Академический Документы
Профессиональный Документы
Культура Документы
Air Cycles
Загружено:
jehadyamИсходное описание:
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
Air Cycles
Загружено:
jehadyamАвторское право:
Доступные форматы
AIR STANDARD POWER CYCLES
INTRODUCTION
Engine cycle analysis is an important tool in the design of n IC engines. This is because, before as
engine is designed and fabricated, one has to have a rough idea on how it would behave and what
performance it would exhibit. Therefore, wed go for cycle analysis in order to have an idea about
the performance limits of an engine.
Thermodynamic cycle is defined as a series of processes through which the working fluid
progresses, which will eventually return to its original state. In another words, it implies a closed
system with no exchange of matter with surroundings.
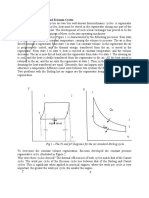
Though, actually speaking, the IC engine operation does not follow the closed system as shown in
Fig. (1) below, however, it is often possible to analyze the open system as though it were a closed
one by imagining one or two processes which would bring the working fluid to its original state.
The actual analysis of the cycle is difficult since it involves a lot of complications like change of
properties of working fluid, chemical reactions, frictional losses etc., however, using certain
assumptions one can simplify the process to make it possible for analysis, though it would bring the
results of this analysis farther from the real situation.
IDEAL or AIR STANDARD POWER CYCLES
Air Standard Cycle is defined as a cycle using perfect gas as the working medium.
ASSUMPTIONS
The assumptions made in order to simplify the cycle for analysis are mentioned below :
Working fluid is a perfect gas which obeys the gas law PV = mRT
The properties of the working fluid is that of the air @ NTP, i.e. C
P
= 1.005, C
V
=
0.718 kJ/Kg.k and = 1.4.
The working fluid has constant specific heats.
No chemical reaction takes place all throughout the process.
There is always fixed mass inside the cylinder.
Heat addition and rejection always takes place in a reversible manner.
Engine operation is frictionless.
Kinetic and Potential energies are neglected.
Compression and expansion processes are reversible adiabatic.
THE CONSTANT VOLUME OR OTTO CYCLE.
Pressure Temperature
3 Reversible Adiabatic 3
PV
= Constant
Q
in
W
out
Q
in
W
out
2 4 2 4
Q
out
W
in
W
in
1 1 Q
out
V
C
= V
2
V
S
= V
1
-V
2
Volume Entropy
V
t
= V
1
= V
S
+ V
C
W.D. in compression 1-2 = area under 12 Heat added during 2-3 = area under 23
W.D. in expansion 3-4 = area under 34 Heat rejected during 4-1 = area under 41
Net W.D. = area 1234 Net Heat = area 1234
Working Principle of Ideal Otto Cycle.(Explain)
Process (1-2) : Adiabatic Compression of air from V1 to V2.
1
Q
2
= 0.
Process (2-3) : Constant Volume heat addition.
2
W
3
= 0.
Process (3-4) : Adiabatic Expansion of air from V3 to V4.
3
Q
4
= 0.
Process (4-1) : Constant Volume heat rejection.
4
W
1
= 0.
After the air is introduced into the engine cylinder during the suction stroke
(not shown here), it immediately undergoes isentropic compression from (1)
to (2) as the piston moves from the BDC (V
1
) to the TDC (V
2
). This results in
volume reduction as pressure as well as temperature rise. Heat is then added
instantaneously at constant volume (process 2-3) while the piston is
momentarily at rest at the TDC.
This raises the pressure and temperature further. This expansion of the air
pushes the piston from TDC to BDC where power is produced and given to its
consumption point. To complete this ideal air power cycle, the working fluid
has to return back to its initial state that is state point (1). This requires that
the air reject its heat, which occur at constant volume when the piston is at
BDC and then the cycle is repeated.
Mathematical Analysis of the cycle.
Let us take m= 1 Kg.
Heat added during process 2-3 is = C
V
(T
3
T
2
) kJ/Kg
Heat Rejected during the process 4-1 is = C
V
(T
4
T
1
) kJ/Kg
Therefore, the net work done is W
net
= C
V
[(T
3
T
2
) - (T
4
T
1
)] kJ/Kg
Calculation of Thermal Efficiency
Thus the thermal efficiency
th
is
,
_
,
_
1
2
T
3
T
1
1
T
4
T
2
T
1
T
1
)
2
T
3
(T
V
C
)
1
T
4
(T
V
C
- 1
add
Q
rej
Q
1
add
Q
rej
Q
add
Q
Supplied Heat
Done Net Work
th
Now,
2
T
3
T
1
T
4
T
; OR
1
CR
4
T
3
T
1
T
2
T
Hence the equation for thermal efficiency can be written as:
1) - (
CR
1
- 1
3
T
4
T
- 1
2
T
1
T
1
th
Example (1-Otto):
In an ideal constant volume cycle the pressure and temperature at the
beginning of compression are 97 kN/m
2
and 40
o
C, respectively. The volume
ratio of compression is 7:1. The heat supplied during the cycle is 1200 kJ/Kg
of working fluid. Calculate the following :
1) The maximum temperature attained by the cycle, 2) The thermal efficiency
of the cycle and 3) Work done during the cycle per Kg of working fluid. Take
C
v
= 0.718 kJ/kg.K and = 1.4.
Given : P
1
= 97 kPa, T
1
= 313
o
K, CR = 7 and q
in
= 1200 kJ/kg
1) T
2
= T
1
* (V
1
/V
2
)
-1
= 313 * (7)
1.4-1
= 681.68 K
q
in
= C
v
* (T
3
T
2
) i.e. 1200 = 0.718 * (T
3
-681.7) T
3
= 2353.989 K
2) Thermal efficiency = 1-(T
1
/T
2
) = 1- (T
4
/T
3
) = 1- (1/CR
-1
) = 0.5408
3) Work done per cycle = q
in
* thermal efficiency OR W.D. = 1200 * 0.541 = 649 kJ/Kg
Example (2-Otto):
In an Otto cycle, air at 15
o
C and 105 kPa is compressed adiabatically until
the pressure is 1300 kPa. Heat is added at constant volume until the pressure
rises to 3500 kPa. Calculate the air standard efficiency, the compression
ratio, the clearance and stroke volume and the mean effective pressure. Take
C
v
= 0.718 kJ/kg.K and R=0.287 kJ/kg.K
Solution : given, P1 = 105 kPa, T1 = 288 K, P2 = 1300 kPa, P3 = 3500 kPa.
1) (V
1
/V
2
) = (P
2
/P
1
)
1/
OR CR = (1300/105)
0.715
= 6.033
2) Thermal efficiency = 1- (1/CR
-1
) = 0.512
3) Mean Effective Pressure = WD/V
S
Further, WD = C
v
[ (T
3
T
2
)] *
From Process (1-2) T
2
= T
1
* (CR
-1
) = 288 * (6.033)
1.4-1
= 591.025 K
From Process (2-3) (P
3
/ T
3
) = (P
2
/ T
2
) T
3
= 1591.22 K
q
in
= 0.718 * (1591.22 591.025) = 1000.196 kJ/kg
WD = q
in
* Ther. Eff. = 512.1 kJ/Kg
V1 = m * R * T1 / P1 = (1 * 0.287 * 288 )/(105) = 0.7872 m
3
/kg
Clearance volume = V
2
= V
1
/CR = 0.787/6.033 = 0.1304 m
3
/kg
Displacement volume = V
1
V
2
= 0.6568 m
3
/kg
Mean Effective Pressure = WD/Vs = (367.688)/(0.6568) = 779.69 kPa
The Constant Pressure or Diesel Cycle.
This is a theoretical cycle for a slow speed engines using diesel as a fuel.
The basic difference between it and the Otto cycle is that heat is added at constant
pressure while in the case of Otto cycle, heat is added at constant volume.
In this cycle, the compression ratio (V
1
/V
2
) the expansion ratio (V
4
/V
3
).
Q
add
P 2 3 Temperature 3
Reversible Adiabatic Q
add
PV
=Constant 4
2
V
3
4 Q
rej
V
C
=V
2
Q
rej
1
V
S
= V
1
-V
2
1
5 6 Volume 5 6 Entropy
W.D. in compression 1-2 = area 1256 Heat added during 2-3 = area 2365
W.D. in expansion 2-4 = area 23465 Heat rejected during 4-1 = area 4156
Net W.D. = area 1234 Net Heat = area 1234
Working Principle of Ideal Diesel Cycle. (Explain)
Process (1-2) : Adiabatic Compression of air from V1 to V2.
1
Q
2
= 0.
Process (2-3) : Constant Pressure heat addition.
2
W
3
0.
Process (3-4) : Adiabatic Expansion of air from V3 to V4.
3
Q
4
= 0.
Process (4-1) : Constant Volume heat rejection.
4
W
1
= 0.
After the air is introduced into the engine cylinder during the suction stroke
(not shown here), it immediately undergoes isentropic compression from (1)
to (2) as the piston moves from the BDC (V
1
) to the TDC (V
2
). This results in
volume reduction and pressure as well as temperature rise. Heat is then
added instantaneously at constant pressure (process 2-3) and the piston is
momentarily moves slightly away from TDC.
This raises the pressure and temperature further. This expansion of the air
pushes the piston from TDC to BDC where power is produced and given to its
consumption point. To complete this ideal air power cycle, the working fluid
has to return back to its initial state that is state point (1). This requires that
the air reject its heat, which occur at constant volume when the piston is at
BDC and then the cycle is repeated.
Mathematical Analysis of the Cycle.
Let us take m= 1 Kg.
Heat added during process 2-3 is = C
P
(T
3
T
2
) kJ/Kg
Heat Rejected during the process 4-1 is = C
V
(T
5
T
1
) kJ/Kg
Therefore, the net work done is W
net
= C
P
(T
3
T
2
) - C
V
(T
5
T
1
) kJ/Kg
Calculation of Thermal Efficiency.
Thus the thermal efficiency
th
is
1
2
T
3
T
1
1
T
4
T
2
T
1
T
1
)
2
T
3
(T
)
1
T
4
(T
1
- 1
add
Q
rej
Q
1
add
Q
rej
Q
add
Q
Supplied Heat
Done Net Work
th
,
_
,
_
Now,
1
4
V
3
V
3
T
4
T
and ,
1
1
V
2
V
2
T
1
T
,
_
,
_
also, V
4
= V
1
Thus,
1
T
2
T
2
T
3
T
3
T
4
T
1
T
4
T
=
1
1
V
2
V
4
V
3
V
2
T
3
T
,
_
1
T
4
T
=
( )
1
V
V
2
V
3
V
2
3
,
_
,
_
2
V
3
V
Hence the equation for thermal efficiency can be written as :
1
1
1
1
1
1
1
]
1
,
_
,
_
,
_
1
2
3
1
2
3
1
2
1
1
1
V
V
V
V
V
V
th
or, finally the equation for thermal efficiency could be written as:
1
1
]
1
1) (r
1
r
1
CR
1
1
th
Analysis of the results.
From the figures below we can conclude the following:
1. Thermal efficiency of Diesel cycle differs from that of Otto cycle by the amount inside
the bracket which is always > 1. Hence
th Diesel
<
th Otto
.
2. Thermal efficiency is a function of the cut-off ratio () which is in turn dependent on
engine load being maximum for maximum load. As the cut-off ratio increases, thermal
2
V
3
V
2
T
3
T
; 3 - 2 process pressure constant the from Further
efficiency decreases. Further, if > 10% of the stroke smoke will tend to appear
because lesser time would be available for the combustion to be completed by the time
EVO.
3. Unlike Otto cycle, the efficiency of diesel cycle decreases as the engine load
decreases.
4. In actual Practice,
th Diesel
>
th Otto
.This is due to the fact that diesel cycles uses
higher compression ratios than Otto cycles.
100 65
80 r=1 (Otto Cycle) CR = 22
r=2
60 r=3 50
CR = 18
40 CR = 14
20 35
0.0 5 10 12 15 20 25 0 2 4 6 8 10
Compression Ratio (CR) Cut-Off Ratio (r)
Example (1-Diesel) :
An air standard diesel cycle has a compression ratio of 14. The pressure at
the beginning of the compression stroke is 100 kPa and the temperature is
27
o
C.The maximum temperature is 2500
o
C. Determine the state of the
system at all points hence the thermal efficiency and mean effective pressure
of the cycle.
Solution:
Given: CR = 14, T1 = 300
o
K, P1 = 100 kPa and T3 = 2773
o
K.
At state point (1):
T
1
= 300
o
K, P
1
= 100 kPa V1 = R*T
1
/P
1
= 0.287 * 300 / 100 = 0.861 m
3
/Kg
At state point (2):
T
2
= T
1
* (V
1
/V
2
)
(-1)
= 300 * 14
0.4
= 300 * 2.88 = 862.129
o
K.
P
2
= P
1
* (T
2
/T
1
)
/-1
= 100*(862.129/300)
3.5
= 100*40.232 = 4023.2 kPa.
V
1
/V
2
= 14 V
2
= 0.861/14 = 0.0615 m
3
/Kg
At state point (3):
P
2
= P
3
= 4023.2 kPa (constant pressure process) and T3 = 2773
o
K.
V
3
/V
2
= T
3
/T
2
= 2773/862.129 = 3.216 V3 = 3.216*0.0615 = 0.1978 m
3
/Kg
At state point (4):
V
4
= V
1
= 0.861 m
3
/Kg (constant volume process).
T
3
/T
4
= (V
4
/V
3
)
-1
= (V
4
/V
2
* V
2
/V
3
)
-1
= (CR * 1/r) = (14 * 1/3.216)
0.4
= 4.36
0.4
= 1.801
Hence, T
4
= 2773/1.801 = 1539.66
o
K.
P
1
/T
1
= P
4
/T
4
P
4
= P
1
* (T
4
/T
1
) = 100*(1539.66/300) = 513.22 kPa
OR we can also use the adiabatic relation P3/P4 = (T3/T4)
/-1
(Check).
Thermal efficiency ( ) = 1 [(T
4
-T
1
)/ (T
3
-T
2
)]
= 1 [(1539.66-300)/1.4*(2773-862.129)] = 1 0.4633 = 0.5366
OR we can use the other relation
1
1
]
1
1) (r
1
r
1
CR
1
1
th
.
Mean Effective Pressure = Net work done per cycle/Stroke volume
= (q
in
q
out
)/ (V
1
V
2
)
= [(C
p
(T
3
-T
2
) C
v
(T
4
-T
1
)) / (V
1
V
2
)]
= [1.005*(2773-862.129)-0.718*(1539.66-300)]/0.7995 = 1288.39 kPa
The Brayton or Joule Cycle.
This is a theoretical cycle in which heat exchange takes place at constant pressure.
P T 3
Q
add
Q
add
2 3 Isothermal
PV=C
2 4
1 Q
rej
1 4
Q
rej
V S
W.D. in compression 1-2 = area under 12 Heat added during 2-3 = area under 23
W.D. in expansion 3-4 = area under 34 Heat rejected during 4-1 = area under 41
Net W.D. = area 1234 Net Heat = area 1234
Working Principle of Ideal Diesel Cycle. (Explain)
Process (1-2) : Isothermal Compression of air from V1 to V2.
1
Q
2
= 0.
Process (2-3) : Constant Pressure heat addition.
2
W
3
0.
Process (3-4) : Isothermal Expansion of air from V3 to V4.
3
Q
4
= 0.
Process (4-1) : Constant Pressure heat rejection.
4
W
1
= 0.
Mathematical Analysis of the Cycle.
Let us take m= 1 Kg.
Heat added during process 2-3 is = h3-h2 = C
P
(T
3
T
2
)
Heat Rejected during the process 4-1 is = h4-h1 = C
P
(T
4
T
1
)
Therefore, the net work done is W
net
= C
P
[ (T
3
T
2
) - (T
4
T
1
) ]
Thus the thermal efficiency
th
is
)
2 3
(
)
1 4
(
- 1
add
Q
rej
Q
1
add
Q
Supplied Heat
Done Net Work
T T
T T
add
Q
rej
Q
th
Now, let us represent all temperatures in terms of T
1
and T
4
.
( ) ( )
1 -
r
3
T
4
T can write we also and
1 -
r
2
T
1
T
substituting into main equation and simplifying we can finally write the equation for thermal
efficiency could be written as:
1
1
1
r
th
Making use of the constant pressure processes, we can write the equation as a function of
the pressure ratio (r
P
= P2/P1) as follows:
4
T
3
T
) 1 (
4
P
3
P
) 1 (
1
P
2
P
1
T
2
T
,
_
,
_
, since (P2 = P3 and P1 = P4)
Therefore we can write ; (T4/T1) = (T3/T2)
From which we can write :
th
2
T
1
T
- 1
1)
2
T
3
T
(
2
T
1)
1
T
4
T
(
1
T
- 1
)
2
T
3
(T
)
1
T
4
(T
- 1
th
1) (
2
P
1
P
- 1
2
T
1
T
- 1
,
_
From which we can write the final equation in terms of pressure ratio as follows:
1)/ (
P
r
1
1
th
Analysis of the result.
The effect of the pressure ratio is clearly shown in the following Figures :
0.6 2 CC 3
0.3 C T
0
0 2 4 6 8 10 12 1 4
Example (1-Brayton):
In a gas turbine air is drawn in at 102 kPa and 15
o
C and is compressed to 612 kPa.
Calculate the thermal efficiency and the work ratio of the ideal constant pressure cycle
when the maximum cycle temperature is limited to 800
o
C. Take =1.4 and C
P
=1.005.
Solution :
Given : P1 = 102 kPa, T1 = 288
o
K and T3 = 1073
o
K.
At state point (1) :
P1 = 102 kPa, T1 = 288
o
K
Taking for a unit mass we can calculate V1 = R*T1/P1 = 0.287*288/1.02*100
V1 = 0.81 m
3
/Kg
At state point (2) :
P2 = 612 kPa
4
T
3
T
) 1 (
4
P
3
P
) 1 (
1
P
2
P
1
T
2
T
,
_
,
_
1.67
0.286
6
4 . 1
) 1 4 . 1 (
102
612
4
T
3
T
1
T
2
T
,
_
T2 = 1.67*288 = 481
o
K
Taking for a unit mass we can calculate V2 = R*T1/P1 = 0.287*481/6.12*100
V2 = 0.2255 m
3
/Kg
At state point (3) :
P3 = P2 = 612 kPa, T3 = 1073
o
K
Taking for a unit mass we can calculate V3 = R*T3/P3 = 0.287*1073/6.12*100
V3 = 0.503 m
3
/Kg
At state point (4) :
P4 = P1 = 102 kPa
T4 = T3/1.67 = 1073/1.67 = 643
o
K
Taking for a unit mass we can calculate V4 = R*T4/P4 = 0.287*643/1.02*100
V4 = 1.845 m
3
/Kg
Thermal efficiency = 1 1/(r
P
(-1)/
) = 0.403 Ans.
Work done by turbine = C
P
*(T
3
T
4
) = 432 kJ/Kg
Work consumed by compressor = C
P
*(T
2
T
1
) = 194 kJ/Kg
Net work = W
net
= C
P
[ (T
3
T
4
) - (T
2
T
1
) ] = 238 kJ/Kg
Back Work Ratio = W
C
/ W
T
= 194/238 = 0.815 Ans.
Вам также может понравиться
- Solution Manual for an Introduction to Equilibrium ThermodynamicsОт EverandSolution Manual for an Introduction to Equilibrium ThermodynamicsОценок пока нет
- PNEUMATICS AND AIR CIRCUITS UNDERSTANDING THE CASCADE VALVE AND PLC UNDERSTANDINGОт EverandPNEUMATICS AND AIR CIRCUITS UNDERSTANDING THE CASCADE VALVE AND PLC UNDERSTANDINGОценок пока нет
- MODULE 3-ICE CyclesДокумент4 страницыMODULE 3-ICE CyclesNigel Ceasar SilvaОценок пока нет
- Carnot's TheorreДокумент12 страницCarnot's Theorremr shantosОценок пока нет
- Thermodynamics All DerivationsДокумент8 страницThermodynamics All Derivationsanuragrabha99Оценок пока нет
- Chapter 6 - IC EnginesДокумент12 страницChapter 6 - IC EnginesAbhijitОценок пока нет
- Theoretical CyclesДокумент49 страницTheoretical CyclesMariaEzzaSyUyОценок пока нет
- Otto CycleДокумент13 страницOtto Cycleoyetunde ridwanОценок пока нет
- ACTUAL 4 CYCLE, 2 Revolutions/cycle: Compression 1-2 Combustion 2-3 Exhaust 4-1 Intake 0-1 Exhaust 1-0 Power 3-4Документ26 страницACTUAL 4 CYCLE, 2 Revolutions/cycle: Compression 1-2 Combustion 2-3 Exhaust 4-1 Intake 0-1 Exhaust 1-0 Power 3-4Christed Aljo BarrogaОценок пока нет
- Thermo Cycles 2Документ16 страницThermo Cycles 2cobalt boronОценок пока нет
- Air-Std CyclesДокумент39 страницAir-Std CyclesKshitij M BhatОценок пока нет
- 490 ' 266+ 29-3 55-9 KJ (E) Work Ratio - R-: - Positive Work Done 36-5Документ6 страниц490 ' 266+ 29-3 55-9 KJ (E) Work Ratio - R-: - Positive Work Done 36-5Jahangir AliОценок пока нет
- Applied Thermodynamics C211 PDFДокумент173 страницыApplied Thermodynamics C211 PDFSubham AcharyaОценок пока нет
- 02 2ndlaw ExessolsДокумент14 страниц02 2ndlaw Exessolsblanca.pegueraОценок пока нет
- Stirling and EricssonДокумент7 страницStirling and EricssonAJ SiosonОценок пока нет
- Volumetric PropertiesДокумент36 страницVolumetric PropertiesRohan BhilkarОценок пока нет
- Reitlinger Thermodynamic CyclesДокумент5 страницReitlinger Thermodynamic Cyclesrays_nitaОценок пока нет
- Heat Engines: First Law: Perfect GasДокумент21 страницаHeat Engines: First Law: Perfect GasMagdy RiadОценок пока нет
- Science Q4 2Документ2 страницыScience Q4 2kageizander7Оценок пока нет
- 4 EngineCyclesAnalysisДокумент9 страниц4 EngineCyclesAnalysisNatthaphon NaosookОценок пока нет
- ME411 (Ideal Models of Engine Cycles) (160215)Документ13 страницME411 (Ideal Models of Engine Cycles) (160215)Eftaykhar AhmedОценок пока нет
- Thermodynamics - ch4Документ17 страницThermodynamics - ch4Hassan AzouzОценок пока нет
- Alhaji Massoud Juma - Thermo AssignmentДокумент12 страницAlhaji Massoud Juma - Thermo AssignmentAlhaj MassoudОценок пока нет
- 30 C 100 Kpa 500 Kpa 500 Kpa 30 C: 4F-5: Performance of An Ideal Gas Cycle 10 PtsДокумент5 страниц30 C 100 Kpa 500 Kpa 500 Kpa 30 C: 4F-5: Performance of An Ideal Gas Cycle 10 PtsVyan IlhamОценок пока нет
- Gas Power CyclesДокумент17 страницGas Power Cycleskimosave99Оценок пока нет
- Unit-Ii Diesel, Gas Turbine and Combined Cycle Power PlantsДокумент71 страницаUnit-Ii Diesel, Gas Turbine and Combined Cycle Power Plantsrsankarganesh MECH-HICETОценок пока нет
- Advanced Thermodynamics: Volumetric Properties of Pure FluidsДокумент36 страницAdvanced Thermodynamics: Volumetric Properties of Pure FluidsArunodhayam NatarajanОценок пока нет
- Lab 2 Application of SFEE To A Blower Duct System 2021Документ2 страницыLab 2 Application of SFEE To A Blower Duct System 2021nomaan7804Оценок пока нет
- CLS JEEAD-19-20 XI Phy Target-5 Level-2 Chapter-11 PDFДокумент28 страницCLS JEEAD-19-20 XI Phy Target-5 Level-2 Chapter-11 PDFVISHVESH GOYAL100% (1)
- Termodinamika, Entropy, Dan Energi Dalam 2014Документ23 страницыTermodinamika, Entropy, Dan Energi Dalam 2014Deriandra MuhyiddinОценок пока нет
- Balingt - Prelim ExamДокумент21 страницаBalingt - Prelim ExamRene Niño Macamay BalingitОценок пока нет
- Kmme Te101-3Документ6 страницKmme Te101-3ayla sözenОценок пока нет
- Carnot CycleДокумент7 страницCarnot CycleAmey DОценок пока нет
- Example 4.8 Thermal Efficiency of The Otto Engine (Continued)Документ3 страницыExample 4.8 Thermal Efficiency of The Otto Engine (Continued)Sunil MОценок пока нет
- HW2 - 2011 SolutionsДокумент10 страницHW2 - 2011 Solutionsrianto prayogo100% (2)
- Lesson 2.2 Rankine-CycleДокумент12 страницLesson 2.2 Rankine-CycleJarina De EspañaОценок пока нет
- Introduction To The Module and The Carnot CycleДокумент17 страницIntroduction To The Module and The Carnot Cycleأحمد صلاح الدينОценок пока нет
- Gas Power Cycles Study Guide in Powerpoint: To AccompanyДокумент68 страницGas Power Cycles Study Guide in Powerpoint: To AccompanyManjunatha TnОценок пока нет
- Power Cycles 1 - 1 PDFДокумент6 страницPower Cycles 1 - 1 PDFclarkmaxОценок пока нет
- Applied Thermodynamics and Heat Transfer - Unit IДокумент37 страницApplied Thermodynamics and Heat Transfer - Unit IArun ShankarОценок пока нет
- Lecture3b (Compatibility Mode)Документ9 страницLecture3b (Compatibility Mode)Maryam MusnadОценок пока нет
- Gas Power CyclesДокумент140 страницGas Power CyclesMohammed AlsirajОценок пока нет
- Gas Cycles Otto, Diesel, Dual CyclesДокумент43 страницыGas Cycles Otto, Diesel, Dual Cyclesprasad5034100% (1)
- PemampatДокумент49 страницPemampatNURUL SYUHADA BT ISMAIL HAJAR100% (3)
- Term Odin A MicaДокумент10 страницTerm Odin A MicaFelipe De Lima RomeroОценок пока нет
- Ch5 - Internal Combustion EnginesДокумент46 страницCh5 - Internal Combustion EnginesShiau FenОценок пока нет
- Internal Combustion Engines: Compiled by PM Muhammad Abd Razak FKM, UitmppДокумент18 страницInternal Combustion Engines: Compiled by PM Muhammad Abd Razak FKM, UitmppFarisОценок пока нет
- Processes of Ideal GasДокумент14 страницProcesses of Ideal GasKevin Baterina40% (5)
- Web6 Combuction SystemДокумент11 страницWeb6 Combuction SystemeswarbobbyОценок пока нет
- Internal Combustion EngineДокумент20 страницInternal Combustion EngineRowin Mark Sabornido33% (3)
- Gas Power Cycle - Part 1Документ46 страницGas Power Cycle - Part 1Shahran IezzatОценок пока нет
- Internal Combustion EnginesДокумент13 страницInternal Combustion EnginesEunice Joy VillegasОценок пока нет
- 3 Ideal Models of Engine Processes and CyclesДокумент58 страниц3 Ideal Models of Engine Processes and Cyclesdinosaur x-drakeОценок пока нет
- Thermodynamics of IC Engines: Chapter TwoДокумент32 страницыThermodynamics of IC Engines: Chapter Twoashenafi tesfayeОценок пока нет
- Thermodynamic Processes and DerivationДокумент10 страницThermodynamic Processes and DerivationAbenayaОценок пока нет
- Fuel Air and Actual CyclesДокумент39 страницFuel Air and Actual CyclesHrithik SОценок пока нет
- Facts and Formulae SheetДокумент7 страницFacts and Formulae SheethenryОценок пока нет
- 03BAB 3 - COMPRESSOR Class 15 Nov21Документ42 страницы03BAB 3 - COMPRESSOR Class 15 Nov21BernadetteОценок пока нет
- The First Law of Thermodynamics (CHAP. 4Документ5 страницThe First Law of Thermodynamics (CHAP. 4mozam haqОценок пока нет
- The First Law of Thermodynamics (CHAP. 4Документ5 страницThe First Law of Thermodynamics (CHAP. 4mozam haqОценок пока нет
- Steam TurbineДокумент3 страницыSteam TurbinejehadyamОценок пока нет
- Figure 6Документ19 страницFigure 6jehadyamОценок пока нет
- Water CirculationДокумент22 страницыWater CirculationjehadyamОценок пока нет
- Rankine Cycle RevisionДокумент20 страницRankine Cycle RevisionjehadyamОценок пока нет
- Impact of Free JetsДокумент7 страницImpact of Free JetsjehadyamОценок пока нет
- Data VSE 1000-3000-BPДокумент3 страницыData VSE 1000-3000-BPjehadyamОценок пока нет
- Review Problems On Gas TurbineДокумент9 страницReview Problems On Gas TurbinejehadyamОценок пока нет
- Load Curves and CalculationsДокумент23 страницыLoad Curves and CalculationsjehadyamОценок пока нет
- Example To Be Solved HWA PDFДокумент1 страницаExample To Be Solved HWA PDFjehadyamОценок пока нет
- Gas Turbine Power Plant PresentationДокумент126 страницGas Turbine Power Plant Presentationjehadyam100% (1)
- Flow Measurement by Drag Effects: RotameterДокумент3 страницыFlow Measurement by Drag Effects: RotameterjehadyamОценок пока нет
- TURBOMACHINES Pumps PerformanceДокумент35 страницTURBOMACHINES Pumps PerformancejehadyamОценок пока нет
- Lec 5Документ24 страницыLec 5jehadyamОценок пока нет
- ChemKin Tutorial 2-3-7 PDFДокумент274 страницыChemKin Tutorial 2-3-7 PDFXavier NavaОценок пока нет
- Drag Force SensorДокумент2 страницыDrag Force SensorjehadyamОценок пока нет
- Bolt & Screw TerminologyДокумент2 страницыBolt & Screw TerminologyjehadyamОценок пока нет
- An Emission and Performance Comparison of The Natural Gas C-Gas Plus Engine in Heavy-Duty TrucksДокумент40 страницAn Emission and Performance Comparison of The Natural Gas C-Gas Plus Engine in Heavy-Duty Trucksjehadyam0% (1)
- Accredited Logos Style Sheet and Usage GuideДокумент13 страницAccredited Logos Style Sheet and Usage GuidejehadyamОценок пока нет
- Drilling Engineering ExpermeintsДокумент50 страницDrilling Engineering ExpermeintsNourden AlОценок пока нет
- CH 10Документ13 страницCH 10patilamardip0078122Оценок пока нет
- Heat ExxxxxДокумент15 страницHeat ExxxxxZak_DaudОценок пока нет
- PH6251Документ245 страницPH6251Praveen KumarОценок пока нет
- EHV AC & DC TRANSMISSION MCQ Unit - 2 - Math TradersДокумент5 страницEHV AC & DC TRANSMISSION MCQ Unit - 2 - Math TradersRit100% (1)
- PHYS Module 2 WorksheetsДокумент16 страницPHYS Module 2 WorksheetsPrathmesh Sinha100% (1)
- DC & AC Machinery AДокумент9 страницDC & AC Machinery AMark julius garciaОценок пока нет
- The Hall Effect in GermaniumДокумент12 страницThe Hall Effect in GermaniumSanele ZiqubuОценок пока нет
- Construction Vibrations and Their Impact On Vibrat PDFДокумент10 страницConstruction Vibrations and Their Impact On Vibrat PDFAngel PaulОценок пока нет
- Structured Question (Forces in Equilibrium II)Документ3 страницыStructured Question (Forces in Equilibrium II)leelee1127Оценок пока нет
- CRE Lab ManualДокумент19 страницCRE Lab ManualMayursinh Solanki100% (1)
- Cable Sizing and Effects of Cable Length OnДокумент6 страницCable Sizing and Effects of Cable Length Onyao100% (1)
- 01 Density PDFДокумент12 страниц01 Density PDFshoaib akhtarОценок пока нет
- Equations For The Soil-Water Characteristic'curve: D.G. XingДокумент12 страницEquations For The Soil-Water Characteristic'curve: D.G. XingburlandОценок пока нет
- Klinkenberg EffectДокумент4 страницыKlinkenberg EffectQaiser Hafeez89% (9)
- Tutorial 1Документ4 страницыTutorial 1SYAFIQAH ISMAILОценок пока нет
- Basic Electrical Circuits DR Nagendra Krishnapura Department of Electrical Engineering Indian Institute of Technology Madras Lecture - 77Документ8 страницBasic Electrical Circuits DR Nagendra Krishnapura Department of Electrical Engineering Indian Institute of Technology Madras Lecture - 77Vikhyath KstОценок пока нет
- Enhanced Science 8 Portfolio 1 Quarter A.Y 2019-220 Ms. Genel R. YutucДокумент3 страницыEnhanced Science 8 Portfolio 1 Quarter A.Y 2019-220 Ms. Genel R. YutucVenicee EveОценок пока нет
- ASU PHY 131 Homework 2 Fall 2020Документ2 страницыASU PHY 131 Homework 2 Fall 2020Vyomika TewaryОценок пока нет
- Plane Stress - MSE 305-1Документ149 страницPlane Stress - MSE 305-1Oloyede RidwanОценок пока нет
- Layout Solns 3Документ12 страницLayout Solns 3VIKRAM KUMARОценок пока нет
- HW1Документ5 страницHW1Nathan YangОценок пока нет
- ANSI ESD SP15.1-2005 Standard Practice For In-Use Resistance Tetsing of Gloves and Finger CotsДокумент16 страницANSI ESD SP15.1-2005 Standard Practice For In-Use Resistance Tetsing of Gloves and Finger Cotssmalnif0% (1)
- Department of Aeronautical Engineering: Answer All The Questions: Part - A (10 X 2 20 MARKS)Документ2 страницыDepartment of Aeronautical Engineering: Answer All The Questions: Part - A (10 X 2 20 MARKS)RajakumariОценок пока нет
- Piezoelectric MaterialsДокумент4 страницыPiezoelectric MaterialsAswathy CjОценок пока нет
- 2nd-Quarter - DLL Grade 9Документ42 страницы2nd-Quarter - DLL Grade 9STEPHEN MILANОценок пока нет
- Evaluation of Design of Rear AxleДокумент6 страницEvaluation of Design of Rear AxleAbhijeet PandeyОценок пока нет
- 0625 s14 QP 33 PDFДокумент20 страниц0625 s14 QP 33 PDFHaider AliОценок пока нет
- 4-Optical Properties of Materials-Dielectrics and MetalsДокумент36 страниц4-Optical Properties of Materials-Dielectrics and MetalsvenkateshsanthiОценок пока нет
- Daftar PustakaДокумент3 страницыDaftar PustakaAwaliyatun Fhathonatuz ZuhriyahОценок пока нет