Академический Документы
Профессиональный Документы
Культура Документы
Estimation of Saturated Liquid Density
Загружено:
ankur2061Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
Estimation of Saturated Liquid Density
Загружено:
ankur2061Авторское право:
Доступные форматы
Estimating Saturated Liquid Density using A) Gunn-Yamada Correlation and B) Rackett Equation
Reference: Section 1.7, Estimation of Liquid Density, 'Handbook of Chemical Engineering Calculations' by Nicholas P.Chopey, 3rd Edition
A. Gunn-Yamada Correlation
where:
V = liquid molar specific volume, cm
3
/g-mol
V
Sc
= See equation A1 below
V
r
(0)
= See equation A2(a) and A2(b) below
e = Acentric factor
I = See equation A-3
-------------- A1
where:
R = 82.06 atm.cm
3
/g-mol.K (universal gas constant)
T
c
= critical temperature, K
P
c
= critical pressure, atm-a
----A2(a)
for 0.2T/T
c
0.8
OR
----A2(b)
for 0.8T/T
c
1.0
where:
T = Actual temperature of the liquid, K
------A3
B. Rackett Equation
where:
V
sat-liq
= molar specific volume for the saturated liquid, cm
3
/g-mol
V
c
= critical molar volume, cm
3
/g-mol
Z
c
= critical compressibility factor
Notes
1. Both the Gunn-Yamada and Rackett equations are limited to saturated liquids.
2. Gunn-Yamada equation is fairly accurate for nonpolar as well as slightly polar compounds.
3. Both the equations give an error of 1% from experimental values for nonpolar compounds.
) 1 (
) 0 (
I = e
r
Sc
V
V
V
( ) e 0967 . 0 2920 . 0
|
|
.
|
\
|
=
c
c
Sc
P
RT
V
( ) ( ) ( ) ( )
4 3 2 ) 0 (
.. 11422 . 1 .. 02512 . 2 .. 51941 . 1 .. 33953 . 0 . 33593 . 0
c c c c r
T T T T T T T T V + + =
( ) ( ) ( ) ( )
2 5 . 0 ) 0 (
1 .. 91534 . 0 1 . 50879 . 0 1 log 1 . 3 . 1 0 . 1
c c c c r
T T T T T T T T V + =
( ) ( )
2
04842 . 0 09405 . 0 29607 . 0
c c
T T T T = I
( )
2857 . 0
1
c
T T
c c liq sat
Z V V
=
Saturated Liquid Density Using the Gunn-Yamada Equation
Inputs
Liquid Ammonia
Molecular weight, M 17.031 g/g-mol
Temperature, T 37 C
Critical Temp., T
c 405.65 K
Critical Press., P
c 111.5 atm-a
Crit. Compressibility, Z
c 0.241
Crit. Molar Volume, V
c 72
cm
3
/g-mol
Acentric Factor, 0.253
Gas Constant, R 82.06 atm.cm
3
/g-mol.K
Calculations
V
Sc
= 79.87
cm
3
/g-mol
T/T
c
= 0.765
V
r
(0)
= 0.440
I = 0.201
V = 33.37 cm
3
/g-mol
Density, = 0.510 g/cm
3
Saturated Liquid Density Using the Rackett Equation
Inputs
Liquid Ammonia
Molecular weight, M 17.031 g/g-mol
Temperature, T 37 C
Critical Temp., T
c 405.65 K
Critical Press., P
c 111.5 atm-a
Crit. Compressibility, Z
c 0.241
Crit. Molar Volume, V
c 72
cm
3
/g-mol
Acentric Factor, 0.253
Gas Constant, R 82.06 atm.cm
3
/g-mol.K
Calculations
T/T
c
= 0.765
V
sat-liq
= 28.09
cm
3
/g-mol
Density, = 0.606 g/cm
3
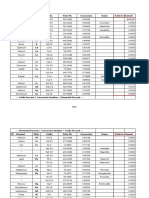
Critical Constants (Reference: Table 2-164: Critical Constants & Acentric Factors of Inorganic and Organic Compounds, Perry's Handbook, 7th Ed.)
NBP or Sat.
Temperature,
C Mol. Wt. T
c
, K P
c
, atm-a
V
c,
cm
3
/g-
mol Z
c
-161.4 16.043 190.564 45.3 99 0.286 0.011
-88.6 30.07 305.32 47.86 146 0.279 0.098
-42.1 44.1 369.83 41.55 200 0.273 0.149
-0.51 58.123 425.12 37.21 255 0.272 0.197
36.07 72.15 469.7 33.16 315 0.271 0.251
68.73 86.177 507.6 30 373 0.269 0.304
-11.79 58.123 408.14 35.73 261 0.278 0.177
27.84 72.15 460.43 33.26 304 0.268 0.226
58 86.177 499.98 30.89 358 0.269 0.246
60.26 86.177 497.5 29.8 366 0.267 0.279
98.42 100.204 540.2 26.84 428 0.259 0.346
-103.74 28.054 282.34 49.64 132 0.283 0.086
-47.69 42.081 365.57 45.69 188 0.286 0.137
-84.7 26.038 308.32 60.69 113 0.271 0.188
80.1 78.114 562.16 48.16 261 0.273 0.209
64.69 32.042 512.64 80.33 117 0.224 0.566
78.3 46.069 513.92 60.4 168 0.240 0.643
-33.3 17.031 405.65 111.5 72 0.241 0.253
Compound
Methane
Ethane
Propane
n-Butane
n-Pentane
n-Hexane
i-Butane
i-Pentane
2,3-Dimethylbutane
i-Hexane (2-Methylpentane)
n-Heptane
Methanol
Ethanol
Ethylene
Propylene
Acetylene
Benzene
Ammonia
Prepared by: Ankur Srivastava
Chemical Engineer
e-mail: ankur_2061@hotmail.com
Disclaimer : The information and methods included within this spreadsheet are presented for 'Saturated Liquid Density' calculations. It
is intended to be used by technically skilled persons at their own discretion. I do not warrant the suitability or accuracy of these
methods.
Notes:
2. Compounds can be added to the table given above by clicking the 'Unprotect' tab and typing the password
ankur2061 in the 'Tools-Protection' Menu.
3. When deviating from nonpolarity i.e. more polar compounds the error compared to experimentally obtained
liquid molar volume or density will be higher.
1. The temperature value in the input cell for temperature should not exceed the value of the "crtitical
temperature" since these equations are meant for saturated liquids and not supercritical liquids.
Вам также может понравиться
- Bubble Dew - Solver IPДокумент33 страницыBubble Dew - Solver IPApsari Puspita AiniОценок пока нет
- Acid Dew PointДокумент1 страницаAcid Dew Pointankur2061Оценок пока нет
- KFHC TPB Reguler ZfactorДокумент9 страницKFHC TPB Reguler ZfactorIlhamRifaldiОценок пока нет
- Jumadiao, Yra Marielle M. Exercise 9: Heat Balance and Theoretical Flame Temperature GivenДокумент4 страницыJumadiao, Yra Marielle M. Exercise 9: Heat Balance and Theoretical Flame Temperature GivenJanelle M. JumadiaoОценок пока нет
- 5729 - 5729 - Bitzer 4T-12.2Документ2 страницы5729 - 5729 - Bitzer 4T-12.2Jose CordovaОценок пока нет
- Convert XRF Data Element %Документ8 страницConvert XRF Data Element %manas773Оценок пока нет
- Pipe Properties FullДокумент16 страницPipe Properties FullJuan P RuizОценок пока нет
- L&T Construction: Rehabilitation of Bhagirathi WTP, Delhi List of Lab EquipmentsДокумент1 страницаL&T Construction: Rehabilitation of Bhagirathi WTP, Delhi List of Lab EquipmentsRamBinodSharmaОценок пока нет
- Aa + BB +CC+DD RR A+ (B/a) B + (C/a) C + (D/a) D (R/a) R R - KC C C C N N (1-X) N N - (B/a) (N - N) N N - (C/a) (N - N) N N - (D/a) (N - N) C N /VДокумент7 страницAa + BB +CC+DD RR A+ (B/a) B + (C/a) C + (D/a) D (R/a) R R - KC C C C N N (1-X) N N - (B/a) (N - N) N N - (C/a) (N - N) N N - (D/a) (N - N) C N /VChemical EngineeringОценок пока нет
- Unit Conversions Between U.S. Customary System & Metric SystemДокумент1 страницаUnit Conversions Between U.S. Customary System & Metric SystemMario Sajulga Dela CuadraОценок пока нет
- Stress Analysis ReportДокумент123 страницыStress Analysis ReportNOXOLO PETUNIA SAMBOОценок пока нет
- Gawish SPE Paper PDFДокумент11 страницGawish SPE Paper PDFEdsonОценок пока нет
- Yanbu: Export Refinery ProjectДокумент5 страницYanbu: Export Refinery ProjectJanakiraman MalligaОценок пока нет
- Specific Heat Chart of WaterДокумент2 страницыSpecific Heat Chart of WaterDaud IfadahОценок пока нет
- BWB Synopsis ReportДокумент37 страницBWB Synopsis ReportMidhun MvОценок пока нет
- Peng Robinson MixturesДокумент1 страницаPeng Robinson MixturesdckristantoОценок пока нет
- Langmuir Isotherm DevelopmentДокумент16 страницLangmuir Isotherm DevelopmentbarlosОценок пока нет
- Hydroulics Calculation and Gas PropertiesДокумент4 страницыHydroulics Calculation and Gas PropertiesMubarizОценок пока нет
- Process Calculation - Purge Gas CalculationДокумент1 страницаProcess Calculation - Purge Gas CalculationmakamahamisuОценок пока нет
- Z - Peng RobinsonДокумент1 страницаZ - Peng RobinsonMuhammadTanzeeLUsmanОценок пока нет
- Reaction Stoichiometry Balancer & Atom Economy CalculatorДокумент14 страницReaction Stoichiometry Balancer & Atom Economy CalculatorshikaswaОценок пока нет
- LPG Gas Press Above 5psi Pipe SizingДокумент1 страницаLPG Gas Press Above 5psi Pipe SizingSopi LabuОценок пока нет
- Boundary Layer CalculatorДокумент10 страницBoundary Layer CalculatorTint TigerОценок пока нет
- Turbine Combustion Kinetics PSR-1 : CVODE Starts at Line 100 On EXCEL SheetДокумент14 страницTurbine Combustion Kinetics PSR-1 : CVODE Starts at Line 100 On EXCEL SheetcymyОценок пока нет
- Converter Atomic Percent To Weight PercentДокумент6 страницConverter Atomic Percent To Weight Percentdiegomez84Оценок пока нет
- Flammability WorksheetДокумент6 страницFlammability WorksheetshailendraОценок пока нет
- fouling factor 중량 비체적: ft^2/Btu m^2/Kcal lb/ft kg/m lb/ft^3 kg/m^3 0.3689556791 1.4881889764 16.018732594Документ27 страницfouling factor 중량 비체적: ft^2/Btu m^2/Kcal lb/ft kg/m lb/ft^3 kg/m^3 0.3689556791 1.4881889764 16.018732594김종민Оценок пока нет
- Exercise 01: Component N-ButaneДокумент17 страницExercise 01: Component N-ButanesadiaОценок пока нет
- Yanbu: Export Refinery ProjectДокумент7 страницYanbu: Export Refinery ProjectJanakiraman MalligaОценок пока нет
- Packed Fluid BedДокумент26 страницPacked Fluid BedgeogeogeoОценок пока нет
- Structural SpecsДокумент42 страницыStructural SpecsJosh Jaymes MasseОценок пока нет
- T500 VeeyesДокумент5 страницT500 VeeyesTimothy TaylorОценок пока нет
- Material PropertiesДокумент2 страницыMaterial PropertiesCelineKevinОценок пока нет
- Basic Pecvd Plasma Processes (Sih Based) : Pecvd Sinx: Sih + NH (+H) or Sih + N (+H)Документ36 страницBasic Pecvd Plasma Processes (Sih Based) : Pecvd Sinx: Sih + NH (+H) or Sih + N (+H)Anonymous lidok7lDiОценок пока нет
- Material Balance of Styrene Production PДокумент12 страницMaterial Balance of Styrene Production PSteve WanОценок пока нет
- Vapor Pressure CalculatorДокумент120 страницVapor Pressure Calculatorhilman_hilmawanОценок пока нет
- Blank Calc Template - Steel Tools1Документ656 страницBlank Calc Template - Steel Tools1magdy.kamel6528Оценок пока нет
- Calalang, Louise Anne E. EXAM # 1-SE533 AT P. 164 Single Degree of Freedom PropertiesДокумент1 713 страницCalalang, Louise Anne E. EXAM # 1-SE533 AT P. 164 Single Degree of Freedom PropertiesRenzel EstebanОценок пока нет
- Basicflowmeasurement 150428100633 Conversion Gate02 PDFДокумент50 страницBasicflowmeasurement 150428100633 Conversion Gate02 PDFankur2061Оценок пока нет
- Natural Gas Homework2Документ42 страницыNatural Gas Homework2Khanz KhanОценок пока нет
- Ideal Gas ConversionДокумент5 страницIdeal Gas ConversionpsaayoОценок пока нет
- Size ReductionДокумент24 страницыSize ReductionKarynne Bernardine Gerona SiclotОценок пока нет
- Bubble and Dew PointДокумент6 страницBubble and Dew PointMawaddah Nur TambakОценок пока нет
- 2.2 Manual Calculation of Materials and Energy Balances 2.2.1 Mass Balance Plant Complete If The Amount That Targeted Is AchievedДокумент34 страницы2.2 Manual Calculation of Materials and Energy Balances 2.2.1 Mass Balance Plant Complete If The Amount That Targeted Is AchievedmmbmnbmnbОценок пока нет
- Mset Engineering Corporation SDN BHD: (Ref:Pressure Vessel Design Manual 3rd Edition by Dennis R. Moss Page 291 295)Документ36 страницMset Engineering Corporation SDN BHD: (Ref:Pressure Vessel Design Manual 3rd Edition by Dennis R. Moss Page 291 295)Karina RoquelОценок пока нет
- Brill Beggs ZДокумент3 страницыBrill Beggs ZFariz AdriansyahОценок пока нет
- Heat Exchanger Project FinalДокумент16 страницHeat Exchanger Project FinalChristopher CameronОценок пока нет
- Report PDFДокумент98 страницReport PDFYuri PaixãoОценок пока нет
- Perancangan CycloneДокумент20 страницPerancangan CycloneDavid LambertОценок пока нет
- Tugas 3 - Laily Fitri Pelawi - 1806154116Документ8 страницTugas 3 - Laily Fitri Pelawi - 1806154116LailyОценок пока нет
- Flash CalculationsДокумент10 страницFlash CalculationsHamza AliОценок пока нет
- Garbage IncineratorДокумент59 страницGarbage IncineratorgsdaundhОценок пока нет
- Group 1 Mass & Energy BalanceДокумент98 страницGroup 1 Mass & Energy BalanceDianah NajeebОценок пока нет
- Note of Use of The Linear Solver in AsterДокумент28 страницNote of Use of The Linear Solver in AsterTran TuyenОценок пока нет
- Feuille Calcul Gas Pipeline Blowdown TimeДокумент6 страницFeuille Calcul Gas Pipeline Blowdown TimeNic RicОценок пока нет
- Thermo AppendixДокумент118 страницThermo AppendixJody Leigh SheldonОценок пока нет
- Chapter 03 PDFДокумент7 страницChapter 03 PDFKyro100% (1)
- MCG 2131 Exam 08Документ6 страницMCG 2131 Exam 08子豪王Оценок пока нет
- GasesДокумент38 страницGaseshОценок пока нет
- Study of A Single Pass Shell and Tube Heat ExchangerДокумент21 страницаStudy of A Single Pass Shell and Tube Heat Exchangermahbub1332Оценок пока нет
- CourseNotes HeatExchangers2008Документ76 страницCourseNotes HeatExchangers2008Jorge Giovanny Vásquez CárdenasОценок пока нет
- Protectoseal Emergency Vent Application Worksheet: Service ConditionsДокумент1 страницаProtectoseal Emergency Vent Application Worksheet: Service Conditionsankur2061Оценок пока нет
- Natural Convection Heat Transfer From Vertical HelДокумент9 страницNatural Convection Heat Transfer From Vertical Helsachins1318Оценок пока нет
- Glycolysis PET WasteДокумент13 страницGlycolysis PET Wasteankur2061Оценок пока нет
- XLXДокумент14 страницXLXVương Đình NamОценок пока нет
- Energies: Physical and Chemical Properties of Waste From PET Bottles Washing As A Component of Solid FuelsДокумент17 страницEnergies: Physical and Chemical Properties of Waste From PET Bottles Washing As A Component of Solid FuelsCQ SHOОценок пока нет
- Recip RISK Rating Chart 2015Документ9 страницRecip RISK Rating Chart 2015romoexОценок пока нет
- Vibration Analysis in Reciprocating CompressorsДокумент11 страницVibration Analysis in Reciprocating Compressorsakamalapuri388Оценок пока нет
- Hyperfocal Distance CalculatorДокумент28 страницHyperfocal Distance Calculatorankur2061Оценок пока нет
- A Loop Thermosyphon For Asphalt Tank HeatingДокумент6 страницA Loop Thermosyphon For Asphalt Tank Heatingankur2061Оценок пока нет
- Tank Steam Heating Overall HTC Heavy FOДокумент2 страницыTank Steam Heating Overall HTC Heavy FOankur2061Оценок пока нет
- Instrument Process Datasheet ChecklistДокумент1 страницаInstrument Process Datasheet Checklistankur2061Оценок пока нет
- LECTURE 06 Equipment Sizing and Capital Cost EstimationДокумент10 страницLECTURE 06 Equipment Sizing and Capital Cost EstimationSomayeh SarabadanОценок пока нет
- Boiling Liquid Expanding Vapor Explosion (BLEVE) Fireball Diameter & DurationДокумент2 страницыBoiling Liquid Expanding Vapor Explosion (BLEVE) Fireball Diameter & Durationankur2061Оценок пока нет
- Centrifugal Compressor SealsДокумент4 страницыCentrifugal Compressor SealsHadi ShahsavanОценок пока нет
- General Guiidelines For Precommissioning and Commissioning A Chemical Process PlantДокумент2 страницыGeneral Guiidelines For Precommissioning and Commissioning A Chemical Process Plantankur2061100% (1)
- High Purity Oxygen ( 99.5%) Production Using Vacuum Pressure Swing Adsorption (VPSA)Документ2 страницыHigh Purity Oxygen ( 99.5%) Production Using Vacuum Pressure Swing Adsorption (VPSA)ankur2061Оценок пока нет
- Characterization of C6 Plus HCs in Natural GasДокумент6 страницCharacterization of C6 Plus HCs in Natural Gasankur2061Оценок пока нет
- Boiloff Gas Calcs For LNG TanksДокумент1 страницаBoiloff Gas Calcs For LNG Tanksankur2061Оценок пока нет
- P&ID Check ListДокумент3 страницыP&ID Check Listankur2061Оценок пока нет
- Equipment Process Data Sheet ChecklistДокумент1 страницаEquipment Process Data Sheet Checklistankur2061100% (1)
- General Guiidelines For Precommissioning and Commissioning A Chemical Process PlantДокумент2 страницыGeneral Guiidelines For Precommissioning and Commissioning A Chemical Process Plantankur2061100% (1)
- PFD Check ListДокумент2 страницыPFD Check Listankur2061Оценок пока нет
- PFD Check ListДокумент2 страницыPFD Check Listankur2061Оценок пока нет
- Fermenter Design: Mahesh BuleДокумент82 страницыFermenter Design: Mahesh BuleSagar DhuriОценок пока нет
- Burner Management System PresentationДокумент63 страницыBurner Management System Presentationankur2061100% (1)
- Design of Hoppers Using Spreadsheet: Journal of Agricultural Engineering Research January 2010Документ7 страницDesign of Hoppers Using Spreadsheet: Journal of Agricultural Engineering Research January 2010ankur2061Оценок пока нет
- Machine Reliability in Parallel Operations: Re (%) 100 PF Liability PДокумент4 страницыMachine Reliability in Parallel Operations: Re (%) 100 PF Liability Pankur2061Оценок пока нет
- Chapter2 ToxicologyДокумент4 страницыChapter2 ToxicologyS JОценок пока нет
- Basicflowmeasurement 150428100633 Conversion Gate02 PDFДокумент50 страницBasicflowmeasurement 150428100633 Conversion Gate02 PDFankur2061Оценок пока нет
- Pre-Admission Math Assessment - SampleДокумент13 страницPre-Admission Math Assessment - SamplePranav BISUMBHERОценок пока нет
- 9th Gravitation SolutionsДокумент104 страницы9th Gravitation SolutionsAjay D KumarОценок пока нет
- Wind Energy in MalaysiaДокумент17 страницWind Energy in MalaysiaJia Le ChowОценок пока нет
- Acid Base SeparationДокумент6 страницAcid Base SeparationAlexandra CatalinaОценок пока нет
- HW 3Документ3 страницыHW 3Siva RamОценок пока нет
- Is 600 MM Sufficient To Keep BDV FunctionalДокумент0 страницIs 600 MM Sufficient To Keep BDV Functionalsachin2010Оценок пока нет
- Musica Universalis First DraftДокумент78 страницMusica Universalis First DraftWilliam1091Оценок пока нет
- 5.random VariableДокумент28 страниц5.random VariableSadman SiamОценок пока нет
- CSE 2261 Structural Analysis II: Course OutlineДокумент38 страницCSE 2261 Structural Analysis II: Course OutlinezakheusОценок пока нет
- Study On Mechanical Properties of Concrete On Partial Replacement of Fine Aggregate With Copper Slag and Granite PowderДокумент4 страницыStudy On Mechanical Properties of Concrete On Partial Replacement of Fine Aggregate With Copper Slag and Granite PowderIJIRST100% (1)
- Dynamical Models of LoveДокумент11 страницDynamical Models of LoveTresor KalambayiОценок пока нет
- Automatic Railway Gate Control by Using Microcontroller - 24 PagesДокумент44 страницыAutomatic Railway Gate Control by Using Microcontroller - 24 PagesSebastin AshokОценок пока нет
- Apa PR l238 I Joist - LPДокумент14 страницApa PR l238 I Joist - LPRodrigo CandeoОценок пока нет
- Oelchecker Winter 2016 enДокумент5 страницOelchecker Winter 2016 enAzhar1109Оценок пока нет
- Example Design of A Cold-Formed Steel Lipped Channel Wall Stud in Compression PDFДокумент4 страницыExample Design of A Cold-Formed Steel Lipped Channel Wall Stud in Compression PDFOprisor Costin100% (1)
- CPRF Analysis PDFДокумент8 страницCPRF Analysis PDFMohd FirojОценок пока нет
- Directions Sense Quiz 12Документ16 страницDirections Sense Quiz 12Arijit GhoshОценок пока нет
- ACA 2010 Abstracts Manish Chandra PathakДокумент602 страницыACA 2010 Abstracts Manish Chandra Pathakpmcy2Оценок пока нет
- Soil CompactionДокумент24 страницыSoil Compactionsyah123Оценок пока нет
- Martini L4 TemperatureControlДокумент11 страницMartini L4 TemperatureControlJubaer JamiОценок пока нет
- A Field Method For Measurement of Infiltration PDFДокумент31 страницаA Field Method For Measurement of Infiltration PDFHamza MamiОценок пока нет
- Development of A Belt Conveyor For Small Scale Industry: September 2017Документ6 страницDevelopment of A Belt Conveyor For Small Scale Industry: September 2017DatОценок пока нет
- Instruction Manual System User's Guide Uv-1800 Shimadzu SpectrophotometerДокумент533 страницыInstruction Manual System User's Guide Uv-1800 Shimadzu SpectrophotometerAdrian Salazar100% (1)
- ENGINEERING - MATHEMATICS - 2 VTU Syllabus PDFДокумент167 страницENGINEERING - MATHEMATICS - 2 VTU Syllabus PDFAdarshОценок пока нет
- Experimental Physics PDFДокумент2 страницыExperimental Physics PDFJessicaОценок пока нет
- EP2CD4T1. Notes - Phys 1D Review With FlexPDE and MapleДокумент63 страницыEP2CD4T1. Notes - Phys 1D Review With FlexPDE and MapleJason wonwonОценок пока нет
- Manufacturing Processes II: Fundamentals of Metal FormingДокумент17 страницManufacturing Processes II: Fundamentals of Metal FormingMohamed Galal MekawyОценок пока нет
- AnimationДокумент9 страницAnimationAruna AruchamiОценок пока нет
- Ferro CementДокумент236 страницFerro Cementpbharadwaj545Оценок пока нет
- Optical Fiber CommunicationДокумент27 страницOptical Fiber CommunicationDrashti ShahОценок пока нет