Академический Документы
Профессиональный Документы
Культура Документы
PS13 Sol
Загружено:
Truong CaiОригинальное название
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
PS13 Sol
Загружено:
Truong CaiАвторское право:
Доступные форматы
3.091 Introduction to Solid-State Chemistry - Fall 2013 Problem Set 13 Evaluation to be held on the final 1.
There are two stereo isomers of butene, cis 2-butene and trans-2-butene. Which molecule has a larger dipole moment? Which has higher boiling point?
2. Two specimens of polyethylene, one composed of linear chains and the other composed of branched chains, are solidified by identical cooling from the melt. The degree of polymerization is the same for both specimens. What is more likely to be suitable for use as a plastic food wrap?
3. Draw a trimer of isotactic polypropylene
4. Acrylonitrile, H2C=CH-CN, can be reacted by addition polymerization to form polyacrylonitrile (PAN). Draw a segment of syndiotactic PAN showing four repeat units.
5. 1-chlorobutene, ClHC=CH-CH2-CH3, can be reacted to form isotactic polychlorobutene (PCB) a. Does the reaction occur by addition or condensation? b. Draw a trimer of isotactic PCB c. What is the value of the degree of polymerization, n, of isotactic PCB with a molecular weight of 3 x 105 g/mole? d. Is isotactic PCB a thermoset or a thermoplastic? Explain e. Draw a schematic representation of the temperature dependence of the volume of linear isotactic PCB and branched PCB assuming both to be of comparable molecular weight. Label the T g of both substances.
6. Calculate the root mean squared length of a polypropylene molecule with molecular weight of 100,000 gm/mole.
7. A particularly strong rigid polyester used for electronic parts is marketed under the trade name Glyptal. It is a polymer of terephthalic acid and glycerol. Draw a segment of the polymer and explain why it is so strong.
8. Explain why a much stiffer foam is obtained if small amount of glycerol is added to the reaction mixture of toluene-2-6-diisocyanate and ethylene glycol during synthesis of a polyurethane foam.
9. Estimate the surface energy of the (100) face of Rh crystals given just the information in the periodic table.
10. Estimate the surface energy of the (110) face of K crystals given just the information in the periodic table.
11. How much larger will the surface energy be for a (100) face of Pt crystal if it is miss cut by 5 degrees?
12. What facets do you expect to appear on single crystals of Pt? What will be the equilibrium shape for these platinum crystals?
13. Two crystals of Pd are sitting next to one another. One has overall dimension of one micron the other has overall dimension of five microns. Which crystal has the larger energy per unit volume? How could the total energy be decreased?
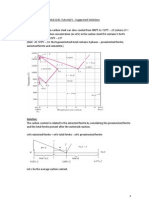
14. The surface tension of an aqueous solution of a molecule is measured as a function of concentration. The data is shown below. Explain what is happening and why. How will the appearance of the solution differ at 0.001 wt% versus 1 wt.%?
15. Sketch how you expect the conductivity of an aqueous solution of SDS (CH3(CH2)11OSO3Na) to vary with concentration. Label all the relevant features of your sketch.
16. Sketch how you expect the surfactant surface concentration at the air water interface to change with aqueous solution concentration of the surfactant. Explain all relevant features of the sketch.
17. A surfactant known as Brij 30 has the structure C12H25(OCH2CH2)4OH. Sodium dodecyl sulfate (SDS) has the structure CH3(CH2)11OSO3Na. Which of these two surfactants has the lower CMC? Briefly explain your reasoning.
18. Why might SDS (CH3(CH2)11OSO3Na) form spherical micelles and phosphatidylcholine (shown below) form bilayer aggregates?
Вам также может понравиться
- +2 ReapДокумент3 страницы+2 ReapRejeena SОценок пока нет
- Half Yearly Examination Paper 2Документ8 страницHalf Yearly Examination Paper 2AëОценок пока нет
- Fly High Group Tuitions Chemistry Test 2Документ3 страницыFly High Group Tuitions Chemistry Test 2Manthan RathodОценок пока нет
- ChemistryДокумент48 страницChemistryDeepan KumarОценок пока нет
- Fabrication of Nanoparticle CsH2PO4 Electrolyte for Fuel Cell ApplicationsДокумент4 страницыFabrication of Nanoparticle CsH2PO4 Electrolyte for Fuel Cell ApplicationsHelen TelilaОценок пока нет
- Practice Test AllДокумент29 страницPractice Test Alljraman2450% (2)
- 2011 Chemistry ExamДокумент24 страницы2011 Chemistry Examduy_ScdОценок пока нет
- Closed-Book Practice-Ch 15 (2015!03!28)Документ21 страницаClosed-Book Practice-Ch 15 (2015!03!28)JuanОценок пока нет
- IJANS - Spectroscopic and Electrical Properties of Phthalocyaninato CobaltII - Nazar A - HusseinДокумент8 страницIJANS - Spectroscopic and Electrical Properties of Phthalocyaninato CobaltII - Nazar A - Husseiniaset123Оценок пока нет
- Isomerism and Coordination Geometries Explored by SpectroscopyДокумент4 страницыIsomerism and Coordination Geometries Explored by SpectroscopymohammedОценок пока нет
- B.tech Question Paper-2017Документ3 страницыB.tech Question Paper-2017aryanr2233Оценок пока нет
- Polymer Properties and Characterization"This title is concise (less than 40 characters) and SEO-optimized by including the main topic of the document ("Polymer Properties and CharacterizationДокумент4 страницыPolymer Properties and Characterization"This title is concise (less than 40 characters) and SEO-optimized by including the main topic of the document ("Polymer Properties and CharacterizationOsama Aadil SaadiОценок пока нет
- RRRRRR Chido ReactHetExamenOrdinarioJunio2020aДокумент4 страницыRRRRRR Chido ReactHetExamenOrdinarioJunio2020aDaniel Moreno CabreraОценок пока нет
- E344 2013 Summer Solution Set 3: (B) What Role Does Each Component Play in The Forming and Firing Procedures?Документ19 страницE344 2013 Summer Solution Set 3: (B) What Role Does Each Component Play in The Forming and Firing Procedures?Firdhaus ZulkifleОценок пока нет
- Final Selection Examination For The 2004 Australian Chemistry Olympiad TeamДокумент6 страницFinal Selection Examination For The 2004 Australian Chemistry Olympiad Teamrajeswar royОценок пока нет
- 2016 Sample Paper 12 Chemistry 02 AnsДокумент7 страниц2016 Sample Paper 12 Chemistry 02 AnsjrajaОценок пока нет
- 2017 AdvancesДокумент8 страниц2017 AdvancesAkshai Ashok KumarОценок пока нет
- 2022 - 350 Homework #1Документ3 страницы2022 - 350 Homework #1MeredithОценок пока нет
- IBO Worksheet ChemistryДокумент26 страницIBO Worksheet ChemistryAarav PatelОценок пока нет
- Problems T9 PolymersДокумент2 страницыProblems T9 PolymersAli PliegoОценок пока нет
- Travaux Diriges 2 Esb 161Документ2 страницыTravaux Diriges 2 Esb 161i98843464Оценок пока нет
- Class XII Assignments (Complete) Chootiya Ho TumДокумент35 страницClass XII Assignments (Complete) Chootiya Ho TumpranavОценок пока нет
- QP PA1 Chemistry SET 1docxДокумент5 страницQP PA1 Chemistry SET 1docxAtharva SrivastavaОценок пока нет
- Edexcel GCE Chemistry Unit-5 June 2014 Question PaperДокумент28 страницEdexcel GCE Chemistry Unit-5 June 2014 Question PaperAvrinoxОценок пока нет
- SR Inter CHEMISTRY IMP-New With 70% Syllabus-Converted-1Документ6 страницSR Inter CHEMISTRY IMP-New With 70% Syllabus-Converted-1B. SwapnaОценок пока нет
- Question Bank Chemistry XI Term - 2Документ4 страницыQuestion Bank Chemistry XI Term - 2GHOSTX GAMERОценок пока нет
- Acetate-Ultrasmooth Organic-Inorganic Perovskite Thin-FilmДокумент10 страницAcetate-Ultrasmooth Organic-Inorganic Perovskite Thin-FilmDanila SaraninОценок пока нет
- 6CH01 01R Que 20140523Документ28 страниц6CH01 01R Que 20140523Celinne TehОценок пока нет
- Exercises Unit IДокумент9 страницExercises Unit INairobi SoultanianОценок пока нет
- Long Exam 1Документ8 страницLong Exam 1Allan DОценок пока нет
- Vivek High School Sector 38, Chandigarh NAME - Class: Xi Date: SUBJECT: Chemistry (Revision) TOPIC: Chap1 and 2Документ5 страницVivek High School Sector 38, Chandigarh NAME - Class: Xi Date: SUBJECT: Chemistry (Revision) TOPIC: Chap1 and 2manseeratОценок пока нет
- Preparatory Problems2 PDFДокумент4 страницыPreparatory Problems2 PDFGerel BayrmagnaiОценок пока нет
- Inclusion Complex Formation of Cyclodextrin and PolyanilineДокумент4 страницыInclusion Complex Formation of Cyclodextrin and PolyanilineElbahi DjaalabОценок пока нет
- Army Public School Dhaula Kuan Half Yearly Examination CHEMISTRY (2019)Документ5 страницArmy Public School Dhaula Kuan Half Yearly Examination CHEMISTRY (2019)YahooОценок пока нет
- 2008 Synthesis, Characterization, and Photovoltaic Properties of Novel Semiconducting Polymers WithДокумент8 страниц2008 Synthesis, Characterization, and Photovoltaic Properties of Novel Semiconducting Polymers WithDoktor transmisionesОценок пока нет
- Chemistry Question BankДокумент21 страницаChemistry Question Bankप्रियांशु मिश्राОценок пока нет
- 3 - The Role of Oxygen-Permeable Ionomer For Polymer Electrolyte Fuel CellsДокумент9 страниц3 - The Role of Oxygen-Permeable Ionomer For Polymer Electrolyte Fuel CellsFaseeh KKОценок пока нет
- Dehradun Public School ASSIGNMENT (2020-21) Subject-Chemistry (043) Class-Xii Unit1:Solid State I-Multiple Choice QuestionsДокумент23 страницыDehradun Public School ASSIGNMENT (2020-21) Subject-Chemistry (043) Class-Xii Unit1:Solid State I-Multiple Choice QuestionsSarvesh Kumar SinghОценок пока нет
- CLASS 12 Chem Practice Sample QP CHEM SET 1Документ20 страницCLASS 12 Chem Practice Sample QP CHEM SET 1Minecraft NoobsОценок пока нет
- Chemistry Set 1Документ7 страницChemistry Set 1krish.meghashriОценок пока нет
- BMEДокумент7 страницBMEJabeen ThazОценок пока нет
- Self-Assembly: From Surfactants to NanoparticlesОт EverandSelf-Assembly: From Surfactants to NanoparticlesRamanathan NagarajanОценок пока нет
- 1060 Hixson-Lied Student Success Center V 515-294-6624 V Sistaff@iastate - Edu V HTTP://WWW - Si.iastate - EduДокумент5 страниц1060 Hixson-Lied Student Success Center V 515-294-6624 V Sistaff@iastate - Edu V HTTP://WWW - Si.iastate - EduRhod Jayson RicaldeОценок пока нет
- Bimolecular Nucleophilic Reaction (S) : NO NOДокумент5 страницBimolecular Nucleophilic Reaction (S) : NO NObhartiyaanujОценок пока нет
- GR XI Term 2 CHEMISTRY Ans KeyДокумент10 страницGR XI Term 2 CHEMISTRY Ans Keyrohan fernandesОценок пока нет
- J. Patrick A. Fairclough Et Al - Chain Length Dependence of The Mean Ðeld Temperature in Poly (Oxyethylene) - Poly (Oxybutylene) Diblock CopolymersДокумент3 страницыJ. Patrick A. Fairclough Et Al - Chain Length Dependence of The Mean Ðeld Temperature in Poly (Oxyethylene) - Poly (Oxybutylene) Diblock CopolymersDremHpОценок пока нет
- Thesis of PHD in Organic ChemistryДокумент7 страницThesis of PHD in Organic Chemistryfjebwgr4100% (1)
- Chapter 4 Practice: Problems 1,2-DichloroethaneДокумент8 страницChapter 4 Practice: Problems 1,2-DichloroethanecwodОценок пока нет
- FSC PaperДокумент2 страницыFSC PaperRana Hassan TariqОценок пока нет
- Spring 2015 OMET Practice Problem Set KEYДокумент12 страницSpring 2015 OMET Practice Problem Set KEYSay sayОценок пока нет
- Engineering Chemistry Exam QuestionsДокумент3 страницыEngineering Chemistry Exam QuestionsSaikat LayekОценок пока нет
- 11 Chemistry23 24sp 01Документ13 страниц11 Chemistry23 24sp 01AbhishekОценок пока нет
- Test Bank For Organic Chemistry 9th Edition Leroy G Wade Isbn 10 032197137x Isbn 13 9780321971371Документ43 страницыTest Bank For Organic Chemistry 9th Edition Leroy G Wade Isbn 10 032197137x Isbn 13 9780321971371oanhkieu5me100% (26)
- Chem Engg Paper-IIДокумент6 страницChem Engg Paper-IIambaneh tzeraОценок пока нет
- Types of Solids, Crystal Structure, Amorphous SolidsДокумент3 страницыTypes of Solids, Crystal Structure, Amorphous SolidsAishwarya RaghavanОценок пока нет
- Half Yearly Exam Paper 1Документ7 страницHalf Yearly Exam Paper 1AëОценок пока нет
- Question Bank of Chemistry (BSC-105) for 2018 onwards Batch Students (1)Документ8 страницQuestion Bank of Chemistry (BSC-105) for 2018 onwards Batch Students (1)interestingfacts2525Оценок пока нет
- MLE1101 - Tutorial 5 - Suggested SolutionsДокумент5 страницMLE1101 - Tutorial 5 - Suggested SolutionsYin HauОценок пока нет
- Common Foundation Organic Q in A LevelДокумент21 страницаCommon Foundation Organic Q in A Level黄维燕Оценок пока нет
- Computational Pharmaceutics: Application of Molecular Modeling in Drug DeliveryОт EverandComputational Pharmaceutics: Application of Molecular Modeling in Drug DeliveryDefang OuyangОценок пока нет
- Failure CriterionДокумент40 страницFailure CriterionTruong CaiОценок пока нет
- Failure Theories..Документ93 страницыFailure Theories..adnanmominОценок пока нет
- MIT Course 10-ENG Curriculum Planning FormДокумент4 страницыMIT Course 10-ENG Curriculum Planning FormTruong CaiОценок пока нет
- AfsdfasdfДокумент15 страницAfsdfasdfTruong CaiОценок пока нет
- Liquid Metal Better yДокумент25 страницLiquid Metal Better yTruong CaiОценок пока нет
- 5.111 Principles of Chemical Science: Mit OpencoursewareДокумент6 страниц5.111 Principles of Chemical Science: Mit OpencoursewareTruong CaiОценок пока нет
- Beth 1Документ274 страницыBeth 1Truong CaiОценок пока нет
- Mathematics Competition PracticeДокумент5 страницMathematics Competition PracticeTruong CaiОценок пока нет
- Conformal Mapping PDFДокумент78 страницConformal Mapping PDFClinton PromotingJesusОценок пока нет
- Energy Conversion AssessmentДокумент10 страницEnergy Conversion AssessmentTruong CaiОценок пока нет
- Ta NotesДокумент25 страницTa NotesTruong CaiОценок пока нет
- Answers To Extra Spectroscopy ProblemsДокумент8 страницAnswers To Extra Spectroscopy ProblemsTruong CaiОценок пока нет
- Lec Notes 01Документ4 страницыLec Notes 01shoulditbeОценок пока нет
- Spring 2012 10.491 Course ScheduleДокумент1 страницаSpring 2012 10.491 Course ScheduleTruong CaiОценок пока нет
- Mohammed AlDajaniE1V1Документ4 страницыMohammed AlDajaniE1V1Truong CaiОценок пока нет
- Plan of Study for Applied Mathematics DegreeДокумент1 страницаPlan of Study for Applied Mathematics DegreeAntonio JeffersonОценок пока нет
- Research Proposal Summer 2014Документ1 страницаResearch Proposal Summer 2014Truong CaiОценок пока нет
- Home1 PDFДокумент1 страницаHome1 PDFTruong CaiОценок пока нет
- D 2 Group 13Документ2 страницыD 2 Group 13Truong CaiОценок пока нет
- Outline 13Документ3 страницыOutline 13Truong CaiОценок пока нет
- Wealth NationsДокумент786 страницWealth NationsTruong CaiОценок пока нет
- Sing ValueДокумент2 страницыSing ValueTruong CaiОценок пока нет
- Clinical Curriculum Chart 2014 2015Документ6 страницClinical Curriculum Chart 2014 2015Truong CaiОценок пока нет
- Sing ValueДокумент2 страницыSing ValueTruong CaiОценок пока нет
- Setup Guide For Modela Pro II MDXДокумент1 страницаSetup Guide For Modela Pro II MDXTruong CaiОценок пока нет
- Topics Covered in ChemistryДокумент6 страницTopics Covered in ChemistryTruong CaiОценок пока нет
- Pset 10Документ1 страницаPset 10Truong CaiОценок пока нет
- Dual DegreesДокумент2 страницыDual DegreesTruong CaiОценок пока нет
- MitДокумент76 страницMitTruong CaiОценок пока нет
- Nuclear PhysicsДокумент9 страницNuclear PhysicsTruong CaiОценок пока нет
- Lifting Lug SampleДокумент1 страницаLifting Lug Sampleabdul marpaung0% (1)
- Heat Transfer Presentation: Modes and LawsДокумент16 страницHeat Transfer Presentation: Modes and LawsmehediОценок пока нет
- Annual Xi Sample Paper 22-23Документ6 страницAnnual Xi Sample Paper 22-23Cat ChanОценок пока нет
- Proof Hi PDFДокумент29 страницProof Hi PDF孙中体Оценок пока нет
- Worksheet N º 6. Sixth Grade. Unit 7.: Colegio San SebastiánДокумент3 страницыWorksheet N º 6. Sixth Grade. Unit 7.: Colegio San Sebastiángenesis llancapaniОценок пока нет
- WPS - 006Документ13 страницWPS - 006MAT-LIONОценок пока нет
- PROTECTIONS & Interlocks Diary 300MWДокумент42 страницыPROTECTIONS & Interlocks Diary 300MWkarthick.gОценок пока нет
- Sound - Wikipedia, The Free EncyclopediaДокумент6 страницSound - Wikipedia, The Free EncyclopediaRoshanaManjunathОценок пока нет
- Determination of Lightfastness (According To Iso 12040) 2.3.2.1Документ2 страницыDetermination of Lightfastness (According To Iso 12040) 2.3.2.1marinaОценок пока нет
- Design of Concrete Structures 15th Edition Ebook PDFДокумент61 страницаDesign of Concrete Structures 15th Edition Ebook PDFmario.becker25297% (37)
- Pipe VibrationДокумент7 страницPipe Vibrationjohn9999_502754Оценок пока нет
- Qip Ice 31 Stirling EnginesДокумент20 страницQip Ice 31 Stirling EnginesChetanPrajapatiОценок пока нет
- Syllabus For Mec 456Документ4 страницыSyllabus For Mec 456ninja1stclassОценок пока нет
- Durham E-Theses: Novel Block Co-Polymers As Potential Photonic MaterialsДокумент118 страницDurham E-Theses: Novel Block Co-Polymers As Potential Photonic MaterialsWassini BensОценок пока нет
- Heat Treating SpecialtiesДокумент4 страницыHeat Treating SpecialtiesYe Wint ThuОценок пока нет
- Factors Affecting PermeabilityДокумент4 страницыFactors Affecting PermeabilityMahesh RamtekeОценок пока нет
- Boussinesq Modeling of Longshore CurrentsДокумент18 страницBoussinesq Modeling of Longshore CurrentsnikifОценок пока нет
- 0625 - w10 - Ms - 22documentation of PhysicsДокумент6 страниц0625 - w10 - Ms - 22documentation of PhysicsAhmed HossainОценок пока нет
- Lecture Sheet PDFДокумент65 страницLecture Sheet PDFFaruk abdullahОценок пока нет
- IKS-Titan ZY-1212 Green Color Technical Proposal 20211206 PDFДокумент9 страницIKS-Titan ZY-1212 Green Color Technical Proposal 20211206 PDFBharath KumarОценок пока нет
- Exportar Páginas Meriam Kraige Engineering Mechanics Statics 7th TXTBKДокумент1 страницаExportar Páginas Meriam Kraige Engineering Mechanics Statics 7th TXTBKabrahamrv44Оценок пока нет
- A5 Catalog CG 091214 Unnop V10Документ10 страницA5 Catalog CG 091214 Unnop V10Tanjim FakirОценок пока нет
- Set2-PetE418-Midterm Examination-SignedДокумент5 страницSet2-PetE418-Midterm Examination-SignedRoxanne NavarroОценок пока нет
- Reinforced Masonry Engineering Handbook .6th - Ed.secДокумент647 страницReinforced Masonry Engineering Handbook .6th - Ed.secEc Ef100% (2)
- M410 Orifice Plate Assemblies: 2. Technical Details 3. Installation RequirementsДокумент8 страницM410 Orifice Plate Assemblies: 2. Technical Details 3. Installation RequirementsIvan RocoОценок пока нет
- Studies On Effect of Change in Dynamic Behavior of Crack Using FemДокумент7 страницStudies On Effect of Change in Dynamic Behavior of Crack Using FemMatthew SmithОценок пока нет
- THE PARTICULATE NATURE OF MATTER: STATES, CHANGES, AND THE KINETIC THEORYДокумент32 страницыTHE PARTICULATE NATURE OF MATTER: STATES, CHANGES, AND THE KINETIC THEORYB R YОценок пока нет
- The Secret of Physics: U U V V U U V VДокумент5 страницThe Secret of Physics: U U V V U U V VVIPscholarОценок пока нет
- Module-2 Fundamentals of SurveyingДокумент2 страницыModule-2 Fundamentals of Surveyingnonononoway100% (1)
- Intermodal Stimulated Brillouin Scattering in TwoДокумент4 страницыIntermodal Stimulated Brillouin Scattering in TwoRamón José Pérez MenéndezОценок пока нет