Академический Документы
Профессиональный Документы
Культура Документы
Structural Characterization and Isomer Differentiation
Загружено:
Sonia SahnounИсходное описание:
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
Structural Characterization and Isomer Differentiation
Загружено:
Sonia SahnounАвторское право:
Доступные форматы
JOURNAL OF MASS SPECTROMETRY J. Mass Spectrom. 2003; 38: 555572 Published online 25 April 2003 in Wiley InterScience (www.interscience.wiley.com).
DOI: 10.1002/jms.472
Structural characterization and isomer differentiation of chalcones by electrospray ionization tandem mass spectrometry
Junmei Zhang and Jennifer S. Brodbelt
Department of Chemistry and Biochemistry, University of Texas, Austin, Texas 78712, USA
Received 15 November 2002; Accepted 9 February 2003
A series of chalcones were characterized by electrospray ionization tandem mass spectrometry (MSn ). Several ionization modes were evaluated, including protonation, deprotonation and metal complexation, with metal complexation being the most efcient. Collision-activated dissociation (CAD) was used to characterize the structures, and losses commonly observed include H2 , H2 O, CO and CO2 , in addition to methyl radicals for the methoxy-containing chalcones. CAD of the metal complexes, especially [CoII (chalcone H) 2,2 -bipyridine]+ , allowed the most effective differentiation of the isomeric chalcones with several diagnostic fragment ions appearing upon activation of the metal complexes. MSn experiments were performed to support identication of some fragment ions and to verify the proposed fragmentation pathways. In several cases, MSn indicated that specic neutral losses occurred by stepwise pathways, such as the neutral loss of 44 u as CH3 and HCO , or CH4 and CO, in addition to CO2 . Copyright 2003 John Wiley & Sons, Ltd.
KEYWORDS: chalcone structural characterization; isomer differentiation; electrospray ionization mass spectrometry; collision-activated dissociation; metal complexation
INTRODUCTION
Owing to their antioxidant abilities and emerging chemopreventive properties against heart disease, aging and cancer,1 3 avonoids have become the focus of increasing numbers of research studies. Flavonoids are a large group of phytochemicals that have the general structure of a 15-carbon skeleton, which consists of two phenyl rings connected by a three-carbon bridge.4,5 More than 4000 known avonoids are classied into subgroups, including avone, avanone, avonol, isoavonoid, anthocyanidin and chalcone. Chalcones, differing from all other avonoids by the absence of the C ring, are still considered a subclass of avonoids.6 Chalcones, along with retrochalcones and dihydrochalcones, in fact have an open structure and a carbon skeleton numbered in a way different from other avonoids (Fig. 1). Chalcones have shown good physicochemical and biological activities similar to other avonoids, including some antibacterial,7 antifungal,7 9 anti-inammatory,10 antimicrobial,11 antitumor,12,13 and anticancer14 properties. Although considered as minor avonoids, chalcones play a key role in avonoid biosynthesis5,15,16 because they are the
Correspondence to: Jennifer S. Brodbelt, Department of Chemistry and Biochemistry, University of Texas, Austin, Texas 78712, USA. E-mail: jbrodbelt@mail.utexas.edu Contract/grant sponsor: National Institutes of Health; Contract/grant number: NIH RO1 GM63512. Contract/grant sponsor: Welch Foundation; Contract/grant number: F-1155.
precursors of other avonoids. Chalcones are rst synthesized in plants by chalcone synthase (CHS) and then cyclized to other avonoids by chalcone isomerase (CHI). Since the chemopreventive properties of avonoids (including chalcones) depend on both the different functional groups and their relative positions, it is important to be able to characterize the structures and differentiate the isomers. Chalcones are commonly found in licorice (liquorice) and apple seeds.6 Native chalcone glycosides tend to transform to avanone glycosides during extraction. Acid hydrolysis, as is used prior to high-performance liquid chromatography (HPLC) in many routine analyses for dietary avonoids, converts the chalcones to the corresponding avanones. This isomerization has been evaluated in solution,17 19 and also by theoretical calculations.20 Although mass spectrometry has been involved in chalcone structural characterization since the early 1960s, most studies have focused on electron ionization21 29 with one report of chemical ionization,30 eld desorption31 and a few on fast atom bombardment studies.32,33 Special attention has been given to 20 -hydroxychalcones21,29 because of their different fragmentation patterns from other chalcones. The cyclization of chalcones to avanones also has been observed in the gas phase.21 23 It was noticed that the mass spectra of chalcones bearing a 20 -hydroxyl functional group were nearly identical with those of the isomeric avanones. Isomerization was rst proposed and later supported by experimental data.21 Based on experiments using the relatively energetic electron ionization method,
Copyright 2003 John Wiley & Sons, Ltd.
556
J. Zhang and J. S. Brodbelt
Chalcones
Flavanones 3 O
6 5 A 4 3 2 6 1 1
2 3 B 5 4 7
1 O 2
2 B
4 5 6
A 6 5
C 3 4 O
Chalcone 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20
Mw 224 240 240 240 240 240 254 254 256 268 270 270 270 284 284 284 284 288 298 298
-OH 2 4,2 2, 4 2, 2 3, 2 2, 5 2 2 4, 2,5 2 2, 6 2, 2 2, 4 2 2 2 2 3,4, 2, 4, 6 2
-OCH3
-CH3 H3CO O
OH 4 4 4 4 3 4 3, 4 3, 4 4, 4 2, 4 3, 4 4, 2,5 5
O FA (Mw 270)
OH OH HO O
OH 5
O FB (Mw 288)
Figure 1. Chalcone and avanone structures studied.
it was concluded that an intramolecular equilibrium exists between a chalcone type and a avanone type molecular ion. There have been no reports on the structural characterization of chalcones by electrospray ionization (ESI) or matrixassisted laser desorption/ionization (MALDI). In this work, we used ESI to form parent ions of interest and then obtain their fragmentation patterns upon collision-activated decomposition (CAD). Metal complexation, a method that has proven to be an effective ionization mode for acidic avonoids,34,35 is used as an alternative to differentiate some isomers.
chloride and cobalt(II), nickel(II), and copper(II) bromide were dissolved in methanol at a concentration of 5 10 3 M and stored at room temperature.
Mass spectrometric conditions
All the experiments were performed on a Thermo Finnigan LCQ Duo quadrupole ion trap mass spectrometer. The spray voltage was kept at 4.5 kV (). The temperature of the heated capillary was set at 200 C. Nitrogen was used as the sheath gas (20 arbitrary units). Auxiliary gas (nitrogen, 30 arbitrary units) was added as needed. The injection time was set at 10 ms except for the tandem mass spectrometric (MSn ) experiments (50100 ms). Each chalcone working solution was delivered to the ESI source at 5 l min 1 . The other parameters, including capillary voltage, octapole offsets and tube lens offset, were optimized for maximum abundance of the ions of interest. In solutions of chalcones without metal addition, the ions optimized were [L H] (deprotonation mode) and [L C H]C or [L C Na]C (positive ion mode). When lithium chloride was added, the ions of interest were [L C Li]C . Upon addition of the auxiliary ligand 2,20 -bipyridine (bipy) and a transition metal, the [MII (L H) bipy]C complex was optimized. The product ion spectra of the ions of interest were obtained by CAD, using helium as the collision gas. The collision energy was varied such that the
EXPERIMENTAL Chemicals and reagents
All the chalcones and avanones listed in Fig. 1 were purchased from Indone (Somerville, NJ). 2,20 -Bipyridine, lithium chloride and cobalt(II), nickel(II) and copper(II) bromide were purchased from Aldrich (Milwaukee, WI, USA). HPLC-grade methanol was purchased from Fisher (Fair Lawn, NJ, USA). All materials were used without further purication. Each chalcone or avanone stock solution was prepared in methanol at a concentration of 1 10 3 M and kept protected from light at 4 C until use. 2,20 -Bipyridine, lithium
Copyright 2003 John Wiley & Sons, Ltd.
J. Mass Spectrom. 2003; 38: 555572
ESI-MS/MS structural characterization of chalcones
557
relative intensity of the surviving parent ions was 510%. For the MSn experiments, the fragment ions of interest from MS/MS were subject to further isolation and CAD, so that only 510% of the selected precursors survived the second stage of activation. The working solutions of chalcones (1 10 5 M) were made in methanol from their stock solutions with/without metal addition. When a metal was added, the nal concentration ratios were 1 : 10 chalcone : lithium or 1 : 1 : 1 chalcone : transition metal : 2,20 -bipyridine.
protonated or alkali metal cationized chalcones was not pursued further.
Deprotonation
Chalcones other than 20 were easily deprotonated due to their acidic nature (Fig. 2(A)). The CAD data of the resulting [L H] ions are summarized in Table 1 for chalcones without methoxy groups and in Table 2 for chalcones with methoxy groups, and typical CAD spectra are shown in Figs 35 for three sets of isomers. To help rationalize the proposed fragmentation mechanisms, MSn experiments were performed on all the deprotonated chalcones (Table 3).
RESULTS AND DISCUSSION
Four ionization modes were evaluated: protonation, deprotonation, lithium cationization and transition metal complexation. CAD was then performed to characterize the resulting ions and assess the ability to differentiate isomers, and MSn was used to assist in elucidating the fragmentation pathways.
A-vs B-type fragments
As shown in Tables 1 and 2 and Figs 35, many of the fragment ions can be classied as A or B type, meaning that these products retain the A or B ring portion of the chalcone while the second ring is lost. The loss of B ring (which results in the A type of ions) has been observed earlier for some chalcones by electron ionization.21,29 Both A- and B-type fragments have also been reported for other subclasses of avonoids by ESI.36 Examples of these types of fragment ions are illustrated in Scheme 1. In Scheme 1, the site of deprotonation is shown to accommodate the product ion structures. The resulting fragment ions may isomerize or intraconvert to other resonance structures, and thus the structures shown in Scheme 1 are only given to help guide the reader in matching up structures that reasonably t the masses of the fragments listed in the tables and gures. MSn provided support for some of the A vs B fragment assignments (Table 3). For example, MS/MS/MS indicated that the product ion m/z 119 of 2 was an A ring fragment even though the A and B rings have the same isomeric composition (the A ring fragment would be C8 H7 O and the B ring fragment would be C7 H3 O2 ). The assignment of the fragment ion as an A ring fragment was veried by
Protonation and lithium cationization
Most of the chalcones did not form detectable ions in the positive ESI mode. Only chalcone 20, which does not have any acidic hydrogens, formed sodium adducts [L C Na]C (m/z 321) and not protonated species in the positive mode (even with addition of acetic acid), or lithium adducts [L C Li]C (m/z 305) with addition of lithium chloride (spectra not shown). The CAD spectra of these alkali metal adducts were relatively simple. The major fragment (m/z 311) of [20 C Na]C (m/z 321) was the loss of CO with subsequent addition of H2 O, whereas [20 C Li]C mainly underwent a methyl radical loss resulting in a fragment at m/z 290. All the other chalcones have at least one hydroxyl group, which makes them slightly acidic. They did not form [L C H]C (even with acid addition), [L C Na]C or [L C Li]C ions that were abundant enough to be isolated. Therefore, CAD of
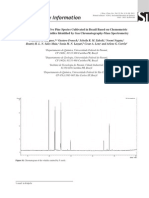
A) 100 Deprotonation 7.7 105
283 [L - H]-
Relative Abundance
0 B) Cobalt complexation 100 1.0 107
498 [CoII(L - H) bipy]+
0 140 180 220 260 300 340 380 420 460 500 540
m/z
Figure 2. ESI spectra of chalcone 16 in both deprotonation and cobalt complexation modes with the relative intensities shown.
Copyright 2003 John Wiley & Sons, Ltd.
J. Mass Spectrom. 2003; 38: 555572
558
J. Zhang and J. S. Brodbelt
Table 1. CAD of deprotonated chalcones without methoxy groups (m/z, %), where A and B denote fragments retaining the A or B ring system 1 Pa P P P P P P A B 223 (6) 221 (41) 205 (18) 195 (100) 193 (3) 2 239 (7) 3 239 (11) 4 239 (8) 221 (1) 211 (4) 197 (100) 195 (15) 91 (4) 135 (44) 109 (3) 148 (21) 5 239 (6) 221 (100) 6 239 (12) 237 (93) 221 (60) 211 (100) 197 (17) 195 (74)b 9 255 (7) 18 287 (5) 269 (4)
H2 H2 O CO H2 CO C2 H2 O 44
195 (55) 119 (100) 93 (3) 145 (2)
195 (22) 93 (100)d 119 (50) 93 (100)d 145 (9) 133 (4)
211 (7) 119 (11) 135 (100) 125 (3) 107 (10) 151 (100)
161 (95) 135 (27) 224 (25) 131 (10)
Othersc
a
P D L H , surviving parent ions. MSn experiments indicate that the loss of 44 u may also be due to the loss of H2 and C2 H2 O. For the other chalcones, there is no evidence for the loss of 44 u being anything other than the direct loss of CO2 . c Only fragment ions with more than 10% relative abundance are listed. d m/z 93 can be either A- or B-type ions owing to the same structure of A and B rings.
b
Table 2. CAD of deprotonated chalcones with methoxy groups (m/z, %), where A and B denote fragments retaining the A or B ring system 7 Pa P P P P P P P P P P P P P P P A 253 (7) 2CH4 C H2 O CH3 b 16 H2 O CO 2CH3 2CH4 H2 O CH3 C2 H2 O CO CH3 44 H2 O CO CO2 CH3 2CH4 CO 2H2 O CO 8 253 (8) 10 267 (8) 11 269 (6) 12 269 (5) 13 269 (7) 14 283 (8) 15 283 (7) 16 283 (8) 17 19 20
238 (100) 238 (100) 252 (100) 254 (100) 254 (15) 237 (15) 237 (10) 251 (7) 251 (73) 225 (9) 225 (3) 239 (4) 237 (3) 236 (16)
283 (13) 297 (10) X 269 (40) 254 (100) 268 (100) 268 (100) 268 (100) 268 (90) 282 (100) 267 (12) 267 (14) 265 (2) 255 (44) 253 (88) 253 (5) 253 (84) 253 (100) 267 (92) 251 (78)
227 (23) 210 (1) 209 (7)c 224 (4) 226 (13) 225 (11)c 225 (7) 223 (5) 210 (8) 240 (5) 225 (2) 254 (2)
B Othersd
a b
191 (5) 165 (23)
175 (9) 149 (100) 123 (19) 93 (33) 148 (30)
223 (30) 219 (17) 145 (26)
175 (4) 123 (5) 236 (10)
P D L H , surviving parent ions. MSn experiments indicate that the loss of 16 u for 7, 8, 10, 14 and 16 may be due to two pathways: the loss of CH4 and the loss of CH3 and H . c n MS experiments indicate that the loss of 44 u for 7 may be due to three pathways: the loss of CO2 , the loss of CH3 and HCO , and n the loss of CH4 and CO. MS experiments indicate that the loss of 44 u for 11 may be due to two pathways: the loss of CO2 and the loss of CH3 and HCO . For the other chalcones, there is no evidence for the loss of 44 u being anything other than the direct loss of CO2 . d Only fragment ions with more than 10% relative abundance are listed.
Copyright 2003 John Wiley & Sons, Ltd.
J. Mass Spectrom. 2003; 38: 555572
ESI-MS/MS structural characterization of chalcones
559
A) Chalcone 2 100
A 119 (C8H7O)
195 (-CO2)
0 B) Chalcone 3 100 A 91 B 109 B 135 (-C8H8) 148 153 195 197 (-C2H2O)
239
239
Relative Abundance
C) Chalcone 4 100 93 (C6H5O) A 119 (C8H7O)
B 145 (-C6H6O)
195 (-CO2)
0 D) Chalcone 5 100 221 (-H2O)
239
0 E) Chalcone 6 100 B 161 (-C6H6) B 135 (-C8H8) 0 70 100 130 160 m/z 190 220 211 (-CO) 195 (-CO2) 197
239
221 (-H2O) 237 (-H2) ,239 250
Figure 3. CAD spectra of deprotonated chalcone isomers 2, 3, 4, 5 and 6. Parent ions [L B denote fragments retaining the A or B ring system.
A) Chalcone 11 100 B 191 (-C6H6) 236 (-CH H O) 3 2 B 225/226 165 (-C8H8) 0 B) Chalcone 12 100 251 (-H2O)
H] are labeled with asterisks. A and
254 (-CH3)
269
Relative Abundance
A 149 (C9H9O2) 210 (-CH3- CO2) 225 (-CO2) A 175 (-C6H6O) 254 (-CH3)
B 93 (C6H5O) A 123 (C7H7O2) 0 C) Chalcone 13 100
269
254 (-CH3)
B 148 (C8H4O3) 0 80 120 160 200
227 (-C2H2O)
269
240 280
m/z
Figure 4. CAD spectra of deprotonated chalcone isomers 11, 12 and 13. Parent ions [L denote fragments retaining the A or B ring system.
H] are labeled with asterisks. A and B
Copyright 2003 John Wiley & Sons, Ltd.
J. Mass Spectrom. 2003; 38: 555572
560
J. Zhang and J. S. Brodbelt
A) Chalcone 14 100
253 (-2CH3)
268 (-CH3)
0 B) Chalcone 15 100
283
268 (-CH3)
Relative Abundance
0 C) Chalcone 16 100
253 (-2CH3)
283
253 (-2CH3)
268 (-CH3)
0 D) Chalcone 17 100 145 0 80 100 120 140
283 255(-CO) 253 (-2CH3) 251(-2CH4) 223 (-2CH4-CO) 219 (-CO2H2O) 160 180 200 220 240 260 268 (-CH3) 269 (-2CH4 + H2O) 283 280 300
m/z
Figure 5. CAD spectra of deprotonated chalcone isomers 14, 15, 16 and 17. Parent ions [L Table 3. MSn data of deprotonated chalcone ions (L H (m/z) Fragment 193 165 145 193 167 119 93 91 169 141 193 169 167 120 91 117 91 65 211 193 165 209 195 193 211 Neutral loss 28 56 76 2 28 76 26 28 28 56 2 26 28 28 44 2 28 28 28, CH2 Ob 28 56 28 42 44 28, CH2 Ob Table 3. (Continued) Chalcone %a 100 13 14 100 100 64 100 16 100 25 75 100 37 100 100 100 18 100 32 100 11 98 39 100 13 10 239 ! 211 ! Route Fragment 193 183 169 167 157 143 133 117 237 209 209 193 210 237 210 209 193 210 183 125 107 93 251 237 224 Neutral loss 28 28 42 44 54 68 28 44 1 29 28 44 15 1 28 28 44 15 28 28, CH2 Ob 28 26 1 15 28 %a 100 100 31 60 14 17 31 100 100 20 22 100 100 100 21 100 20 100 100 30 100 100 100 22 37 H] are labeled with asterisks.
Chalcone 1
Route 223 ! 221 !
223 ! 195 ! 2 239 ! 195 ! 239 ! 119 ! 3 239 ! 197 ! 239 ! 195 !
239 ! 161 ! 7 253 ! 238 ! 253 ! 237 ! 253 ! 225 ! 8 253 ! 238 ! 253 ! 237 ! 253 ! 225 ! 9 255 ! 211 ! 255 ! 135 ! 255 ! 119 ! 267 ! 252 !
239 ! 148 ! 239 ! 135 ! 4 239 ! 119 ! 239 ! 93 ! 5 239 ! 221 !
239 ! 237 !
239 ! 221 !
Copyright 2003 John Wiley & Sons, Ltd.
J. Mass Spectrom. 2003; 38: 555572
ESI-MS/MS structural characterization of chalcones
561
Table 3. (Continued) Fragment 253 226 225 177 163 236 253 236 134 108 148 212 120 267 253 225 209 253 267 253 235 225 211 209 185 253 240 238 209 236 223 208 117 107 267 252 239 223 Neutral loss 1 28 29 77 91 15 1 18 15 15 106 15 28 1 15 28 44 15 1 15 18 28 42 44 68 15 15 15 44 15 28 15 28 44 15 15 28 44
Chalcone 11
Route 269 ! 254 !
%a 25 100 86 18 18 100 100 60 100 100 100 100 100 3 100 100 56 100 2 100 16 100 43 81 18 100 100 56 100 17 100 100 100 100 100 25 100 51
Fragment ions at m/z 149 and 123 in the CAD spectrum of deprotonated 12 were assigned as A ring fragments because of the exclusive loss of 15 u observed in the MS/MS/MS of m/z 123 and 149. The sole methoxy group of 12 is located on the A ring.
Small neutral losses
In addition to the cleavages due to A or B ring losses (see Fig. 1 for the location of the A and B rings), small neutral losses of H2 O, CO and CO2 were commonly observed and sometimes dominant for chalcones with only hydroxyl groups (Table 1). In addition to the expected neutral losses of CO and CO2 and the formation of A and B ring fragments, there was an unusual loss of H2 for 1 and 6, as well as a prominent loss of H2 O for 5, 6 and 11. The pathways for these losses of H2 or H2 O are not readily explained, nor is it evident why these unusual losses are observed only for certain chalcones. Another small neutral loss of C2 H2 O (42 u) was observed in the CAD spectra of deprotonated chalcones 3, 6 and 13, and was especially prominent for 3. Each of these three chalcones has two hydroxyl groups on the B ring, either in the meta- or para-position. The same loss has also been observed for some other deprotonated avonoids.36 Extensive isotopic labeling is impractical owing to the high cost of the chalcones (up to $200 per mg) and the synthetic difculty of selective deuterium, 18 O or 13 C labeling, in addition to the possibility of solution or gasphase isomerization of the chalcones, as shown in Scheme 2 for one simple deprotonated chalcone. Neutral losses such as CO, CO2 , and C2 H2 O also have been observed for other deprotonated avonoids,36 such as for avonols, avones and avanones. In fact, the loss of CO2 from deprotonated avones has been proposed to involve a carbonyl group and an oxygen of the C ring in a rather unusual rearrangement reaction.36 For chalcones with both hydroxyl and methoxy groups, the most dominant losses were methyl radicals (and/or
A) MS2 100 253 (-2CH3)
269 ! 251 ! 12 269 ! 254 ! 269 ! 149 ! 269 ! 123 ! 13 269 ! 254 ! 269 ! 227 ! 269 ! 148 ! 283 ! 268 ! 283 ! 253 ! 15 16 283 ! 268 ! 283 ! 268 ! 283 ! 253 !
14
17
283 ! 268 ! 283 ! 255 ! 283 ! 253 ! 283 ! 251 ! 283 ! 223 ! 283 ! 145 !
268 (-CH3)
18 19
287 ! 151 ! 297 ! 282 ! 297 ! 267 !
283 0
Relative Abundance
100
B) MS3
253 (-CH3)
Only fragments with more than 10% relative abundance are listed, except for 14 and 16. b The observed loss of 10 u is due to loss of CO followed by addition of H2 O.
268 0 C) MS4 100 225 (-CO) 209 (-CO2) 253 0 100 120 140 160 180 200 220 240 260 280 300
the loss of 26 u upon CAD of m/z 119, and this loss of 26 u (HC CH) must involve the carbons adjacent to the A ring. The assignments of m/z 135 of 3 and 161 of 6 as B ring fragments were supported by the subsequent loss of CO2 in the MS/MS/MS of 135 and 161 , respectively, which would not be possible for A ring species owing to the lack of oxygen atoms on the A ring.
m/z
Figure 6. of deprotonated chalcone 14. Parent ions are labeled with asterisks. MSn
Copyright 2003 John Wiley & Sons, Ltd.
J. Mass Spectrom. 2003; 38: 555572
562
J. Zhang and J. S. Brodbelt
methane for 7, 8, 10, 14 and 16) in addition to other small neutral losses, such as CO (Table 2). The consecutive loss of methyl radicals during CAD was conrmed by MSn experiments (Fig. 6 and Table 3), which showed sequential loss of 15 u. Even though it is unusual to have radical losses from even-electron molecular ions, this was observed not only for deprotonated chalcones but also previously for other avonoids.36 39 As shown in Fig. 6 and Table 3, MS/MS/MS experiments proved to be useful in conrming some of the suspected fragmentation pathways, including support for sequential losses. For example, for the deprotonated chalcones without methoxy groups, the loss of 30 u in MS/MS/MS was (H2 C CO). This could be seen by examining the sequence: parent ion P ! P 2 ! P 30 or P ! P 28 ! P 30 , where P represents the deprotonated precursor chalcone. For the deprotonated chalcones with two methoxy groups (14, 15, 16, 17 and 19), the loss of 30 u was in part due to sequential loss of CH3 based on the consecutive loss of 15 u seen in MS/MS/MS (Fig. 6). Note that this MS/MS/MS sequence does not rule out other pathways for the loss of
30 u, such as the loss of CO with H2 . Even though 10 has only one methoxy group and one methyl group, the loss of 30 u seen for deprotonated 10 was likewise conrmed to be at least in part due to loss of two methyl radicals in the MS/MS/MS experiments. MS/MS/MS experiments also showed that the loss of 44 u from deprotonated 7 is actually in large part due to consecutive losses of CH3 and HCO or consecutive losses of CH4 and CO (Table 3). The consecutive losses of CH4 and CO are also observed in MS/MS/MS experiments for deprotonated 8 even though this [P 44] fragment is not observed upon CAD of this deprotonated chalcone (Table 2). Similarly, the loss of 44 u from deprotonated 11 was found to be due in part to consecutive losses of CH3 and HCO . Furthermore, for deprotonated 6, one route for the loss of 44 was proven to be consecutive losses of H2 and C2 H2 O, as conrmed by MS/MS/MS experiments (Table 3). For the other deprotonated chalcones that showed a loss of 44 u in their CAD spectra, these unusual consecutive losses (i.e. CH3 and HCO , CH4 and CO, H2 and C2 H2 O) were not indicated based on MS/MS/MS experiments, hence the
O-
O A 1
OB
O-
93- (B) O OH
145- (B)
-O
-O
2 O O-
119- (A) OCH2
-
OC O 135- (B) O O-
OH 3 O OH
91- (A) O-
OH 109- (B)
O4 93- (Aor B)
O119- (A) O O145- (B)
OH
O C
O6
O135- (B) O C
OH 161- (B) O C
O-
HO 9 OH
O107- (B)
-O
119- (A)
O135- (B)
Scheme 1. Structures of the A- and B-type fragment ions of some deprotonated chalcones.
Copyright 2003 John Wiley & Sons, Ltd.
J. Mass Spectrom. 2003; 38: 555572
ESI-MS/MS structural characterization of chalcones
563
OH
O C
-O
O-
-O
OCH3
OCH3 165- (B)
11 O OH O-
HO 191- (B)
OCH3
C OOOCH3 12 O O-O
OOCH3 149- (A)
OOCH3 175- (A)
OCH3 93- (B) 123- (A) O
H3CO 13 O O-
OH
.
148- (B) O-
O C C
O-
OCH3 17 O
OCH3 123 OO
OCH3
- (B)
O145
- (A)
O 175- (B)
OCH3
OC O
HO OH
HO 18
OH
HO 151- (B)
Scheme 1. (Continued).
4' 6 5 4 3 I 2 6' 5' O O3' 4' 5 4 3 II 2 6 3' O 5' 6' O
4' 3' 6 5 4 3 III 2 O 5' 6' O-
Scheme 2. Proposed resonant structures of deprotonated chalcones. Note that avanones have a different numbering system other than the chalcones as shown in Fig. 1. However, the same numbering system according to the original chalcone is used for the whole scheme to emphasize the positions involved in the ring closure.
most likely pathway is simple loss of CO2 , which has been conrmed for other avonoids.
Deprotonated 7, 8, 10, 14 and 16 all dissociate by the loss of CH3 and the loss of CH4 upon collisional activation. MS/MS/MS experiments conrm that the loss of CH4 is in part due to consecutive losses of CH3 and H . MS/MS/MS experiments show that sequential losses of CH3 and H may also occur for chalcone 11 even though the fragment [P 16] is not directly observed in the CAD spectrum of this deprotonated chalcone (Table 2). As discussed above for the MSn experiments, some of the neutral losses observed upon CAD of the deprotonated chalcones may actually involve several pathways. For example, MSn experiments indicate that the loss of 44 u may be loss of CO2 , loss of H2 with C2 H2 O, loss of CH3 with HCO , and loss of CH4 with CO, depending on the identity of the chalcone. Likewise, in some cases the loss of 16 u is due to consecutive loss of H and CH3 , not exclusively the loss of CH4 . These alternative pathways are shown in the footnotes of Tables 1 and 2. In general, the MS/MS/MS data indicated that some of the fragment ions observed in the CAD spectra of the deprotonated chalcones stemmed from consecutive losses of small neutrals, thus adding a layer of complexity to the rational interpretation of the fragmentation patterns of chalcones. In addition, MSn experiments proved to be useful in verifying some of the A versus B ring assignments. Scheme 2 indicates the possible isomerization pathway of chalcones to avanones in order to explain some of the A and B type fragments in Scheme 1, which is in
Copyright 2003 John Wiley & Sons, Ltd.
J. Mass Spectrom. 2003; 38: 555572
564
J. Zhang and J. S. Brodbelt
accordance with earlier studies.21 23 To verify further the existence of avanone isomeric forms of the chalcones, two avanones isomeric to chalcones 11 and 18 were chosen for ESI-MS and CAD. 5-Hydroxyl-7-methoxy avanone (FA, Fig. 1) is an isomer of chalcone 11. The fragments upon CAD of the deprotonated FA were the same as that of deprotonated chalcone 11 (Table 2) but the abundances were very different, with m/z 191 (100%) becoming the base fragment ion followed by m/z 165 (84%). This could be explained by the much easier loss of the B ring in the avanone. The other major fragments were P CH3 (42%), P H2 O (21%) and P CO (14%). The second avanone tested was 5,7,30 ,40 -tetrahydroxyavanone (eriodictyol, FB, Fig. 1), an isomer of chalcone 18. The CAD spectra of the deprotonated ions of these two compounds were identical (Table 1). The similarity of the CAD patterns of the above two isomer pairs is further strong evidence for possible isomerization between the chalcone and avanone forms.
of CO2 , 94 u (loss of C6 H6 O, the B ring), 120 u (loss of C7 H2 O4 , another B ring loss) and 146 u (loss of C9 H6 O2 , another B ring loss) upon collisional activation, which clearly simplies the interpretation of the fragmentation patterns of these related chalcones. In short, the chalcones do not follow consistent structure-based fragmentation rules, thus suggesting that creation of mass spectral libraries will be critical for identifying chalcones in mixtures.
Isomer differentiation
Five chalcone isomer series were included in this study: chalcones 2, 3, 4, 5 and 6 (Series 1, each containing two hydroxyl groups); chalcones 7 and 8 (Series 2, each containing one hydroxyl group and one methoxy group); chalcones 11, 12 and 13 (Series 3, each containing two hydroxyl groups and one methoxy group); chalcones 14, 15, 16 and 17 (Series 4, each containing one hydroxyl group and two methoxy groups), and chalcones 19 and 20 (Series 5, containing a combination of hydroxyl, methoxy and methyl groups). Chalcones in each series differ only by positions of the same functional groups except in Series 5, the latter compounds having the same chemical formulas but different combinations of functional groups. It was our goal to differentiate these isomers by ESI-tandem mass spectrometry. CAD of the deprotonated chalcones was sufcient to differentiate the chalcones in Series 1 and 3, which have more than one hydroxyl group (Figs 3 and 4). Although some common fragments are observed for each isomer series, the individual fragmentation patterns are unique. For example, in Series 1, chalcone 3 gives unique A- and B-type fragment ions at m/z 91, 109 and 135 (with structures proposed in Scheme 1). Chalcone 4 produces a distinctive A and B fragment ions at m/z 93 and 145 and chalcone 6 gives a very rich, diagnostic CAD pattern with two B-type ions (see Scheme 1). Chalcones 2 and 5, which have only one or two fragment ions, can nonetheless be distinguished from the others owing to their lack of key ions present in the CAD spectra of the other three chalcones. For Series 3, deprotonated 11 and 12 each produce an array of diagnostic ions upon CAD, including several A- and B-type ions shown in Scheme 1, whereas deprotonated 13 is the only one of this series that dissociates by loss of C2 H2 O, in addition to a unique B-type ion. CAD spectra of the deprotonated chalcones in Series 2 and 4 did not provide adequate differentiation (Table 2 and Fig. 5). The deprotonated chalcones 7 and 8 predominantly dissociate by loss of CH3 , and the unique loss of 44 u that is observed only for deprotonated 7 is a minor pathway. For the Series 4 chalcones, a comparison of CAD patterns of isomeric chalcones 14, 15, 16 and 17 showed that 15 only showed a minor loss of 2CH3 , while 14, 16 and 17 showed signicant losses of 2CH3 (Table 2 and Fig. 5). However, even more striking was the variety of fragments observed for deprotonated 17, which not only had neutral losses such as CH3 , CH4 , CO, 2CH3 , and 2CH4 , but also gave one diagnostic fragment ion at lower mass range (i.e. m/z 145), an A ring species (Table 2 and Fig. 5). Chalcones 16 and 17 differ only in the position of one methoxy group, and the
Fragmentation patterns vs structural features
It is interesting that the fragmentation patterns of the various chalcones may change dramatically upon rather modest changes of substituents. For examples, substitution of a methoxy group for a hydroxyl group, such as the case for chalcones 3 versus 7, signicantly changes the observed CAD patterns. Deprotonated 3 produces diagnostic A and B ions upon CAD, in addition to the characteristic loss of C2 H2 O, whereas deprotonated 7 dissociates predominantly by loss of CH3 , CH4 , or CO, and forms no detectable A or B ions. The different CAD patterns of 3 and 7 might be at least partially due to the different numbers of possible isomeric forms: 3 has two different hydroxyl groups that can be deprotonated upon ESI which results in two possible isomeric forms, whereas 7 only has one hydroxyl group available for deprotonation. A similar lack of parallel fragmentation patterns occurs for the pair 13 and 16. These chalcones each have three substituents in the same three positions, except that one has two hydroxyl groups and one methoxy group whereas the other has two methoxy groups and one hydroxyl group. Deprotonated 13 dissociates via three pathways, including loss of CH3 , loss of C2 H2 O and formation of one diagnostic B ion. Deprotonated 16 only undergoes loss of one or two CH3 units and gives no A or B fragment ions. The addition of an extra substituent can also promote changes in the fragmentation patterns, as illustrated by the comparison of deprotonated 3 and 13 (the latter having a methoxy group at the 4-position). The CAD spectrum of deprotonated 13 lacks the detail of that observed for deprotonated 3. In fact, the methoxy group in 13 is key for the dominant fragmentation pathway (loss of CH3 ), and the observed B fragment is not an analog of any of those observed for deprotonated 3. The comparisons summarized above are examples of how the interpretation of the fragmentation patterns of related chalcones is not always straightforward. However, there are cases in which the fragmentation patterns of complementary pairs show logical similarities. For example, chalcones 4 and 12 both have two hydroxyl substituents at the 20 - and 2-positions, and 12 also has one methoxy group at the 3-position. Each of these undergoes the loss
Copyright 2003 John Wiley & Sons, Ltd.
J. Mass Spectrom. 2003; 38: 555572
ESI-MS/MS structural characterization of chalcones
565
greater array of dissociation pathways for 17 is not readily explained based on this minor structural difference of the isomers. Deprotonated 17 gives a rich CAD spectrum that is diagnostic, but the other three isomers are not differentiated with condence owing to the dominant or exclusive methyl radical losses upon CAD. Therefore, metal complexation was examined as an alternative ionization method in an effort to produce complexes that would give distinctive fragmentation patterns. Differentiation of the two chalcones in Series 5 was an easy case because these two chalcones differed signicantly in their ionization efciencies in both the protonation and deprotonation modes. Chalcone 20 does not deprotonate and 19 does not protonate.
7 dissociated predominantly by loss of a methyl radical, with minor losses of methane, CO or 44 u. In contrast, the metal complex [CoII (7 H) bipy]C produced several diagnostic fragment ions with possible structures proposed in Scheme 3, in addition to characteristic losses of CH3 and CH3 with one or two CO units. An example of the comparative CAD spectra for the complexes of chalcone 14 containing different metals (CoII , NiII , CuII ) is shown in Fig. 8. Both the cobalt and nickel complexes, presumably owing to the similar metal binding strength of the deprotonated chalcone and the auxiliary ligand and the similar coordination structures, dissociated to
468 (-2CH3) 465 (-CH3 - H2O) 482 (-CH4) 454 (-CO2) II + 308 [Co (C6H5O) bipy] 307 498
A) Cobalt complexation 100
Metal complexation
Since avonoids in general deprotonate easily and have a tendency to coordinate with metals, metal complexation has been used as an alternative for structural characterization.34,35 2,20 -Bipyridine, which is neutral and has a similar metal binding strength to that of the deprotonated avonoids, is used as an auxiliary ligand to promote stable complexes and prevent the formation of neutral dimeric species between the metal and two deprotonated avonoids.34,35 The addition of a transition metal (CoII , NiII or CuII ) and 2,20 -bipyridine to the chalcone solutions produced [MII (L H) bipy]C complexes. The signal intensities of the metal complexes were similar to or higher than that of the corresponding deprotonated chalcones (Fig. 2). CAD was then performed on the metal complexes. In many cases, the metal complexes give much richer fragmentation patterns than the corresponding deprotonated chalcones. An example is shown in Fig. 7 for chalcone 7. Upon collisional activation, deprotonated
232/233 247 216
Relative Abundance
100
B) Nickel complexation 231/232 307 [NiII (C H O) bipy]+ 6 5 246 369 214
495 (-H2) 497
0 C) Copper complexation 237 219 251 0 100 150 200 250 300 350 400 450
100
502 500 550
m/z
Figure 8. CAD mass spectra of [MII (14 H) bipy]C , where MII D Co2C , Ni2C or Cu2C . Parent ions are labeled with asterisks.
A) Chalcone 14 232/233 247 216 0 468 (-2CH3) 465 (-CH3 H2O) 482 (-CH4) 454 (-CO2) 308 [CoII (C6H5O) bipy]+ 498 307
A) (L H)100
238 (-CH3) 100
237 (-16) 225 (-CO) 209 (-44) B) Chalcone 15 253 250 270 100 468 (-2CH3) 466 (-2CH4) 352 0 C) Chalcone 16 100 216 0 D) Chalcone 17 100
468
483 (-CH3) 498
Relative Abundance
0 70 90 110 130 150 170 190 210 230
m/z
B) [CoII (L H) bipy]+ 100
228 216 233 247
Relative Abundance
468 (-2CH3) 455 (-CH3 - CO) 338 427
483 (-CH3) 498
425 (-CH3 CO)
453 (-CH3)
397 (-CH3 - 2CO) 338 [CoII (C7H7O2) bipy]+
292 [CoII (C6H5) bipy]+
362
232/233 247
157
348 180 220 260 300 340 380 420
232/233 247 216 0 140 180 220 260 300
337
483 (-CH3) 498
0 140 460 500
340
380
420
460
500
540
m/z
m/z
Figure 7. CAD spectra of chalcone 7 in both deprotonation and cobalt complexation modes. Parent ions are labeled with asterisks. The losses of 16 and 44 u are explained in Table 2.
Figure 9. CAD spectra of chalcone 14, 15, 16 and 17 complexes with cobalt and 2,20 -bipyridine. Parent ions [CoII (L H) bipy]C are labeled with asterisks.
Copyright 2003 John Wiley & Sons, Ltd.
J. Mass Spectrom. 2003; 38: 555572
566
J. Zhang and J. S. Brodbelt
N Co2+ O
N Co2+ O-
N Co2+ -
N Co -
N
2+
O-
OCH3 7 338+
OCH3 318+ 292+
N Co2+ O
N N Co O
2+
N Co2+ CH2O N Co2+ ON
O-
O-
H3CO 8 360+ 322+ 308+
Scheme 3. Proposed structures of the unique fragment ions of the cobalt complexes of the chalcones in Series 2. Table 4. CAD of cobalt complexes of chalcones without methoxy groups: [CoII (L fragments retaining the A or B ring system 1 Pa P P P P P P P A 438 (9) 436 (10) 420 (2) 410 (10) 408 (6) 2 454 (10) 452 (18) 436 (18) 426 (9) 3 454 (10) 452 (8) 436 (20) 426 (6) 4 454 (5) 436 (100) H) bipy]C (m/z, %), where A and B denote
5 454 (10) 452 (1) 436 (100)
6 454 (8) 452 (11) 436 (51) 426 (16) 424 (2) 410 (2)
9 470 (9) 468 (13) 452 (61) 442 (3) 440 (2) 424 (6) 308 (9)
18 502 (7) 500 (28) 484 (36)
H2 H2 O CO H2 CO CO2 H2 O CO 2CO
408 (19) 382 (13) 318 (16) 292 (26) 308 (62) 248 (24) 247 (32) 246 (10) 233 (38) 232 (47) 228 (14) 216 (100) 157 (12) 332 (24) 334 (15) 308 (91) 248 (26) 247 (43) 246 (10) 233 (52) 232 (37) 228 (17) 216 (100) 157 (15) 332 (27) 309 (14)
408 (8) 292 (23) 324 (31) 248 (22) 247 (33) 246 (10) 233 (40) 232 (40) 228 (50) 216 (100) 157 (12) 348 (29) 334 (12) 334 (34) 308 (36)
408 (14)
B Common ionsb
248 (1) 247 (1) 246 (13) 232 (77) 233 (2) 232 (2) 216 (5) 157 (1)
Othersc
324 (28) 248 (9) 247 (24) 246 (8) 233 (33) 232 (30) 228 (9) 216 (14) 157 (5) 437 (100) 370 (10)
324 (29) 248 (12) 247 (25) 246 (4) 233 (29) 232 (20) 228 (4) 216 (55) 157 (6)
376 (35) 247 (2) 246 (2) 233 (6) 232 (23) 228 (6) 216 (11) 157 (2) 466 (36) 417 (11) 380 (24) 350 (100) 346 (17) 337 (24)
P D [CoII (L H) bipy]C , surviving parent ions. Common product ions identied are [CoI (CH3 OH) bipy]C (m/z 247), [CoII (CH3 O) bipy]C (m/z 246), [CoI (H2 O) bipy]C (m/z 233), [CoII (OH) bipy]C (m/z 232), [bipy C H]C (m/z 157). c Only fragment ions with more than 10% relative abundance are listed.
a b
Copyright 2003 John Wiley & Sons, Ltd.
J. Mass Spectrom. 2003; 38: 555572
ESI-MS/MS structural characterization of chalcones
567
similar fragment ions upon CAD. Note that the fragment ions seen in the range m/z 200255 are non-specic products containing 2,20 -bipyridine, the metal, and up to one solvent (water or methanol) molecule. For most of the chalcones, the [CoII (chalconeH) bipy]C fragmentation patterns give the most useful array of diagnostic ions (see Fig. 9(A) as an example). Since 2,20 -bipyridine has a much greater copper binding energy compared to that of each chalcone, the whole chalcone molecule is generally lost upon collisional activation of the copper complexes, resulting in non-diagnostic fragmentation patterns for the [CuII (chalconeH) bipy]C complexes (Fig. 9(C)).
Cobalt complexation
The CAD data for the cobalt complexes are provided in Tables 4 and 5, and a representative series of CAD spectra
are shown in Fig. 9. For the chalcones without methoxy groups, commonly observed small neutral losses included H2 , H2 O, and CO (Table 4), similar to the losses seen for the deprotonated chalcones. Dominant losses observed for the chalcones with methoxy groups were CH3 , CH4 , H2 , H2 O, CO and CO2 (Table 5). In addition, several less informative products were observed for all the chalcones, such as [CoI (CH3 OH) bipy]C (m/z 247); [CoII (CH3 O) bipy]C (m/z 246); [CoI (H2 O) bipy]C (m/z 233); [CoII (OH) bipy]C (m/z 232); [bipy C H]C (m/z 157). In fact, ions below m/z 250 are generally cobalt/2,20 -bipyridine species that are non-specic. Relative to the CAD patterns of the deprotonated chalcones, the losses of H2 O, H2 and CO are generally more prominent for the metal complexes, [CoII (chalconeH) bipy]C , and the relative loss of 44 u is signicantly lower. Moreover, the types of A and B ions are different for the deprotonated
H) bipy]C (m/z, %), where A and B denote
Table 5. CAD of cobalt complexes of chalcones with methoxy groups: [CoII (L fragments retaining the A or B ring system 7 P P P P P P P P P P P P P P P P P P A B Common ionsb
a
10
11
12
13
14
15
16
17
19
20
H2 CH3 CH4 H2 CH3 H2 O CO H2 CO 2CH3 CH4 CH3 2CH4 H2 O CH3 CO CH3 CO2 H2 O CO 2CO H2 O CH3 2CO CH3
468 (10) 466 (4) 453 (92) 452 (17) 450 (5) 440 (6) 438 (8)
468 (8) 482 (11) 484 (10) 484 (9) 466 (8) 482 (100) 453 (100) 467 (100) 469 (1) 469 (1) 452 (21) 451 (10) 450 (3) 466 (5) 466 (52) 440 (5) 456 (2) 438 (4) 452 (21)
484 (9) 498 (11) 498 (12) 498 (11) 498 (12) 512 (9) X 482 (8) 496 (7) 496 (6) 496 (2) 510 (3) 469 (100) 483 (19) 483 (100) 483 (100) 483 (100) 497 (100) 468 (17) 482 (100) 482 (12) 466 (11) 456 (2) 454 (4) 468 (46) 468 (58) 468 (11) 468 (11) 482 (71) 467 (10) 466 (24) 466 (12) 465 (11) 465 (11) 441 (13) 455 (16) 455 (9) 468 (8) 454 (27) 454 (6) 452 (10) 437 (8) 437 (8) 427 (11) 427 (3)
425 (57) 425 (4) 424 (6) 424 (3) 412 (5) CO 397 (36) 318 (5) 322 (6) 292 (13) 338 (34) 360 (9) 308 (30) 248 (16) 248 (7) 247 (77) 247 (20) 246 (7) 246 (3) 233 (100) 233 (27) 232 (30) 232 (17) 228 (31) 228 (4) 216 (72) 216 (35) 157 (8) 157 (4) 362 (23)
441 (3) 440 (4)
318 (4) 292 (9) 322 (6) 248 (1) 247 (3) 246 (1) 233 (3) 232 (2) 216 (5)
364 (100) 308 (34) 376 (5) 308 (15) 338 (12) 390 (3) 337 (69) 248 (6) 248 (7) 247 (25) 247 (41) 246 (3) 246 (6) 233 (28) 233 (53) 232 (11) 232 (19) 228 (9) 216 (30) 216 (37) 157 (4) 157 (4) 322 (8) 248 (1) 247 (21) 246 (2) 233 (29) 232 (10) 216 (7) 157 (1)
Othersc
248 (11) 248 (8) 248 (4) 247 (11) 247 (17) 247 (84) 246 (2) 246 (2) 246 (3) 246 (6) 233 (15) 233 (27) 233 (95) 232 (15) 232 (30) 232 (19) 232 (29) 228 (18) 228 (15) 216 (50) 216 (43) 216 (24) 157 (5) 157 (4) 157 (3) 393 (18) 348 (15) 465 (11) 378 (12) 324 (18) 307 (14) 354 (21)
247 (5) 246 (1) 233 (5) 232 (5) 216 (5) 352 (11)
P D [CoII (L H) bipy]C , surviving parent ions. Common product ions identied are [CoI (CH3 OH) bipy]C (m/z 247), [CoII (CH3 O) bipy]C (m/z 246), [CoI (H2 O) bipy]C (m/z 233), [CoII (OH) bipy]C (m/z 232), [bipy C H]C (m/z 157). c Only fragment ions with more than 10% relative abundance are listed.
a b
Copyright 2003 John Wiley & Sons, Ltd.
J. Mass Spectrom. 2003; 38: 555572
568
J. Zhang and J. S. Brodbelt
chalcones compared to the metal complexes, a reasonable result considering the vastly different structures of these two types of molecular ions. The CAD spectra of the metal complexes gave a greater array of fragment ions, including at least one diagnostic ion for each isomer, more than observed for the deprotonated chalcones. The chalcones in Series 2, which relied on minor fragment ions (m/z 209 via loss of 44 u (7%) for 7 vs m/z 210 via loss of CH3 and CO (1%) for 8) for differentiation in the deprotonation mode, had distinctive fragment ions resulting from the cobalt complexes: 7, m/z 425, 397, 376, 362, 338, 318, 292; and 8, m/z 412, 360, 332, 322, 308 (Table 5) (m/z 376 and 332 are fragment ions with less than 10% relative abundance and are thus omitted in the Others column in Table 5). Structures for some of these diagnostic ions are suggested in Scheme 3. Likewise, all the isomers in Series 4, which were not distinguishable by CAD in the deprotonation mode due to the dominant loss of one or two methyl radicals for 14, 15 and 16, had both distinctive CAD patterns and unique fragments upon CAD of the metal complexes: 14, m/z 307/308; 15, m/z 352; 16, m/z 455, 427, 338; and 17, m/z 337 (Fig. 9), which led to easy differentiation. Dissociation of the metal complex of 16 results in an even-electron ion at m/z 338, while the metal complex of 17 gives a radical ion at m/z 337 that could be rationalized by the availability of a hydrogen at the 2-position. For 16, the hydrogen at the 2-position could migrate to the 10 -position while the bond between the ketone and 10 -carbon is broken, resulting in a fragment ion at m/z 338 and a ring closure between the ketone and the A-ring at the 2-position. 17 has a methoxy group instead of hydrogen at the 2-position, which makes the hydrogen donation impossible. The structures of the most unique diagnostic ions are suggested in Scheme 4. Differentiation of the other isomer series was also possible based on the CAD patterns of the metal complexes (Tables 4 and 5). Therefore, the CAD spectra of the cobalt complexes served as distinctive ngerprints of the chalcones.
N Co2+
N Co2+ O-
O-
H3CO OCH3 14 308+
N Co2+
N Co2+ OOCH3 O. H 352+
OOCH3 OCH3 H
15
N Co2+ O-
N Co2+
OCH3 338+
O-
H3CO 16
N OCH3 O
N Co2+ O-
O. 427+
N Co2+
N Co2+ O
Nickel complexation
The fragmentation patterns of the nickel complexes (Tables 6 and 7) were similar to that of the cobalt complexes, but with H2 losses enhanced. The CAD mass spectra of these complexes could also serve as ngerprints of the chalcones.
O-
OCH3 17
OCH3 337+
OCH3
Copper complexation
The copper complexes, in contrast to the cobalt and nickel complexes, did not give signicant chalcone-related fragments upon CAD (Tables 8 and 9 and Fig. 8(C)). The main products upon CAD were complexes of copper and the auxiliary bipyridine ligand. Even when compound-specic fragments existed in the spectra, their abundances were too low to be analytically useful. MSn experiments were also performed on some selected metal complexes (Table 10). MS/MS/MS experiments of the cobalt and nickel complexes were also found to be useful in further differentiation of the isomers in Series 4. For example, 15 and 16 shared a major common fragment at m/z 483, but the MS/MS/MS patterns of m/z 483 were unique for both chalcone complexes (Table 10). MSn experiments were also
Scheme 4. Proposed structures of the unique fragment ions of the cobalt complexes of the chalcones in Series 4.
performed on those fragments that allowed differentiation of the isomers (m/z 307 and 308 for 14, m/z 352 for 15, m/z 338 and 427 for 16 and m/z 337 for 17). The structures of those unique diagnostic fragments proposed in Scheme 4 were based on the supporting MSn data.
CONCLUSION
Different ionization modes were compared for their utility for formation of chalcone ions and the subsequent CAD mass spectra. Owing to their slightly acidic nature, chalcones were easily deprotonated. CAD upon the resulting deprotonated
Copyright 2003 John Wiley & Sons, Ltd.
J. Mass Spectrom. 2003; 38: 555572
ESI-MS/MS structural characterization of chalcones
569
Table 6. CAD of nickel complexes of chalcones without methoxy groups: [NiII (L fragments retaining the A or B ring system 1 Pa P P P P P P P A H2 H2 O CO H2 CO H2 O CO 2CO H2 2CO 437 (8) 435 (75) 409 (6) 407 (28) 381 (8) 379 (1) 317 (4) 291 (32) 307 (81) 246 (36) 245 (14) 232 (49) 231 (100) 214 (4) 2 453 (14) 451 (56) 425 (7) 423 (24) 397 (4) 395 (2) 3 453 (10) 451 (66) 425 (6) 423 (44) 407 (2) 397 (3) 395 (3) 291 (33) 323 (86) 246 (41) 245 (16) 232(56) 231 (100) 214 (5) 307 (100) 246 (1) 245 (6) 231 (43) 297 (29) 4 453 (6) 435 (9)
H) bipy]C (m/z, %), where A and B denote
5 453 (9) 451 (45) 435 (20) 425 (3) 423 (30) 407 (9) 397 (5) 395 (1)
6 453 (5) 451 (42) 425 (4) 423 (17) 407 (1) 397 (2) 291 (13)
9 469 (8) 467 (100) 441 (15) 439 (43) 423 (20) 413 (3) 307 (72) 323 (83) 246 (66) 245 (8) 232 (88) 231 (74) 214 (8) 243 (12)
18 501 (6) 499 (100) 483 (13) 471 (8) 455 (6)
B Common ionsb
307 (100) 246 (24) 245 (5) 232 (32) 231 (32) 214 (3)
Othersc
a
307 (100) 246 (40) 245 (3) 232 (51) 231 (36) 214 (3) 297 (42)
323 (32) 246 (14) 245 (5) 232 (18) 231 (36) 214 (2) 297 (100)
349 (10) 323 (21) 375 (11) 246 (9) 232 (36) 231 (10) 214 (13) 339 (18)
P D [NiII (L H) bipy]C , surviving parent ions. Common product ions identied are [NiI (CH3 OH) bipy]C (m/z 246), [NiII (CH3 O) bipy]C (m/z 245), [NiI (H2 O) bipy]C (m/z 232), [NiII (OH) bipy]C (m/z 231), [NiI bipy]C (m/z 214). c Only fragment ions with more than 10% relative abundance are listed.
b
Table 7. CAD of nickel complexes of chalcones with methoxy groups: [NiII (L fragments retaining the A or B ring system 7 Pa P P P P P P P P P P P P P A 467 (11) 465 (76) 452 (6) 8 467 (11) 465 (82) 452 (7) 10 481 (11) 479 (85) 466 (41) 11 12 13 483 (8) 481 (77) 468 (11)
H) bipy]C (m/z, %), where A and B denote
14 497 (6) 495 (72) 482 (10) 481 (14) 479 (6) 469 (9) 467 (8)
15
16
17 497 (12) 495 (36) 482 (18)
19 511 (10) 509 (85) 496 (40) 495 (12) 483 (13) 481 (23)
20 X
H2 CH3 CH4 H2 O CO H2 CO 2CH3 CO CH3 CO2 H2 O CO 2CO H2 2CO CO2 CH3
483 (11) 483 (9) 481 (100) 481 (3) 468 (2) 468 (6) 465 (2) 455 (1) 453 (3) 440 (4) 437 (2) 427 (1) 465 (12) 455 (1) 453 (1) 440 (2)
497 (11) 497 (10) 495 (12) 495 (81) 482 (100) 482 (14)
439 (9) 437 (27)
439 (12) 437 (38)
453 (18) 451 (47)
455 (11) 453 (44)
469 (11) 467 (19) 454 (11) 453 (8) 451 (3) 467 (31)
469 (4) 467 (12)
411 (5) 409 (2) 291 (39)
411 (8) 409 (3)
425 (6) 423 (1) 291 (8) 321 (100) 246 (37) 245 (13) 232 (51) 231 (84) 214 (4)
437 (3) 427 (4) 425 (1) 363 (9) 307 (100) 246 (7) 245 (5) 232 (10) 231 (51) 348 (12)
453 (4) 451 (4) 441 (8) 439 (2) 438 (6)
451 (1) 441 (6) 439 (2) 321 (14) 291 (11) 337 (80) 246 (54) 245 (17) 232 (74) 231 (100) 214 (8) 321 (22) 291 (30) 337 (100) 246 (72) 245 (11) 232 (100) 231 (75) 214 (13) 336 (65)
465 (1) 455 (4) 452 (8)
B Common ionsb
321 (12) 291 (10) 337 (79) 307 (81) 246 (37) 246 (44) 245 (15) 245 (13) 232 (50) 232 (62) 231 (100) 231 (100) 214 (5)
246 (7) 232(14) 231 (17) 214 (2) 353 (12)
246 (54) 245 (11) 232 (77) 231 (100) 323 (87) 321 (13) 291 (10)
Othersc
307 (69) 246 (73) 245 (20) 232 (100) 231 (83) 214 (11) 369 (30) 243 (13)
246 (20) 232 (28) 231 (8) 214 (3) 352 (13)
321 (84) 246 (73) 245 (18) 232 (100) 231 (85) 214 (11) 369 (38) 243 (13)
P D [NiII L H bipy]C , surviving parent ions. Common product ions identied are [NiI (CH3 OH) bipy]C (m/z 246), [NiII (CH3 O) bipy]C (m/z 245), [NiI (H2 O) bipy]C (m/z 232), [NiII (OH) bipy]C (m/z 231), [NiI bipy]C (m/z 214). c Only fragment ions with more than 10% relative abundance are listed.
a b
Copyright 2003 John Wiley & Sons, Ltd.
J. Mass Spectrom. 2003; 38: 555572
570
J. Zhang and J. S. Brodbelt
Table 8. CAD of copper complexes of chalcones without methoxy groups: [CuII (L 1 Pa P H2 O P CO Common ionsb 442 (5) 2 458 (5) 3 458 (5) 4 458 (8) 5
H) bipy]C (m/z, %) 6 458 (8) 9 474 (6) 18 506 (3) 478 (40) 251 (9) 237 (100) 219 (86) 247 (6)
458 (5) 440 (1) 251 (11) 237 (100) 219 (33) 365 (5) 312 (2)
251 (10) 237 (100) 219 (30) 365 (5) 237 (100) 219 (29) 365 (2) 320 (2)
251 (12) 237 (100) 219 (30) 381 (5)
Othersc
251 (2) 250 (10) 237 (16) 236 (100) 219 (6) 337 (3) 312 (8)
251 (12) 237 (100) 219 (32) 381 (5) 302 (2)
251 (11) 237 (100) 219 (37) 381 (2) 318 (2) 247 (2)
P D [CuII (L H) bipy]C , surviving parent ions. Common product ions identied are: [CuI (CH3 OH) bipy]C (m/z 251), [CoII (CH3 O) bipy]C (m/z 250), [CuI (H2 O) bipy]C (m/z 237), [CuII (OH) bipy]C (m/z 236), [CuI bipy]C (m/z 219). c Only fragment ions with more than 2% relative abundance are listed
a b
Table 9. CAD of copper complexes of chalcones with methoxy groups: [CuII (L 7 Pa P P P P 472 (7) CH3 H2 O CO 444 (1) CH3 CO Common 251 (10) ionsb 237 (100) 219 (25) 395 (7) 337 (3) 8 472 (7) 10 486 (4) 471 (1) 11 488 (10) 12 488 (5) 470 (1) 444 (1) 460 (1) 445 (1) 251 (14) 251 (5) 251 (11) 13 488 (7) 14
H) bipy]C (m/z, %) 15 502 (12) 487 (12) 16 502 (12) 17 502 (6) 19 516 (8) 501 (1) 20 X
502 (12) 487 (1) 474 (1) 459 (2) 251 (10)
459 (2)
474 (2) 459 (1) 251 (10) 251 (11) 251 (12)
Othersc
251 (8) 250 (10) 237 (100) 237 (100) 237 (100) 237 (74) 236 (100) 219 (36) 219 (32) 219 (74) 219 (27) 365 (2) 379 (3) 350 (3) 368 (11) 351 (2) 348 (2) 332 (2) 367 (1) 337 (5) 330 (2) 272 (2) 339 (2) 326 (2) 326 (2) 247 (5) 312 (10) 247 (2) 241 (2)
251 (11)
251 (10) 250 (9) 237 (100) 237 (100) 237 (100) 236 (97) 219 (37) 219 (37) 219 (36) 381 (2) 381 (2) 425 (3) 351 (2) 365 (2) 364 (6) 350 (2) 364 (6) 346 (2) 356 (3) 217 (3)
237 (100) 237 (100) 237 (100) 219 (37) 473 (2) 395 (3) 364 (5) 351 (3) 346 (3) 326 (2) 219 (37) 395 (5) 364 (5) 346 (16) 219 (34) 379 (3) 378 (3) 360 (3) 356 (3)
P D [CuII (L H) bipy]C , surviving parent ions. Common product ions identied are [CuI (CH3 OH) bipy]C (m/z 251), [CoII (CH3 O) bipy]C (m/z 250), [CuI (H2 O) bipy]C (m/z 237), [CuII (OH) bipy]C (m/z 236), [CuI bipy]C (m/z 219). c Only fragment ions with more than 2% relative abundance are listed.
a b
Table 10. MSn data for selected chalcone complex ions [MII (L H) bipy]C (m/z) Fragment 408 247 233 216 464 454 Neutral loss 28 189 203 220 18 28
Table 10. (Continued) Fragment 452 436 424 410 362 344 Neutral loss 30 46 58 72 120 138
Complex % 100 33 30 10 100 19
Chalcone
Route
% 72 10 12 52 21 20
Complex [CoII (L H) bipy]C
Chalcone 2
Route 454 ! 436 !
14
498 ! 482 !
Copyright 2003 John Wiley & Sons, Ltd.
J. Mass Spectrom. 2003; 38: 555572
ESI-MS/MS structural characterization of chalcones
571
Table 10. (Continued) Fragment 326 247 233 232 216 498 ! 308 ! 280 247 233 306 289 279 468 465 454 437 352 281 247 233 232 268 246 232 482 468 455 454 427 390 247 232 399 397 247 233 295 247 233 336 309 308 280 279 249 247 23 480 479 451 232 231 Neutral loss 156 235 249 250 266 28 61 75 1 18 28 15 18 29 46 131 202 236 250 251 84 106 120 1 15 28 29 56 93 236 251 28 30 80 194 43 91 105 1 28 29 57 58 88 90 104 15 16 44 263 138
Table 10. (Continued) Fragment 246 232 337 281 246 232 214 246 232 246 232 246 232 Neutral loss 61 75 15 71 106 120 138 91 105 91 105 90 104
Complex
Chalcone
Route
% 36 14 20 21 12 10 69 100 18 12 100 57 18 39 100 88 14 23 29 12 51 15 100 44 19 67 51 37 32 82 100 27 13 85 100 25 85 100 55 27 21 55 22 17 80 100 15 100 21 12 100
Complex
Chalcone
Route 497 ! 307 !
% 71 100 100 25 81 98 28 76 100 74 100 75 100
15
497 ! 352 !
16 17
497 ! 337 ! 497 ! 337 ! 497 ! 336 !
498 ! 307 !
15
498 ! 483 !
498 ! 352 !
16
498 ! 483 !
species gave simple and compound-specic fragments, which were sufcient in differentiating most of the isomers. The use of metal complexation as an alternative ionization mode resulted in equal or greater signal intensities and led to more specic fragmentation patterns so that all the isomeric series were distinguished. MSn experiments proved to be useful in elucidating proposed fragmentation pathways and conrmed that many of the neutral losses occurred through stepwise pathways. Finally, it is clear that the chalcones do not follow consistent structure-specic rules for fragmentation, thus complicating the identication of new chalcones in mixtures.
Acknowledgements
This work was supported by the National Institutes of Health (NIH RO1 GM63512) and the Welch Foundation (F-1155).
498 ! 427 !
REFERENCES
1. Bohm BA. Introduction to Flavonoids. Harwood Academic Publishers: Singapore, 1998; 365. 2. Middleton E Jr, Kandaswami C. In The Flavonoids: Advances in Research Since 1986, Harborne JB (ed). Chapman & Hall: London 1986; 619. 3. Kaur C, Kapoor HC. Antioxidants in fruits and vegetablesthe millenniums health. Int. J. Food Sci. Technol. 2001; 36: 703. 4. Bohm BA. Introduction to Flavonoids. Harwood Academic Publishers: Singapore, 1998; 5. 5. Robards K, Antolovich M. Analytical chemistry of fruit bioavonoids: a review. Analyst 1997; 122: 11R. 6. Tomas-Barberan FA, Clifford MN. Flavanones, chalcones and dihydrochalconesnature, occurrence and dietary burden. J. Sci. Food Agric. 2000; 80: 1073. 7. Mishra S, Rao JT. Biological activities of chalcones. J. Instit. Chem. (India) 1995; 67: 181. 8. Lopez SN, Castelli MV, Zacchino SA, Dominguez JN, Lobo G, Charris-Charris J, Cortes JCG, Ribas JC, Devia C, Rodriguez AM, Enriz RD. In vitro antifungal evaluation and structureactivity relationships of a new series of chalcone derivatives and synthetic analogues, with inhibitory properties against polymers of the fungal cell wall. Bioorg. Med. Chem. 2001; 9: 1999. 9. Chowdhury A, Mukherjee N, Adityachaudhury N. Sensitivity of some plant pathogenic fungi towards plant metabolites. Antifungal activity of some chalcones, dihydrochalcones, and avanones. Experientia 1974; 30: 1022.
498 ! 338 !
17
498 ! 337 !
[NiII (L H) bipy]C
14
497 ! 495 !
497 ! 369 !
Copyright 2003 John Wiley & Sons, Ltd.
J. Mass Spectrom. 2003; 38: 555572
572
J. Zhang and J. S. Brodbelt
10. Hsieh HK, Tsao LT, Wang JP, Lin CN. Synthesis and antiinammatory effect of chalcones. J. Pharm. Pharmacol. 2000; 52: 163. 11. Vibhute YB, Wadje SS. Antimicrobial activity of some chalkones and avones. Indian J. Exp. Biol. 1976; 14: 739. 12. Iwata S, Nishino T, Inoue H, Nagata N, Satomi Y, Nishino H, Shibata S. Antitumorigenic activities of chalcones (II). Photoisomerization of chalcones and the correlation with their biological activities. Biol. Pharm. Bull. 1997; 20: 1266. 13. Okuyama T, Takata M, Takayasu J, Hasegawa T, Tokuda H, Nishino A, Nishino H, Iwashima A. Anti-tumor-promotion by principles obtained from Angelica keiskei. Planta Med. 1991; 57: 242. 14. De Vincenzo R, Ferlini C, Distefano M, Gaggini C, Riva A, Bombardelli E, Morazzoni P, Valenti P, Belluti F, Ranelletti FO, Mancuso S, Scambia G. In vitro evaluation of newly developed chalcone analogues in human cancer cells. Cancer Chemother. Pharmacol. 2000; 46: 305. 15. Bohm BA. Introduction to Flavonoids. Harwood Academic Publishers: Singapore, 1998; 285. 16. Heller W, Forkmann G. In The Flavonoids: Advances in Research Since 1986, Harborne JB (ed). Chapman & Hall: London, 1986; 499. 17. Old KB, Main L. The kinetics and mechanism of the cyclisation of some 20 -hydroxychalcones to avanones in basic aqueous solution. J. Chem. Soc., Perkin Trans. 2 1982; 1309. 18. Nudelman NS, Furlong JJP. Conversion of avanones and chalcones in alkaline medium. Kinetic and spectroscopic studies. J. Phy. Org. Chem. 1991; 4: 263. 19. Arcus VL, Simpson CD, Main L. An enolate intermediate in chalcone-avanone isomerisation; saturation kinetics and a change in rate-limiting step in the general-acid-catalysed cyclisation of 20 ,60 -dihydroxy-3,4,40 -trimethoxychalcone in amine buffer solutions. J. Chem. Res. (S) 1992; 80. 20. Yamin LJ, Blanco SE, Luco JM, Ferretti FH. Theoretical study of cyclization of 20 -hydroxychalcone. J. Mol. Struct. (Theochem) 1997; 390: 209. 21. Van De Sande C, Serum JW, Vandewalle M. Studies in organic mass spectrometryXII: mass spectra of chalcones and avanones. The isomerisation of 20 -hydroxyl-chalcone and avanone. Org. Mass Spectrom. 1972; 6: 1333. 22. Mentlein R, Vowinkel E. Ortho effect in the fragmentation of 2-acetoxychalcones under electron impact. Org Mass Spectrom. 1984; 19: 330. 23. Ardanaz CE, Traldi P, Vettori U, Kavka J, Guidugli F. The iontrap mass spectrometer in ion structure studies. The case of [M H]C ions from chalcone. Rapid Commun. Mass Spectrom. 1991; 5: 5.
24. Yenesew A, Midiwo JO, Waterman PG. Rotenoids, isoavones and chalcones from the stem bark of Millettia usaramensis subspecies usaramensis. Phytochemistry 1998; 47: 295. 25. McCormick SP, Bohm BA, Ganders FR. Methylated chalcones from Bidens torta. Phytochemistry 1984; 23: 2400. 26. Lin YL, Chen YL, Kuo YH. Two new avanones and two new chalcones from the root of Derris laxiora benth. Chem. Pharm. Bull. 1992; 40: 2295. 27. do Nascimento MC, Mors WB. Chalcones of the root bark of Derris sericea. Phytochemistry 1972; 11: 3023. 28. Nesmeyanov AN, Zagorevskii DV, Nekrasov YS, Sizoi VF, Postnov VM, Baran AM, Klimova EI. Mass spectrometry of complexes of transition metals XII. Ferrocenyl and cymantrenyl derivatives of chalcones. J. Organomet. Chem. 1979; 169: 77. 29. Parmar VS, Sharma SK, Vardhan A, Sharma RK. New fragmentation pathways in the electron impact mass spectrometry of derivatized pyrano-1,3-diphenylprop-2-enones. Org. Mass Spectrom. 1993; 28: 23. 30. Bankova VS, Mollova NN, Popov SS. Chemical ionization mass spectrometry with amines as reactant gases. Org. Mass Spectrom. 1986; 21: 109. 31. Hoffmann B, Holzl J. Chalcone glycosides from Bidens pilosa. Phytochemistry 1989; 28: 247. 32. Prasain JK, Tezuka Y, Li JX, Tanaka K, Basnet P, Dong H, Namba T, Kadota S. Six novel diarylheptanoids bearing chalcone or avanone moiety from the seeds of Alpinia blepharocalyx. Tetrahedron 1997; 53: 7833. 33. Redl K, Davis B, Bauer R. Chalcone glycosides from Bidens campylotheca. Phytochemistry 1993; 32: 218. 34. Sattereld M, Brodbelt JS. Enhanced detection of avonoids by metal complexation and electrospray ionization mass spectrometry. Anal. Chem. 2000; 72: 5898. 35. Sattereld M, Brodbelt JS. Structural characterization of avonoid glycosides by collisionally activated dissociation of metal complexes. J. Am. Soc. Mass Spectrom. 2001; 15: 537. 36. Fabre N, Rustan I, de Hoffmann E, Quetin-Leclercq J. Determination of avone, avonol, and avanone aglycones by negative ion liquid chromatography electrospray ion trap mass spectrometry. J. Am. Soc. Mass Spectrom. 2001; 12: 707. 37. Justesen U, Arrigoni E, Larsen BR, Amado R. Degradation of avonoid glycosides and aglycones during in vitro fermentation with human faecal ora. Food Sci. Technol./LWT 2000; 33: 424. 38. Justesen U. Negative atmospheric pressure chemical ionization low-energy collision activation mass spectrometry for the characterization of avonoids in extracts of fresh herbs. J. Chromatogr. A 2000; 902: 369. 39. Justesen U. Collision-induced fragmentation of deprotonated methoxylated avonoids, obtained by electrospray ionization mass spectrometry. J. Mass Spectrom. 2001; 36: 169.
Copyright 2003 John Wiley & Sons, Ltd.
J. Mass Spectrom. 2003; 38: 555572
Вам также может понравиться
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryОт EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryРейтинг: 3.5 из 5 звезд3.5/5 (231)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)От EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Рейтинг: 4.5 из 5 звезд4.5/5 (120)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaОт EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaРейтинг: 4.5 из 5 звезд4.5/5 (266)
- The Little Book of Hygge: Danish Secrets to Happy LivingОт EverandThe Little Book of Hygge: Danish Secrets to Happy LivingРейтинг: 3.5 из 5 звезд3.5/5 (399)
- Never Split the Difference: Negotiating As If Your Life Depended On ItОт EverandNever Split the Difference: Negotiating As If Your Life Depended On ItРейтинг: 4.5 из 5 звезд4.5/5 (838)
- The Emperor of All Maladies: A Biography of CancerОт EverandThe Emperor of All Maladies: A Biography of CancerРейтинг: 4.5 из 5 звезд4.5/5 (271)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeОт EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeРейтинг: 4 из 5 звезд4/5 (5794)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyОт EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyРейтинг: 3.5 из 5 звезд3.5/5 (2259)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersОт EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersРейтинг: 4.5 из 5 звезд4.5/5 (344)
- Team of Rivals: The Political Genius of Abraham LincolnОт EverandTeam of Rivals: The Political Genius of Abraham LincolnРейтинг: 4.5 из 5 звезд4.5/5 (234)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreОт EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreРейтинг: 4 из 5 звезд4/5 (1090)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceОт EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceРейтинг: 4 из 5 звезд4/5 (895)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureОт EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureРейтинг: 4.5 из 5 звезд4.5/5 (474)
- The Unwinding: An Inner History of the New AmericaОт EverandThe Unwinding: An Inner History of the New AmericaРейтинг: 4 из 5 звезд4/5 (45)
- The Yellow House: A Memoir (2019 National Book Award Winner)От EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Рейтинг: 4 из 5 звезд4/5 (98)
- Lewis David On The Plurality of WorldsДокумент279 страницLewis David On The Plurality of Worldsdjoseph_1Оценок пока нет
- E2906E2906M-13 Standard Practice For Acoustic Pulse Reflectometry Examination of Tube BundlesДокумент6 страницE2906E2906M-13 Standard Practice For Acoustic Pulse Reflectometry Examination of Tube BundlesMohamed100% (1)
- Modelling and Simulation of The Three-Phase Induction Motor Using SimulinkДокумент10 страницModelling and Simulation of The Three-Phase Induction Motor Using SimulinkÁron FehérОценок пока нет
- GRP-FT Maintenance ManualДокумент20 страницGRP-FT Maintenance ManualSaleh EttehadiОценок пока нет
- Astm C469 - C469M - 14Документ5 страницAstm C469 - C469M - 14adil Rahman hassoon100% (1)
- X-Ray Sources Diffraction: Bragg's Law Crystal Structure DeterminationДокумент62 страницыX-Ray Sources Diffraction: Bragg's Law Crystal Structure DeterminationSrimanthula SrikanthОценок пока нет
- 2010 551752Документ10 страниц2010 551752Sonia SahnounОценок пока нет
- Chemical Composition of The Essential Oil of Phlomis Linearis Boiss. & Bal., and Biological Effects On The CAM-Assay: A Safety EvaluationДокумент4 страницыChemical Composition of The Essential Oil of Phlomis Linearis Boiss. & Bal., and Biological Effects On The CAM-Assay: A Safety EvaluationSonia SahnounОценок пока нет
- 1 s2.0 S0960852409010876 MainДокумент4 страницы1 s2.0 S0960852409010876 MainSonia SahnounОценок пока нет
- Differentiation of Five Pine Species Cultivated in Brazil Based On ChemometriclДокумент8 страницDifferentiation of Five Pine Species Cultivated in Brazil Based On ChemometriclSonia SahnounОценок пока нет
- Interpretation of Mass Spectra: Choose A Reasonable StructureДокумент16 страницInterpretation of Mass Spectra: Choose A Reasonable Structuredjdr0Оценок пока нет
- Profiling of The Resin GlycosideДокумент7 страницProfiling of The Resin GlycosideSonia SahnounОценок пока нет
- Flavonoid Analysis of Heloniopsis OrientalisДокумент5 страницFlavonoid Analysis of Heloniopsis OrientalisSonia SahnounОценок пока нет
- Profiling of The Resin GlycosideДокумент7 страницProfiling of The Resin GlycosideSonia SahnounОценок пока нет
- 1Документ7 страниц1Sonia SahnounОценок пока нет
- Flavonoid Composition in The Leaves of TwelveДокумент8 страницFlavonoid Composition in The Leaves of TwelveSonia SahnounОценок пока нет
- Flavonoids and Terpenoids From Helichrysum ForskahliiДокумент5 страницFlavonoids and Terpenoids From Helichrysum ForskahliiSonia SahnounОценок пока нет
- Quinolizidine Alkaloids As Systematic Markers of The GenisteaeДокумент7 страницQuinolizidine Alkaloids As Systematic Markers of The GenisteaeSonia SahnounОценок пока нет
- Flavonoids and Terpenoids From Helichrysum ForskahliiДокумент5 страницFlavonoids and Terpenoids From Helichrysum ForskahliiSonia SahnounОценок пока нет
- Chemical Constituents From The Root BarkДокумент6 страницChemical Constituents From The Root BarkSonia SahnounОценок пока нет
- HPLCДокумент6 страницHPLCSonia SahnounОценок пока нет
- Analytical Separation and Detection Methods For FlavonoidsДокумент33 страницыAnalytical Separation and Detection Methods For FlavonoidsrenatacnОценок пока нет
- Isolation and Structural Characterization of New Glycolipid EsterДокумент16 страницIsolation and Structural Characterization of New Glycolipid EsterSonia SahnounОценок пока нет
- ASTM A320 - A320M-22aДокумент8 страницASTM A320 - A320M-22a1965karanfil6Оценок пока нет
- Cancer Management 7e (2003)Документ992 страницыCancer Management 7e (2003)ccvmdОценок пока нет
- 342 B.sc.b.ed. Mdsu PDF 4yrДокумент135 страниц342 B.sc.b.ed. Mdsu PDF 4yrDINESH SALVIОценок пока нет
- Chapter 2 Concrete ManualДокумент10 страницChapter 2 Concrete ManualnguyenkhachiepvnОценок пока нет
- Flow Around SphereДокумент11 страницFlow Around SpheremiladОценок пока нет
- FS-l6S: Instruction ManualДокумент25 страницFS-l6S: Instruction ManualFazrulОценок пока нет
- Analysis of VOltage and Power Interactions in Multi-Infeed HVDC SystemsДокумент9 страницAnalysis of VOltage and Power Interactions in Multi-Infeed HVDC SystemstinazdrilicОценок пока нет
- Quick Panel BrochureДокумент11 страницQuick Panel BrochuretantanОценок пока нет
- Mid Term Paper - DynamicsДокумент3 страницыMid Term Paper - DynamicsMălíķ ĂsfęnđýårОценок пока нет
- Lect 1 & Lect 2 Selection of Materials: October 2018Документ14 страницLect 1 & Lect 2 Selection of Materials: October 2018Enriqe PuentesОценок пока нет
- Enhanced Degradation of Persistent Pharmaceuticals Found in Wastewater Treatment Ef Uents Using Tio2 Nanobelt PhotocatalystsДокумент14 страницEnhanced Degradation of Persistent Pharmaceuticals Found in Wastewater Treatment Ef Uents Using Tio2 Nanobelt PhotocatalystsSourav SutradharОценок пока нет
- 3107 - Nurture - Phase-II - Answerkey & SolutionДокумент11 страниц3107 - Nurture - Phase-II - Answerkey & Solution06 boymaxxОценок пока нет
- Notification APSWREIS Teacher PostsДокумент14 страницNotification APSWREIS Teacher PostsRuthvik ReddyОценок пока нет
- Grade 7 Chem NF 1st Term (1) - 1-6Документ6 страницGrade 7 Chem NF 1st Term (1) - 1-6zizo gamingОценок пока нет
- MA103 Lab 6 SolutionsДокумент2 страницыMA103 Lab 6 Solutionssubway9113Оценок пока нет
- LV TrafoДокумент38 страницLV TrafoApik SubagyaОценок пока нет
- Temperature SensorsДокумент14 страницTemperature SensorsManmeet RajputОценок пока нет
- GEOTECH 1 Module 8Документ12 страницGEOTECH 1 Module 8Earl averzosaОценок пока нет
- Self-Instructional Manual (SIM) For Self-Directed Learning (SDL)Документ24 страницыSelf-Instructional Manual (SIM) For Self-Directed Learning (SDL)Vine OrtegaОценок пока нет
- Mandavya Integrated Pu CollegeДокумент4 страницыMandavya Integrated Pu CollegeSahaana VMОценок пока нет
- Pharmaceutical Powders, Blends, Dry Granulations, and Immediate-Release TabletsДокумент9 страницPharmaceutical Powders, Blends, Dry Granulations, and Immediate-Release TabletsOgunjimi Abayomi Tolulope50% (2)
- Strength of Materials 2016 by S K Mondal PDFДокумент472 страницыStrength of Materials 2016 by S K Mondal PDFSai Subrahmanyam PvkОценок пока нет
- Conventional LatheДокумент19 страницConventional LatheArif TajulОценок пока нет
- Lab Report Strength Tensile TestДокумент3 страницыLab Report Strength Tensile TestAbdul KarimОценок пока нет