Академический Документы
Профессиональный Документы
Культура Документы
COX3
Загружено:
Masludi S SopriyadiИсходное описание:
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
COX3
Загружено:
Masludi S SopriyadiАвторское право:
Доступные форматы
COMMENTARY
Rashid A, Khanna A, Gowar JP, Bull JP. Revised estimates of mortality from burns in the last 20 years at the Birmingham Burns Centre. Burns 2001; 27: 72330. British Association of Plastic Surgeons. National burn care review. 2001: http://www.baps.co.uk (accessed Mar 17, 2003).
COX-3: enigma behind COX-1
Phospholipids Arachidonic acid
COX-1 antibody or molecular in-situ probes do not distinguish between COX-1 and COX-3:
COX-3: just another COX or the solitary elusive target of paracetamol?
In 1899, acetylsalicylic acid, aspirin, was one of the first analgesic and anti-inflammatory drugs. However, it took a further 70 years and at least a dozen new drugs, many of which are still widely used today, such as indometacin and ibuprofen, before the common mechanism of action of all these drugs was established. In 1971, John Vane identified cyclo-oxygenase (COX-1) as a molecular target.1 Inhibition of COX-1 and consecutive reduction of prostaglandins and thromboxanes (figure) explained both the pharmacological activity (analgesic, anti-inflammatory, antipyretic, and antiplatelet) and side-effects (gastrointestinal ulceration). However, some questions still remained unanswered. Metamizole and paracetamol (acetaminophen), two wellestablished analgesic and antipyretic drugs, had only weak inhibitory activity against COX and no antiplatelet effect, suggesting a distinct mode of action. In 198992, a new isoform, COX-2, was discovered, which offered a simple and attractive hypothesis.2 The constitutively expressed COX-1 is the housekeeping enzyme, responsible for physiological activities of prostaglandins while COX-2, whose expression is induced under inflammatory conditions, is responsible for pathological prostaglandins that produce pain and high temperature. On the basis of these observations, COX-2-selective drugs were rapidly developed and became one of the most commercially successful class of drugs. However, after only a few years of clinical use, the COX-2 hypothesis turned out to be more complicated than initially thought. Both COX-1 and COX-2 have physiological and pathological roles.3 COX-2 inhibitors, which have a better gastrointestinalsafety profile (mucosal protection), were found to have cardiovascular and renal side-effects.4 For some years, there were rumours of the existence of further isoforms. An anti-inflammatory COX-3 was postulated by Willoughby et al5 as an inducible isoenzyme expressed in later, resolving stages of inflammation. During the past decade, Brune and Botting6 independently speculated on the existence of a COX-3 in the brain, mainly responsible for pain and high temperature. It took until 2002, and Chandrasekharan and colleagues from Dan Simmons group,7 to identify COX-3 (which is not a genetic isoform like COX-2) in the brain. Alternative splicing generates at least four different mRNA variants derived from the COX-1 gene; COX-3, COX-1, and the two truncated, partial PCOX-1 sequences, PCOX-1a and PCOX-1bencoding novel variants of the COX-1 protein family. However, apart from COX-1, Chandrasekharan and colleagues7 found that only canine COX-3a 65 kDa membrane-bound proteinhas glycosylation-dependent cyclo-oxygenase activity. Human COX-3 is a 52 kb transcript encoded on chromosome 9 that is expressed in specific tissues with highest levels in the brain (reaching 5% of the total amount of COX-1 mRNA) and in the heart.7 In the brain Chandrasekharan and colleagues find that the truncated, partial COX-1 mRNAs (PCOX-1) are equally expressed. COX-3 harbours the COX-1 structure with retained intron 1 and a signal peptide (figure). These investigators
COX-1 PGG2 COX PGH2 PGE2, prostacyclin, thromboxane. COX-3
1
Structure of catalytic active COX-1 gene-splice variants redrawn from reference 7. COX-3 comprises COX-1 . structure including catalytic structure (C), retaining intron 1 (1). PGG2=prostaglandin G2, PGH2= prostaglandin H2.
also claim that COX-3 mRNA is abundantly expressed in the mature brain and in the spinal cord, whereas it is absent in fetal central nervous system tissue. In earlier reports a COX-1/PGHS-1 (prostaglandin synthase 1) transcript of almost similar length (51 kb) to COX-3 was shown to be expressed by monocytes8 and endothelial cells.9 The presence of COX-3 mRNA in the adult human brain, but lack of expression in fetal tissue, identified COX-3 as a conserved differentiationassociated protein of the human central nervous system. Retention of intron 1 could alter folding and may affect dimerisation and the active site, mediating structural changes.7 Therefore, although COX-3 contains all the COX-1 transcript, the retained intron sequence could significantly alter its enzymatic properties as shown by a lower potency (1/5th) in generating PGE2. That Chandrasekharan and colleagues find COX-3 to act differently from COX-1 is further emphasised by a 310 fold higher IC50 for diclofenac or ibuprofen to block COX-1 compared with its ability to inhibit COX-3 (IC50 is the concentration of enzyme needed to inhibit production of substrate by 50%). The different IC50 values between COX-1 and canine COX-3 imply a distinct active centre in COX-3 and thus suggest the feasibility of inhibiting COX-3 specifically. With the discovery of COX-3, attention turns back to COX-1. Was there any specific detection of COX-1 in the past, since available antibodies do not distinguish between COX-1 and COX-3, and detection proposed as specific for COX-1 also included COX-3 signals (figure)? Moreover, results from homozygous mice with a knockedout gene locus for COX-110 have to be reassessed since they also represent COX-3 knockouts. Because COX-3 is a spliced COX-1 variant, a specific COX-3 knock-out (not distinguishing between COX-3 or PCOX-1a) is probably unachievable in practice. The most significant implication of Chandrasekharan and colleagues findings is that multiple COX-1 isoenzymes could be derived from just one gene providing a range of COX enzymes and products, at least in the dog.11 Other than the option of inhibiting COX-1 or COX-3 at the protein level, it should be possible to modify COX-1 splicing at the RNA level, leading to the generation of enzymatically inactive PCOX-1a or PCOX-1b variants. COX-3 inhibition by paracetamol is not specific and only weak, and therefore does not completely solve the mystery of paracetamol being analgesic without affecting COX-1 or COX-2. This finding opens up the search for further COX variants or paracetamol-inducible antipyretic and analgesic-acting proteins. COX-3 does not appear to be the solitary elusive target of paracetamol. So what does COX-3 offer for the future? The answer is 981
THE LANCET Vol 361 March 22, 2003 www.thelancet.com
For personal use. Only reproduce with permission from The Lancet Publishing Group.
COMMENTARY
probably more insight into the analgesic activity of COX inhibition. Paracetamol seems to be an inhibitor of canine COX-3, but the IC50 for inhibiting COX-3 is high and difficult to achieve with an oral dose of 0510 g. More interestingly, well-established COX inhibitors, such as ibuprofen, diclofenac, or indometacin that are widely used as analgesics, show the most powerful COX-3 inhibition. However, the limited number of drugs tested so far allows no final conclusions. Another interesting observation is that selective COX-2 drugs have no effect on COX-3 (data not shown by Chandrasekharan and colleagues but mentioned in the text). COX-2-selective drugs are suspected to be less analgesic than unselective COX-1 or COX-2 inhibitors like ketoprofen,12 naproxen, or diclofenac.13 An important future aspect of human COX-3 research might be the promise of a new target for analgesic drug discovery, enriching the oversimplified COX-1 and COX-2 debate.
JMS is supported by the Young Scientists Program of the German Israeli Foundation for Scientific Research and Development (GIF) no G-20241065.1/2000.
*Jan M Schwab, Hermann J Schluesener, Stefan Laufer
Institute of Brain Research, Medical School, D-72076 Tbingen, Germany (JMS, HJS); and Institute of Pharmacy, University of Tbingen, Tbingen (SL) (e-mail: jmschwab@med.uni-tuebingen.de) 1 2 3 Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol 1971; 23: 23235. Crofford LJ. COX-1 and COX-2 tissue expression: implications and predictions. J Rheumatol 1997; 24 (suppl 49): 1519. Laulederkind SJ, Thompson-Jaeger S, Goorha S, et al. Both constitutive and inducible prostaglandin H synthase affect dermal wound healing in mice. Lab Invest 2002; 82: 91927. Bombardier C, Laine L, Reicin A, et al. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis: VIGOR Study Group. N Engl J Med 2000; 343: 152028. Willoughby DA, Moore AR, Colville-Nash PR. COX-1, COX-2, and COX-3 and the future treatment of chronic inflammatory disease. Lancet 2000; 355: 64668. Botting RM. Mechanism of action of acetaminophen: is there a cyclooxygenase 3? Clin Infect Dis 2000; 31 (suppl 5): S20210. Chandrasekharan NV, Dai H, Roos KL, et al. COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: cloning, structure, and expression. Proc Natl Acad Sci USA 2002; 99: 1392631. Plant MH, Laneuville O. Characterization of a novel transcript of prostaglandin endoperoxide H synthase 1 with a tissue-specific profile of expression. Biochem J 1999; 344: 67785. Hla T. Molecular characterization of the 52 KB isoform of the human cyclooxygenase-1 transcript. Prostaglandins 1996; 51: 8185. Langenbach R, Morham SG, Tiano HF, et al. Prostaglandin synthase 1 gene disruption in mice reduces arachidonic acid-induced inflammation and indomethacin-induced gastric ulceration. Cell 1995; 83: 48392. Warner TD, Mitchell JA. Cyclooxygenase-3 (COX-3): filling in the gaps toward a COX continuum? Proc Natl Acad Sci USA 2002; 99: 1337173. Mazario J, Gaitan G, Herrero JF. Cyclooxygenase-1 vs cyclooxygenase-2 inhibitors in the induction of antinociception in rodent withdrawal reflexes. Neuropharmacology 2001; 40: 93746. US Food and Drug Administration Arthritis Advising Committee, Dec 1, 1997: http://www.fda.gov/ohrms/dockets/ac/98/slides/ 3480s1d.pdf (accessed on March 17, 2003).
6 7
10
11
12
13
leaked news that in the final adventure our hero succumbs to Dark Forces and is condemned to eternity as a retarded adolescent on the film set of Lord of the Rings. And a first edition fetches 25 000. Only the last is true but with pottermania, an affliction to which I am immune, anything is possible. True, the Philosophers Stone film did provide a welcome diversion from plastic airline food but after a few dozen pages of the text of that and a short-lived stab at Chamber of Secrets it seemed reasonable to abandon both tales to a more appreciative age group. Yet otherwise sensible adults do worship at the feet of this precocious child, and numbered among the obsessed is the science correspondent of Londons Daily Telegraph.1 Roger Highfield draws on his considerable personal knowledge and the views of 100 consultants from the sciences to seek rational explanations for happenings that Master Potter and the Hogwarts school would see as magic. This is all good entertainment and the authors skills in getting across sometimes difficult science are amply demonstrated. However, Highfields extrapolations often have to be as elastic as the boot of a Weasley Ford Anglia. Teleportation by Austrian physicists of the quantum state of a photon sounds far short of Beam me up, Scottie. As a model for Quidditch we are invited to look at the ritualised violence of a low-scoring ballgame popular in Mesoamerican civilisations. From what I have read about these two bizarre pastimes the differences outweigh the similarities, and the Eton wall game would have served as well. We canwell, I cant but you know what I meanproject bricks onto a foggy screen and walk through them but what sort of fog holds up a station roof? Highfield rightly hopes that his revelations will not disillusion the young reader. His publisher, though, sees this book as the perfect guide for parents who want to teach their children science through the adventures of their favourite hero. No child worthy of the name would fall for that, surely. Lois Gresh and Robert Weinbergs publisher does not make this mistake.2 These authors, in doing for Batman, Spider-Man, the Incredible Hulk, and other superheroes of the comic strip what Highfield does for Potter, distance themselves from Hogwarts. Supernatural and magical characters, they declare cant be explained by logic or scientific expertise. However, the two books are not that far apart really. The change from high school weakling to the surprised possessor of magic powers is a publicity quote, not for Rowlings skinny hero but on a video of Spider-Man the movie. Furthermore, both books pay tribute to the late Carl Sagans Contact, a literary experiment in space/time travel, and they note the advisory role of CalTech professor Kip Thorne and the grandfather paradox in which you return in time and kill your grandfather before your father is conceived. Gresh and Weinberg are happy to concede when science fails to explain some fantastic deed, and this book is intriguing for the light it throws on readers taste and the development of this type of comic strip from the first appearance of Superman in the 1930s. David Sharp
c/o The Lancet, London NW1 7BY, UK 1 Highfield R. The science of Harry Potter. New York and London: Viking Penguin, 2002. Pp 322. ISBN 0-670-03153-4. US$23.95, 12.99. Gresh L, Weinberg R. The science of superheroes. Hoboken, NJ, and Chichester, UK: Wiley, 2002. Pp 200. ISBN 0-471-02460-0. US$24.95, 14.99.
Hogwash from Hogwarts
Boys are admitted to hospital with head injuries after running luggage trolleys into a railway station wall. An American college accepts a PhD on Ovidian Metamorphosis and Post-Modernist Influences in Writings of J K Rowling. Schoolgirl hysteria follows the 982
THE LANCET Vol 361 March 22, 2003 www.thelancet.com
For personal use. Only reproduce with permission from The Lancet Publishing Group.
Вам также может понравиться
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeОт EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeРейтинг: 4 из 5 звезд4/5 (5794)
- The Yellow House: A Memoir (2019 National Book Award Winner)От EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Рейтинг: 4 из 5 звезд4/5 (98)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryОт EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryРейтинг: 3.5 из 5 звезд3.5/5 (231)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceОт EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceРейтинг: 4 из 5 звезд4/5 (895)
- The Little Book of Hygge: Danish Secrets to Happy LivingОт EverandThe Little Book of Hygge: Danish Secrets to Happy LivingРейтинг: 3.5 из 5 звезд3.5/5 (400)
- Never Split the Difference: Negotiating As If Your Life Depended On ItОт EverandNever Split the Difference: Negotiating As If Your Life Depended On ItРейтинг: 4.5 из 5 звезд4.5/5 (838)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureОт EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureРейтинг: 4.5 из 5 звезд4.5/5 (474)
- The Emperor of All Maladies: A Biography of CancerОт EverandThe Emperor of All Maladies: A Biography of CancerРейтинг: 4.5 из 5 звезд4.5/5 (271)
- Team of Rivals: The Political Genius of Abraham LincolnОт EverandTeam of Rivals: The Political Genius of Abraham LincolnРейтинг: 4.5 из 5 звезд4.5/5 (234)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaОт EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaРейтинг: 4.5 из 5 звезд4.5/5 (266)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersОт EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersРейтинг: 4.5 из 5 звезд4.5/5 (344)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyОт EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyРейтинг: 3.5 из 5 звезд3.5/5 (2259)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreОт EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreРейтинг: 4 из 5 звезд4/5 (1090)
- The Unwinding: An Inner History of the New AmericaОт EverandThe Unwinding: An Inner History of the New AmericaРейтинг: 4 из 5 звезд4/5 (45)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)От EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Рейтинг: 4.5 из 5 звезд4.5/5 (121)
- DNA Denaturation: Biochemistry Department. Course Code:701 Instructor: Dr/OmneyaДокумент16 страницDNA Denaturation: Biochemistry Department. Course Code:701 Instructor: Dr/OmneyaahmedalynasrОценок пока нет
- Emma 130805031509 Phpapp02 PDFДокумент52 страницыEmma 130805031509 Phpapp02 PDFBheru LalОценок пока нет
- Operating Manifold Services in Hospitals: A Costly Affair?: Kumar P, Gupta SK, Sharma DK, Batra RKДокумент9 страницOperating Manifold Services in Hospitals: A Costly Affair?: Kumar P, Gupta SK, Sharma DK, Batra RKPankaj Mittal100% (1)
- Patient Transfer Policy 3.1Документ25 страницPatient Transfer Policy 3.1Innas DoankОценок пока нет
- High Power CommitteeДокумент22 страницыHigh Power CommitteeTHONDYNALUОценок пока нет
- Socialization and Career Development and Professional Association (Pearl Grace B. Romeo)Документ9 страницSocialization and Career Development and Professional Association (Pearl Grace B. Romeo)garaОценок пока нет
- ACEM Training HandbookДокумент186 страницACEM Training HandbookBlackhawk1976Оценок пока нет
- Jammu Kashmir PSC RecruitmentДокумент15 страницJammu Kashmir PSC RecruitmentHiten BansalОценок пока нет
- A Comparative Study of Job Satisfaction of Government and Private Hospitals - For MRS - AroraДокумент87 страницA Comparative Study of Job Satisfaction of Government and Private Hospitals - For MRS - Arorakamleshaarora100% (6)
- MBTI - Personality Type Medical SpecialtiesДокумент66 страницMBTI - Personality Type Medical SpecialtiesCorneliu Coman100% (1)
- How To Pass MRCOG Part 3Документ5 страницHow To Pass MRCOG Part 3Be MRCOG100% (1)
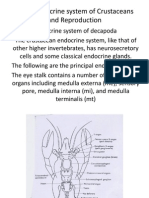
- Neuroendocrine of CrustaceaДокумент29 страницNeuroendocrine of CrustaceaAnirudh Acharya100% (1)
- Guidelines For Distt Hospitals - Indian Public Health StandardsДокумент124 страницыGuidelines For Distt Hospitals - Indian Public Health StandardsTejinder SinghОценок пока нет
- Filipino Nurse Code of EthicsДокумент3 страницыFilipino Nurse Code of EthicsPaulo de Jesus100% (3)
- Clinical 20manual 20for 20oral 140206021148 Phpapp02 PDFДокумент561 страницаClinical 20manual 20for 20oral 140206021148 Phpapp02 PDFAnca MartinoviciОценок пока нет
- Narayana Hrudayalaya Cardiac Care Hospital For The Poor: Leadership Case Study Analysis and Key Lessons For JordanДокумент11 страницNarayana Hrudayalaya Cardiac Care Hospital For The Poor: Leadership Case Study Analysis and Key Lessons For JordanSuhaib KhanОценок пока нет
- Field Trip 2Документ13 страницField Trip 2Raghvendra Singh KhichiОценок пока нет
- Aoa Accredited Fellowship Website Summary: Title of Fellowship Joint Replacement & Tumour Fellowship (Nsw18399)Документ2 страницыAoa Accredited Fellowship Website Summary: Title of Fellowship Joint Replacement & Tumour Fellowship (Nsw18399)SaurabhОценок пока нет
- Patho A 1. 1 The Cell As A Unit of Health and Disease (2015)Документ8 страницPatho A 1. 1 The Cell As A Unit of Health and Disease (2015)Ellese Say100% (2)
- Penis Size - Does It Matter ?Документ4 страницыPenis Size - Does It Matter ?Dr. Muhammad TayubОценок пока нет
- Doctor's ListДокумент10 страницDoctor's ListMrinal ChakrabortyОценок пока нет
- GEHealthcare LOGIQ P Series Transducer GuideДокумент3 страницыGEHealthcare LOGIQ P Series Transducer GuideGigi DuruОценок пока нет
- Basic Surgical Skills Manual PDFДокумент66 страницBasic Surgical Skills Manual PDFMihai MihalacheОценок пока нет
- PrepositionsДокумент4 страницыPrepositionsJelai M. BarreraОценок пока нет
- Appendix B. ESI Triage Algorithm, v.4Документ2 страницыAppendix B. ESI Triage Algorithm, v.4damaris1Оценок пока нет
- Bachelor of Science in MidwiferyДокумент6 страницBachelor of Science in MidwiferyJonas Marvin Anaque67% (3)
- Fa Q Internship ProgramДокумент5 страницFa Q Internship ProgramMariam A. KarimОценок пока нет
- Pres Handbook 2016Документ46 страницPres Handbook 2016Ahmed GendiaОценок пока нет
- Master of Surgery (Surgical Oncology) - Courses - The University of SydneyДокумент4 страницыMaster of Surgery (Surgical Oncology) - Courses - The University of SydneyErlan SantosОценок пока нет
- NPMCN Act PDFДокумент8 страницNPMCN Act PDFAnonymous 9QxPDpОценок пока нет