. Further, the primary transcript for each gene can be alternatively spliced, generating different alpha and beta receptor isoforms. Currently, four different thyroid hormone receptors are recognized: alpha-1, alpha-2, beta-1 and beta-2. Like other members of the nuclear receptor superfamily, thyroid hormone receptors encapsulate three functional domains:
A transactivation domain at the amino terminus that interacts with other transcription factors to form complexes that repress or activate transcription. There is considerable divergence in sequence of the transactivation domains of alpha and beta isoforms and between the two beta isoforms of the receptor. A DNA-binding domain that binds to sequences of promoter DNA known as hormone response elements. A ligand-binding and dimerization domain at the carboxyterminus.
As depicted in the figure below, the DNA-binding domains of the different receptor isoforms are very similar, but there is considerable divergence among transactivation and ligand-binding domains. Most notably, the alpha-2 isoform has a unique carboxy-terminus and does not bind triiodothyronine (T3).
The different forms of thyroid receptors have patterns of expression that vary by tissue and by developmental stage. For example, almost all tissues express the alpha-1, alpha-2 and beta-1 isoforms, but beta-2 is synthesized almost exclusively in hypothalamus, anterior pituitary and developing ear. Receptor alpha-1 is the first isoform expressed in the conceptus, and there is a profound increase in expression of beta receptors in brain shortly after birth. Interestingly, the beta receptor preferentially activates expression from several genes known to be important in brain development (e.g. myelin basic protein), and upregulation of this particular receptor may thus be critical to the well known effects of thyroid hormones on development of the fetal and neonatal brain. The presence of multiple forms of the thyroid hormone receptor, with tissue and stage-dependent differences in their expression, suggests an extraordinary level of complexity in the physiologic effects of thyroid hormone.
Interaction of Thyroid Hormone Receptors with DNA
Thyroid hormone receptors bind to short, repeated sequences of DNA called thyroid or T3 response elements (TREs), a type of hormone response element. A TRE is composed of two AGGTCA "half sites" separated by four nucleotides. The half sites of a TRE can be arranged as direct repeats, pallindromes or inverted repeats. The DNA-binding domain of the receptor contains two sets of four cysteine residues, and each set chelates a zinc ion, forming loops known as "zinc fingers". A part of the first zinc finger interacts directly with nucleotides in the major groove of TRE DNA, while residues in the second finger interact with nucleotides in the minor groove of the TRE. Thus, the zinc fingers mediate specificity in binding to TREs. Thyroid hormone receptors can bind to a TRE as monomers, as homodimers or as heterodimers with the retinoid X receptor (RXR), another member of the nuclear receptor superfamily that binds 9-cis retinoic acid. The heterodimer affords the highest affinity binding, and is thought to represent the major functional form of the receptor.
Thyroid hormone receptors bind to TRE DNA regardless of whether they are occupied by T3. However, the biological effects of TRE binding by the unoccupied versus the occupied receptor are dramatically different. In general, binding of thyroid hormone receptor alone to DNA leads to repression of transcription, whereas binding of the thyroid hormonereceptor complex activates transcription. Ligand-free state: The transactivation domain of the T3-free receptor, as a heterodimer with RXR, assumes a conformation that promotes interaction with a group of transcriptional corepressor molecules. A part of this corepressor complex has histone deacetylase activity (HDA), which is associated with formation of a compact, "turned-off" conformation of chromatin. The net effect of recruiting these types of transcription factors is to repress transcription from affected genes. Ligand-bound state: Binding of T3 to its receptor induces a conformational change in the receptor that makes it incompetent to bind the corepressor complex, but competent to bind a group of coactivator proteins. The coactivator complex contains histone transacetylase (HAT) activity, which imposes an open configuration on adjacent chromatin. The coactivator complex associated with the T3-bound receptor functions to activate transcription from linked genes. A growing number of specific proteins have been identified as members of the corepressor and coactivator complexes described. It should also be mentioned that there are several exceptions to the scheme described above. As mentioned, the alpha-2 receptor is unable to bind T3 and acts as similarly to a dominant-negative mutant of the receptor, but its carboxy-terminus can be differentially phosphorylated, which affects DNA binding and dimerization. Also, the beta-2 isoform apparently fails to function as a repressor in the absence of T3.
Disorders of Thyroid Hormone Receptors
A number of humans with a syndrome of thyroid hormone resistance have been identified, and found to have mutations in the receptor beta gene which abolish ligand binding. Clinicially, such individuals show a type of hypothyroidism characterized by goiter, elevated serum concentrations of T3 and thyroxine and normal or elevated serum concentrations of TSH. More than half of affected children show attention-deficit disorder, which is intriguing considering the role of thyroid hormones in brain development. In most affected families, this disorder is transmitted as a dominant trait, which suggests that the mutant receptors act in a dominant negative manner.
Mice with targeted deletions in thyroid receptor genes have provided additional understanding of the possible roles of different forms of thyroid hormone receptors. Knockout mice that are unable to produce the alpha-1 receptor showed subnormal body temperature and mild abnormalities in cardiac function. Other mice which lacked expression of both alpha isoforms were severely hypothyroid and died within the first few weeks of life. Mice with disruptions of the entire beta gene exhibited elevated TSH levels and deafness, while mice with mutations that disrupted only beta-2 expression had elevated TSH, but normal hearing. Such experiments are beginning to allow determination of which functions of the different receptor isoforms are redundant and which are not. Inactivating mutations in thyroid hormone receptors do not produce a syndrome analogous to the lack of thyroid hormones. This is the case even in mice with targeted deletions in both alpha and beta receptor genes. The most likely explanation for the relative mild effects of receptor deficiency is that responsive genes are left in a "neutral" state, rather than being chronically suppressed as happens with hormone deficiency. Thyroid hormones are orally active, which means that consumption of thyroid gland tissue can cause thyrotoxicosis, a type of hyperthyroidism. Several outbreaks of thyrotoxicosis have been attributed to a practice, now banned in the US, called "gullet trimming", where meat in the neck region of slaughtered animals is ground into hamburger. Because thyroid glands are reddish in color and located in the neck, it's not unusual for gullet trimmers to get thyroid glands into hamburger or sausage. People, and presumably pets, that eat such hamburger can get dose of thyroid hormone sufficient to induce disease. A report by Hedberg and colleagues (1987) on this topic is one of several in the literature. They described an outbreak of thyrotoxicosis in Minnesota and South Dakota that was traced to thyroid-contaminated hamburger. A total of 121 cases were identified in nine counties, with the highest incidence in the county having the offending slaughter plant. The patients complained of sleeplessness, nervousness, headache, fatique, excessive sweating and weight loss. The graph below shows serum concentrations of thyroxine and thyroid-stimulating hormone in a volunteer that consumed a well-cooked, 227 g hamburger (admittedly, a large meal) prepared from the contaminated meat. Note how TSH levels were suppressed during the time when thyroxine (T4) concentrations were elevated.
The ability of the thyroid gland to transport and concentrate iodide from blood is absolutely necessary for the synthesis of thyroid hormones. The key player in this process is the sodium-iodide symporter, an integral membrane protein that resides in the basolateral membrane of thyroid epithelial cells. Considering critical role of iodine trapping in thyroid function, it is not surprising that abnormalities in expression or function of the symporter can lead to thyroid diesase. Two such situations have been identified in humans:
Inactivating mutations in the symporter gene result in congenital hypothyroidism. In several patients with this disorder, specific missense mutations in the symporter mRNA have been characterized. Autoantibodies to the symporter protein adversely affect iodide transport. A substantial number of patients with autoimmune (Hasimoto's) thyroiditis have anti-symporter antibodies, and application of these antibodies to cultured cells expressing the symporter inhibits iodide uptake.
The sodium-iodide symporter cannot distinguish between normal and radioactive iodide, thus providing a useful exploit for diagnosis and treatment of certain thyroid disease. Small amounts of radioactive iodine injected into patients are rapidly concentrated in the thyroid, providing a means to image the thyroid for detection of tumors and other abnormalities. Administration of higher doses of radioiodine is widely used for treatment of hyperthyroidism and some types of thyroid cancer; in this case the radioactivity is concentrated rather precisely in the tissue requiring destruction.
Structure and Function
As its name indicates, the sodium-iodide symporter simultaneously transports both Na+ and I- ions from extracellular fluid (i.e. blood) into the thryoid epithelial cell. This process is an example of secondard active transport. Energy is provided by the electrochemical gradient of sodium across the cell membrane; the low intracellular
concentration of sodium is maintained by sodium pumps.
Recently, cDNAs for the rat and human sodium-iodide transporter have been cloned. Analysis of their deduced protein sequence indicates that the symporter has 13 membrane-spanning domains. The human and rat proteins are 643 and 618 amino acids in length and both contain N-linked glycosylation sites. Although the symporter appears to be glycosylated, those modifications are appearently not required for full activity. The functional receptor may be a multimer.
Regulation of Expression
The sodium-iodide symporter is most highly expressed in thyroid epithelial cells. Lower levels of expression can be detected in mammary gland, salivary gland, stomach and colon, but none of these tissues is known to organify iodide. The presence of the symporter in mammary gland leads to secretion of iodine in milk, which is probably important for thyroid function in neonatal animals. The most important stimulator of symporter gene and protein expression is thyroidstimulating hormone, similar to what is observed with other important thyroid proteins
such as thyroglobulin and thyroid peroxidase. The human sodium-iodide symporter gene has 15 exons. It has a basal promoter region that extends roughly 500 bp upstream from the transcription start site and thyroidspecific enhancer elements that span positions of approximately -2200 to -2500.
Вам также может понравиться
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryОт EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryРейтинг: 3.5 из 5 звезд3.5/5 (231)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)От EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Рейтинг: 4.5 из 5 звезд4.5/5 (119)
- Never Split the Difference: Negotiating As If Your Life Depended On ItОт EverandNever Split the Difference: Negotiating As If Your Life Depended On ItРейтинг: 4.5 из 5 звезд4.5/5 (838)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaОт EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaРейтинг: 4.5 из 5 звезд4.5/5 (265)
- The Little Book of Hygge: Danish Secrets to Happy LivingОт EverandThe Little Book of Hygge: Danish Secrets to Happy LivingРейтинг: 3.5 из 5 звезд3.5/5 (399)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyОт EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyРейтинг: 3.5 из 5 звезд3.5/5 (2219)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeОт EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeРейтинг: 4 из 5 звезд4/5 (5794)
- Team of Rivals: The Political Genius of Abraham LincolnОт EverandTeam of Rivals: The Political Genius of Abraham LincolnРейтинг: 4.5 из 5 звезд4.5/5 (234)
- The Emperor of All Maladies: A Biography of CancerОт EverandThe Emperor of All Maladies: A Biography of CancerРейтинг: 4.5 из 5 звезд4.5/5 (271)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreОт EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreРейтинг: 4 из 5 звезд4/5 (1090)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersОт EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersРейтинг: 4.5 из 5 звезд4.5/5 (344)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceОт EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceРейтинг: 4 из 5 звезд4/5 (890)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureОт EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureРейтинг: 4.5 из 5 звезд4.5/5 (474)
- The Unwinding: An Inner History of the New AmericaОт EverandThe Unwinding: An Inner History of the New AmericaРейтинг: 4 из 5 звезд4/5 (45)
- The Yellow House: A Memoir (2019 National Book Award Winner)От EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Рейтинг: 4 из 5 звезд4/5 (98)
- Pharmacology Tyroid and Antitiroid HormonДокумент21 страницаPharmacology Tyroid and Antitiroid HormonPuterinugraha Wanca ApatyaОценок пока нет
- Thyroid UltrasoundДокумент62 страницыThyroid UltrasoundYoungFanjiens100% (1)
- Forxiga Slide RTD 2017 - 2Документ64 страницыForxiga Slide RTD 2017 - 2Budi WirawanОценок пока нет
- Clinical Tips For The Management of Perimenopausal Syndrome by DR - Deepti ChawlaДокумент19 страницClinical Tips For The Management of Perimenopausal Syndrome by DR - Deepti ChawlaHomoeopathic PulseОценок пока нет
- Handout Step 2 GYN Sakala Jan 2014.ppt4-Part 2 PDFДокумент9 страницHandout Step 2 GYN Sakala Jan 2014.ppt4-Part 2 PDFAhmedОценок пока нет
- CSE Grade 10 Science Lesson PlanДокумент6 страницCSE Grade 10 Science Lesson PlanJelly Marie Baya FloresОценок пока нет
- 10 EndocrinologyДокумент223 страницы10 Endocrinologythisar100% (1)
- CCДокумент9 страницCCFritzie BlancheОценок пока нет
- Bio 2004Документ6 страницBio 2004Bharti NawalpuriОценок пока нет
- Cell Biology II LS1 2 1Документ27 страницCell Biology II LS1 2 1Bharti NawalpuriОценок пока нет
- Tnset 3Документ9 страницTnset 3Bharti NawalpuriОценок пока нет
- Msc. ZoologyДокумент30 страницMsc. ZoologyBharti NawalpuriОценок пока нет
- Bioinformatics in Cell Biology FinalДокумент32 страницыBioinformatics in Cell Biology FinalBharti NawalpuriОценок пока нет
- Bhu Genetics 2011Документ34 страницыBhu Genetics 2011Bharti NawalpuriОценок пока нет
- Bioinformatics in Cell Biology FinalДокумент32 страницыBioinformatics in Cell Biology FinalBharti NawalpuriОценок пока нет
- Kuby Immunology: Kindt - Goldsby - OsborneДокумент35 страницKuby Immunology: Kindt - Goldsby - OsborneBharti NawalpuriОценок пока нет
- Unit 1 Theoretical Perspectives of Development: StructureДокумент11 страницUnit 1 Theoretical Perspectives of Development: StructureTanyaОценок пока нет
- Biology: Sexual ReproductionДокумент7 страницBiology: Sexual ReproductionAllan BustamanteОценок пока нет
- Askep DM Seminar KesdamДокумент29 страницAskep DM Seminar KesdamResty PermatasariОценок пока нет
- Congenital and Acquired Malformations in Vulva and Vagina - Pediatric AgeДокумент2 страницыCongenital and Acquired Malformations in Vulva and Vagina - Pediatric AgePramedicaPerdanaPutraОценок пока нет
- Family Planning Methods and Birth Control PillsДокумент6 страницFamily Planning Methods and Birth Control PillsnutriОценок пока нет
- Changes in abdominal fat linked to weight loss and insulin sensitivityДокумент7 страницChanges in abdominal fat linked to weight loss and insulin sensitivityAres Santiago R. NoguedaОценок пока нет
- The Endocrinopathies of Anorexia NervosaДокумент13 страницThe Endocrinopathies of Anorexia NervosaCarla MesquitaОценок пока нет
- CT findings and complications of subdural and epidural haematomasДокумент3 страницыCT findings and complications of subdural and epidural haematomasKay BristolОценок пока нет
- 2018 Sec 4 Science Biology SA2 - KranjiДокумент35 страниц2018 Sec 4 Science Biology SA2 - Kranji19Y1H GAO CHENZHANGОценок пока нет
- Understanding Pathophysiology e BookДокумент61 страницаUnderstanding Pathophysiology e Bookjames.crandle290100% (37)
- Review on Estrous Synchronization in EthiopiaДокумент22 страницыReview on Estrous Synchronization in EthiopiaTiruneh Mossie mossie100% (1)
- Pathophysiology OF HEART FAILUREДокумент2 страницыPathophysiology OF HEART FAILUREJessa AdenigОценок пока нет
- Anatomy of Male Reproductive SystemДокумент35 страницAnatomy of Male Reproductive SystemDr. Anoop SinghОценок пока нет
- Etiology of Diabetes Mellitus Tipe 2 PDFДокумент2 страницыEtiology of Diabetes Mellitus Tipe 2 PDFGinoОценок пока нет
- ProlactinomasДокумент9 страницProlactinomastvleeeОценок пока нет
- Gizi Pranikah QR 2020Документ28 страницGizi Pranikah QR 2020rianОценок пока нет
- Review On Miracle of Herbals in Treatment and Regulation of ThyroidДокумент5 страницReview On Miracle of Herbals in Treatment and Regulation of ThyroidEditor IJTSRDОценок пока нет
- PertanyaanДокумент12 страницPertanyaanBaskoro Adi NugrohoОценок пока нет
- Primary HyperparathyroidismДокумент13 страницPrimary Hyperparathyroidismqayyum consultantfpscОценок пока нет
- Electrolytes Part 2 11Документ3 страницыElectrolytes Part 2 11Joshoua MalanaОценок пока нет
- Breast Cancer KelseyДокумент12 страницBreast Cancer Kelseyapi-3802092Оценок пока нет