Академический Документы
Профессиональный Документы
Культура Документы
QUT RTI 2012 03 No 5 Errors Manton 1
Загружено:
cormacklИсходное описание:
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
QUT RTI 2012 03 No 5 Errors Manton 1
Загружено:
cormacklАвторское право:
Доступные форматы
Released by QUT under Right to Information Act
Detailed below is a full listing of all errors that have been identified in the Manton et al. (2010) manuscript published in the journal Stem Cells and Development. These errors were identified during the QUT investigation into allegations of research misconduct initiated following a complaint fromMr.LukeCormacklodgedinApril2012. OriginalMantonetal.,2010Figure1:
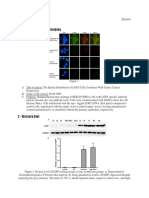
OriginalwordingofFigure1legend: FIG. 1. Human embryonic stem (hES) cells cultured in feedercellfree and serumfree conditions retain expression of pluripotent cell surface and intracellular markers. hES cells that had been propagated for at least 30 passages were fixed and probed for cell surface and intracellular markers using immunofluorescence. ( A ) Morphology only, ( B ) DAPI, ( C ) SSEA4, ( D ) Oct4, ( E ) SSEA1, and(F)TRA160(allphotosatmagnification200). ExplanationoferrorinFigure1: Thepassage numbersreportedin Figure1are incorrect.Thecurrentwording in theFigure1legend shouldbechangedfrom,atleast30 passagesto14passages.Moreover,themorphologyimage in Figure 1A is incorrect. It is from p=6 hES cells. We provide a replacement Figure 1A morphology image,thatisfromp=14hEScells.
Released by QUT under Right to Information Act
SuggestedchangetotheFigure1legend: FIG. 1. Human embryonic stem (hES) cells cultured in feedercellfree and serumfree conditions retain expression of pluripotent cell surface and intracellular markers. hES cells that had been propagated for at least 30 14 passages were fixed and probed for cell surface and intracellular markers using immunofluorescence. ( A ) Morphology only, ( B ) DAPI, ( C ) SSEA4, ( D ) Oct4, ( E ) SSEA1,and(F)TRA160(allphotosatmagnification200). OriginalMantonetal.,2010Figure2:
OriginalwordingofFigure2legend: FIG. 2. Human embryonic stem (hES) cells cultured in feedercellfree and serumfree conditions retain expression of pluripotent cell surface and intracellular markers. hES cells that had been propagated for at least 30 passages in serumfree and feedercellfree conditions were analyzed for expression of cell surface (SSEA4, SSEA1, and TRA180) and intracellular (Oct4) markers using fluorescenceactivated cell sorter (FACS). Unstained cells are also shown. The graph shows the percentage of cells that were positive for undifferentiated (Oct4, SSEA4, TRA180) and differentiated(SSEA1)hESmarkers.
Released by QUT under Right to Information Act
ExplanationoferrorinFigure2legend: The passage numbersreported in Figure2are incorrect.The legendfor Figure2states thatthecells were at least 30 passages. We have now established that the Oct4 data in Figure 2 was from FACS analysis conducted on hES cells that were p=27. The FACS data for the other hES markers was conducted on p=37 hES cells. Therefore, the current wording in the Figure 2 legend should be changedfrom,atleast30passagestoatleast27passages. SuggestedchangetotheFigure2legend: FIG. 2. Human embryonic stem (hES) cells cultured in feedercellfree and serumfree conditions retain expression of pluripotent cell surface and intracellular markers. hES cells that had been propagated for at least 30 27 passages in serumfree and feedercellfree conditions were analyzed for expression of cell surface (SSEA4, SSEA1, and TRA180) and intracellular (Oct4) markers using fluorescenceactivated cell sorter (FACS). Unstained cells are also shown. The graph shows the percentage of cells that were positive for undifferentiated (Oct4, SSEA4, TRA180) and differentiated(SSEA1)hESmarkers. OriginalMantonetal.,2010Figure5:
OriginalwordingofFigure5legend: FIG. 5. Human embryonic stem (hES) cells cultured for up to 30 passages in feedercellfree and serumfree conditions retain the ability to differentiate down the germ lineages. Differentiated hES cells were probed for markers of ectoderm (nestin) and mesoderm (smooth muscle actin, troponin) using immunofl uorescence (top row). Realtime polymerase chain reaction (PCR) was used to determine fold increase in expression of markers of the 3 germ lineages as compared with undifferentiatedhES(bottomtable);p=passagenumber. ExplanationoferrorinFigure5: In reviewing the manuscript we have identified a mistake in the figure 5 table and legend. The legend for figure 5 states that the cells were cultured for up to 30 passages. However, in the table displaying the realtime PCR data, the passage numbers indicates the hES cells were p=20 and p=25. In addition, we have now identified that the p25 hES cells were in fact p22 hES cells. Therefore, the current wording in the Figure 5 legend should be changed from cultured for up to 30 passages to
Released by QUT under Right to Information Act
cultured for 20 or 22 passages. In addition, the title of the right hand column in the table should be changed from P = 25 to P = 22. We provide a replacement Figure 5 table that contains this correction.
SuggestedchangetotheFigure5legend: FIG. 5. Human embryonicstem (hES) cellscultured forup to 3020 or 22 passages in feedercellfree and serumfree conditions retain the ability to differentiate down the germ lineages. Differentiated hES cells were probed for markers of ectoderm (nestin) and mesoderm (smooth muscle actin, troponin)usingimmunofluorescence(toprow).Realtimepolymerasechainreaction(PCR)wasused to determine fold increase in expression of markers of the 3 germ lineages as compared with undifferentiatedhES(bottomtable);p=passagenumber. OriginalMantonetal.,2010manuscript: As a result of the changes to the passage numbers identified in the figure 1, figure 2 and figure 5 legend, the attached pdf file highlights changes that may also be necessary in the text of the manuscript. Where highlighted,the wordingshouldbe changed from 30passagesto14 passages in regards to the immunoflourence experiment (ie: Fig. 1); from 30 passages to 27 or 37 passages depending on the marker in regards to the FACS experiment (ie: Fig. 2) and from 30 passages to 20 passagesinregardstothedifferentiationexperiment(ie:Fig.5).
Released by QUT under Right to Information Act
STEM CELLS AND DEVELOPMENT Volume 19, Number 9, 2010 Mary Ann Liebert, Inc. DOI: 10.1089/scd.2009.0504
A Chimeric Vitronectin: IGF-I Protein Supports Feeder-Cell-Free and Serum-Free Culture of Human Embryonic Stem Cells
Kerry J. Manton, Sean Richards, Derek Van Lonkhuyzen, Luke Cormack, David Leavesley, and Zee Upton
The therapeutic use of human embryonic stem (hES) cells is severely limited by safety concerns regarding their culture in media containing animal-derived or nondefined factors and on animal-derived feeder cells. Thus, there is a pressing need to develop culture techniques that are xeno-free, fully defined, and synthetic. Our laboratory has discovered that insulin-like growth factor (IGF) and vitronectin (VN) bind to each other resulting in synergistic short-term functional effects in several cell types, including keratinocytes and breast epithelial cells. We have further refined this complex into a single chimeric VN:IGF-I protein that functionally mimics the effects obtained upon binding of IGF-I to VN. The aim of the current study was to determine whether hES cells can be serially propagated in feeder-cell-free and serum-free conditions using medium containing our novel chimeric VN:IGF-I protein. Here we demonstrate that hES cells can be serially propagated and retain their undifferentiated state in vitro for up to 35 passages in our feeder-cell-free, serum-free, chemically defined media. We have utilized real-time polymerase chain reaction (PCR), immunofluorescence, and fluorescence-activated cell sorter (FACS) analysis to show that the hES cells have maintained an undifferentiated phenotype. In vitro differentiation assays demonstrated that the hES cells retain their pluripotent potential and the karyotype of the hES cells remains unchanged. This study demonstrates that the novel, fully defined, synthetic VN:IGF-I chimeracontaining medium described herein is a viable alternative to media containing serum, and that in conjunction with laminin-coated plates facilitates feeder-cell-free and serum-free growth of hES.
Introduction
he successful derivation of human embryonic stem (hES) cells by Thomson and colleagues in 1998 [1] was hailed as the beginning of a new era of clinical therapies for the treatment of a range of human diseases. However, currently no hES therapy has been used in clinical settings. This is mainly due to concerns about the introduction of xenoderived pathogens (ie, bovine spongiform encephalopathy) through the use of hES cells cultured on animal feeder cell layers (most often inactivated mouse embryonic fibroblasts [iMEF]) and in media containing xeno-derived components. Indeed, these xeno-derived components have been shown to introduce immunogenic agents into hES cells [2,3]. Progress has been made toward successful xeno-free hES culture through removal of the animal-derived feeder cell layer and bovine serum. In early studies, the iMEF feeder cell layer was replaced with the use of Matrigel or laminin along with media conditioned by the MEF [4]. This was
further refined by Ludwig and colleagues by production of the commercially available mTESR1 media, consisting of Matrigel-coated plates supplemented with large amounts of bovine serum albumin (BSA) [5,6]. Additional studies have since been performed utilizing recombinant vitronectin [7] or synthetic surfaces [8] to replace the animal-derived Matrigel in combination with the mTESR1 media. The issue with these protocols is while they have successfully removed the need for animal feeder cell layers, they still require the inclusion of undefined human and/or animal products such as BSA. Additional work by Lu and colleagues in 2006 removed the bovine BSA and replaced it with undefined human-derived products such as albumin purified from human serum [9], but again, this media still does not meet the gold standard of completely defined, synthetic, safe, and viable culture media. Previous work by our laboratory has determined that synergistic effects exist between growth factors and the
Institute of Health and Biomedical Innovation, Queensland University of Technology, Kelvin Grove, Australia.
1297
Released by QUT under Right to Information Act
1298 extracellular matrix (ECM) protein vitronectin (VN) [10,11]. This led to the development of novel dimeric, trimeric, and multimeric molecular complexes incorporating growth factors such as insulin-like growth factors (IGFs) and insulinlike growth factor binding proteins (IGFBPs) in conjunction with VN. Furthermore, the addition of these complexes to chemically defined media formulations has been demonstrated to stimulate short-term migration and proliferation in a range of cells, including adult keratinocyte and cornealderived epithelial cells [12,13]. These responses are dependent upon the co-activation of VN-binding integrins and the insulin-like growth factor I receptor (IGF-IR) [13]. These observations have led us to design and produce novel chimeric proteins containing domains of VN linked to IGF-I, which can mimic the actions of multimeric complexes by co-activation of the VN-binding integrins and the IGF-IR [14,15]. We have previously reported the use of this chimeric protein in the serum-free growth of keratinocytes and hES cells [16,17] and the focus of the studies herein was on removing the requirement for feeder cells in hES culture. We report that a VN:IGF-I chimeric protein can facilitate the successful long-term cultivation (up to 35 passages) of hES cells in feeder-cell-free and serum-free conditions. Importantly, the hES cells remain undifferentiated and retain pluripotent potential during this entire culture period. MANTON ET AL.
Establishment of a feeder-cell-free and serum-free hES culture
hES cells that had been passaged 5 times on iMEF feeder cells in KSR media (as described earlier) were transferred to feeder-cell-free culture in our serum-free media (described below). The 6-well plates utilized for hES feeder-free culture were precoated with purified murine laminin (Millipore; 100 g laminin/9.6 cm2) in 2 mL of DMEM media for a minimum of 2 h at 37C. The DMEM media was removed and the hES cells were immediately added.
hES feeder-cell-free and serum-free culture
The hES cells were cultured in 6-well tissue culture plates that were precoated with purified mouse laminin (described above). The hES cells were cultured in 2.5 mL of serum-free media/well containing: Dulbeccos modified Eagles medium/ F-12 (DMEM/F-12; Gibco, Grand Island, NY); 1,000 IU/mL penicillin and 1,000 g/mL streptomycin (Invitrogen, Carlsbad, CA); 1 mM glutamax (Invitrogen); 1% nonessential amino acids (Gibco); 0.1 mM -mercaptoethanol (Gibco); 1 mM LiCl (Gibco); 0.4% trace elements B and C (CELLGRO); 0.4% cholesterol lipid concentrate (protein-free; Gibco); 30 ng/mL recombinant human activin-A (PeproTech); 4.0 mg/mL recombinant human albumin (HA; Albucult; Novozymes); and 100 ng/mL bFGF (Chemicon, Billerica, MA). The hES culture was also supplemented with 1.0 g/mL of recombinant human VN:IGF-I chimeric protein (VN(164)LinkerIGF-I), which was produced in our laboratory [14]. hES cells cultured in feeder-cell-free and serum-free conditions had their media changed daily and were sub-cultivated every 79 days (based on morphological evaluation) by mechanical dissociation. In brief, the media was removed from the hES cells that were to be passaged and 1.5 mL of fresh serum-free media was added to the well. The hES cells were detached from the culture vessel surface by gentle scraping with a 5-mL sterile pipette tip and were then resuspended by repeated pipetting. hES cells that displayed indications of differentiation (through morphological evaluation) were not passaged. The cells were diluted at a ratio of 1:2 for weekly passaging and maintenance of optimal individual undifferentiated colonies.
Materials and Methods Ethics
Ethics approval to conduct this project was provided by the Queensland University of Technology (QUT) Human Research Ethics Committee (ID: 2943H). The hES cell line used, BGO1V (BresaGen Inc., Melbourne, Australia), was obtained with strict adherence to the state, federal, and National Health and Medical Research Council (NHMRC) guidelines regarding the conduct of research using hES cells.
Culture of hES cells on iMEF in KSR media
BGO1V hES cells were cultured on inactivated mouse embryonic fibroblasts (iMEFs) (Millipore) in knockout serum replacement (KSR) medium (Dulbeccos modified Eagles medium [DMEM; Invitrogen, Carlsbad, CA]; 20% KSR [Invitrogen]; 1,000 IU/mL penicillin/1,000 g/mL streptomycin [Invitrogen]; 1 mM glutamax [Invitrogen]; 1% nonessential amino acids; 0.1 mM -mercaptoethanol; 20 ng/mL recombinant human basic fibroblast growth factor [bFGF; Chemicon, Billerica, MA]). These hES cells (ie, on iMEF feeders in KSR media) also act as the control culture for the real-time polymerase chain reaction (PCR) experiments. hES cells were passaged by washing in 2 mL PBS per well (Invitrogen) followed by exposure to 0.05% trypsin/ EDTA (Invitrogen) for a 12 min incubation (37C, 5% CO2) to remove the iMEF feeder layer. The trypsin was removed and an additional 2 mL of media/well was added. The hES cells were detached from the culture vessel surface by gentle scraping with a 5-mL sterile pipette tip. The cells were then resuspended in KSR media and spun at 500600g for 5 min. The supernatant was removed and the hES cell pellet was resuspended in KSR media. The cells were diluted at a ratio of 1:12 for weekly passaging and maintenance of optimal individual undifferentiated colonies.
hES in vitro differentiation
hES cells that had been cultured in serum-free and feeder-cell-free conditions for at least 30 passages were cultured for a further 2 weeks in monolayer in differentiation media (DMEM media containing 10% FCS, 1,000 IU/mL penicillin/1,000 g/mL streptomycin [Invitrogen], and 1 mM glutamax [Invitrogen]) as previously described [18]. The media was changed every 3 days for 2 weeks, after which the hES cells were either fixed for immunofluorescence analysis or lysed for RNA extraction for RT-PCR.
hES cell immunofl uorescence
SSEA-1 expressed by differentiated hES cells and SSEA-4, TRA-160, and Oct-4 expressed by undifferentiated hES cells were used to monitor the differentiation status of the hES cells. hES cells at greater than passage 30 were cultured on laminincoated coverslips in 12-well plates for 6 days. The cells were fixed using 4% paraformaldehyde/phosphate-buffered saline for 20 min and were then washed twice for 5 min/wash in PBS. The
Released by QUT under Right to Information Act
SERUM-FREE AND FEEDER-CELL-FREE HES CULTURE cells were permeabilized with 0.1% Triton X-100/PBS for 10 min prior to a further washing step. The cells were blocked in 4% BSA (Sigma, St. Louis, MO) for 30 min at room temperature, followed by incubation with mouse IgG primary antibodies (1:100 dilution; Chemicon) in 4% BSA (or diluent alone) for 1 h. The primary antibodies were removed and the wash steps repeated. The Alexa-Fluor 488 or Alexa-Fluor 555 anti-mouse secondary antibodies (1:100 dilution; Invitrogen) were incubated for 1 h. The secondary antibodies were removed, the wash steps repeated, and the cells were mounted on microscope slides under mounting medium (containing DAPI). The stained hES colonies were viewed with a Nikon TE-2000 epifluorescent microscope. 1299 expression units (REU) were calculated by normalizing the Ct values of the genes to the Ct of GAPDH. A no cDNA template was added as a control for contamination. A 2-way ANOVA with Bonferroni post-test analysis was used to compare differences in gene expression in feedercell-free, serum-free culture versus serum-free, iMEF feeder culture. Significance was set at P < 0.05 and the analysis was performed using GraphPad PRISM version 4.00 for windows software (GraphPad Software, USA).
hES karyotype analysis
BGO1V hES cells that had been cultured for at least 40 passages in our feeder-cell-free and serum-free culture conditions were analyzed for their karyotype by the GTG banding method [20] (performed by Sullivan Nicolaides Pathology, Cytogenetics Laboratory, Toowong, Queensland, Australia).
hES real-time PCR analysis
The genes (Nanog, hTERT, UTF1, SOX2, FOXD4, Oct-4, Dppa, and REX-1) that are accepted markers for undifferentiated hES cells [19] were assayed using real-time PCR. In addition, genes (HAND1, Troponin, AFP, GATA, nestin, and MAP2) that are accepted markers for differentiated hES cells [19] were also assayed using real-time PCR. To perform this total RNA was extracted from the cells using the Qiagen RNeasy plus mini kit (Qiagen, Valencia, CA) as per the manufacturers instructions. cDNA was then synthesized from 2 g of total RNA using SuperScript III Reverse Transcriptase (Invitrogen) and random oligonucleotide primers (Invitrogen). In brief, random primers were added to 2 g of total RNA and allowed to anneal for 5 min at 65C. Samples were then held on ice for 2 min before the addition of the SuperScript III Reverse Transcriptase and buffers. cDNA synthesis was formed by heating these samples for 5 min at 25C followed by 1 h incubation at 50C and then 15 min at 70C. Real-time PCR was performed on 100 ng of cDNA template using the SYBR Green PCR Master Mix (Qiagen) and the primers (Sigma-Aldrich) described in Table 1. These primers were designed using the Invitrogen OligoPerfect software design program and span an exon/intron boundary. PCRs were performed in triplicate with an initial 10-min activation step at 95C followed by 45 cycles of 95C for 20 s, 55C for 10 s, 60C for 30 s, and 72C for 40 s. Relative
hES FACS analysis
hES cells that had been cultured for at least 30 passages in our serum-free and feeder-cell-free culture system were examined for the expression of cell surface (SSEA-1, SSEA-4, TRA180) and intracellular (Oct-4) markers using flow cytometric analysis. Fluorescence-activated cell sorter (FACS) analysis was performed as described previously [21]. In brief, all primary antibodies (1.0 g of mouse IgG; Chemicon, Billerica, MA) were preincubated for 30 min at 4C with 0.5 g of AlexaFluor 488 anti-mouse IgG (Molecular Probes, Eugene, OR) in a final volume of 100 L FACS buffer (2% FCS in PBS). For cell surface markers, 1 106 dissociated hES cells were stained with preincubated antibody for 30 min at 4C, followed by 3 wash steps in FACS buffer (2% FCS in PBS). For intracellular staining (Oct-4), cells were fixed and permeabilized prior to antibody incubation using CALTAG Fix-and-Perm as per the manufacturers instructions (Invitrogen). The cells were stained with 7-AAD for 20 min at room temperature just prior to FACS analysis. Live cells were identified by 7-AAD exclusion and analyzed for cell marker expression using the FC500 FACSCalibur (BD) and Cell Quest Software (BD).
Table 1. Sequences of Primers Used in Real-Time PCR to Examine Gene Expression in hES Cells Cultured in Feeder-Cell-Free and Serum-Free Conditions Gene Rex1 Nanog hTERT UTFI SOX2 FoxD3 Oct4 Dppa5 GAPDH HAND1 Troponin AFP GATA Nestin MAP2 Forward Primer (5 to 3) ACCAGCACACTAGGCAAACC GATTTGTGGGCCTGAAGAAA CGGTGTGCACCAACATCTAC CGCCGCTACAAGTTCCTTA CAAGATGCACAACTCGGAGA TCTGCGAGTTCATCAGCAAC GAAGGATGTGGTCCGAGTGT AAGTGGATGCTCCAGTCCAT ACAGTCAGCCGCATCTTCTT TGAACTCAAGAAGGCGGATG TGAGGAGCGATACGACATTG GCAGAGGAGATGTGCTGGAT TTCCCAAGATGTCCTTGTCC ACTTCCCTCAGCTTTCAGGA GAGAATGGGATCAACGGAGA Reverse Primer (5 to 3) TCTGTTCACACAGGCTCCAG CAGATCCATGGAGGAAGGAA GGGTTCTTCCAAACTTGCTG ATGAGCTTCCGGATCTGCT GCTTAGCCTCGTCGATGAAC TTGACGAAGCAGTCGTTGAG GCCTCAAAATCCTCTCGTTG TCACTTCATCCAAGGGCCTA ACGACCAAATCCGTTGACTC AGGGCAGGAGGAAAACCTT GGTCCATCACCTTCAGCTTC GTGGTCAGTTTGCAGCATTC TCCGCTTGTTCTCAGATCCT TGGGAGCAAAGATCCAAGAC CTGCTACAGCCTCAGCAGTG PCR Product 109 100 I00 86 95 103 90 111 94 81 80 112 96 84 110
hES, human embryonic stem.
Released by QUT under Right to Information Act
1300 MANTON ET AL. nitrogen and the culture was terminated. The morphology of the cells appeared undifferentiated at this time point. hES cells cultured in our feeder-cell-free and serum-free culture conditions showed no significant changes in the expression of Nanog, TERT, and Oct-4 as compared with hES cells cultured in KSR media on a iMEF feeder layer (Fig. 3). FOXD4 only showed significant changes in expression levels following 35 passages (P < 0.01). SOX2 expression varied throughout the culture period with expression levels ranging from being almost identical between the 2 different culture techniques (at p14 and p24) to significantly different at p17 (P < 0.05) and p35 (P < 0.01). UTF1 and Dappa expression were found to be equivalent in the 2 culture methods during the initial stages of culture (up to passage 15). However, continued culture revealed significantly different levels of expression of both these genes in the feeder-cell-free, serumfree culture compared with hES cells cultured in KSR media on iMEF feeder layer. REX-1 expression was found to be significantly lower (P < 0.001) in the hES cells cultured in feeder-cell-free and serum-free conditions throughout the entire culture period (Fig. 3).
Results hES cells maintain expression of pluripotent cell surface and intracellular markers
We determined that use of our new media containing the novel chimeric VN:IGF-I allowed hES cells to survive and be serially propagated in feeder-cell-free and serum-free culture conditions for up to 35 passages (based on marker analysis) and up to 50 passages (based on morphology alone). hES cells cultured in the feeder-cell-free and serum-free culture conditions maintained their undifferentiated state as demonstrated by high levels of expression of SSEA-4, Tra160, and Oct-4, and low or undetectable levels of expression of SSEA-1, a marker of differentiation (Fig. 1). A no primary antibody control was included to detect nonspecific binding between the secondary antibodies and the cells, and immunoreactivity was found to be minimal (data not shown). The hES cells cultured in our feeder-cell-free and serumfree conditions were observed to retain expression of pluripotent markers as shown by FACS analysis of hES cells after culture for at least 30 passages (Fig. 2). hES cells remained positive for TRA-180, SSEA-4, and Oct-4 expression (markers of undifferentiated hES cells), while the SSEA-1 (marker of differentiated cells) was undetectable.
Feeder-cell-free culture did not produce hES chromosomal abnormalities
To ensure that culturing the hES cells in our feeder-cellfree and serum-free conditions did not produce additional chromosomal abnormalities, we performed a karyotype analysis on the hES after passage 40. The karyotype of BGO1V hES cells was found to be 49, XXY, +12, +17 (ISCN Nomenclature 2009; Fig. 4). This is the original karyotype of the BGO1V cell line [22], hence no additional chromosome abnormalities were induced following culture in our feedercell-free and serum-free conditions.
hES cells retain expression of pluripotent genes
To determine the effect of our feeder-cell-free and serumfree culture conditions on hES gene expression, we compared pluripotent gene expression in hES cells grown on an irradiated iMEF feeder cell layer in KSR media (described in Materials and Methods) and hES cells grown in our new feeder-cell-free and serum-free conditions. We examined gene expression at passages 14, 17, 24, and 35 (Fig. 3). As the hES cells were passaged every 89 days, this represents ~126, 153, 216, and 315 days, respectively, of continuous feedercell-free and serum-free culture. We cultured the hES cells to ~400 days of continuous culture (representing up to 50 passages), at which point the hES cells were frozen in liquid
hES cells grown in serum-free and feeder-cell-free conditions retain the ability to differentiate
To determine if hES cells cultured in our feeder-cell-free and serum-free culture conditions retain their pluripotent
FIG. 1. Human embryonic stem (hES) cells cultured in feeder-cell-free and serum-free conditions retain expression of pluripotent cell surface and intracellular markers. hES cells that had been propagated for at least 30 passages were fixed and probed for cell surface and intracellular markers using immunofluorescence. (A) Morphology only, (B) DAPI, (C) SSEA-4, (D) Oct-4, (E) SSEA-1, and (F) TRA160 (all photos at magnification 200).
Released by QUT under Right to Information Act
SERUM-FREE AND FEEDER-CELL-FREE HES CULTURE
412 496
1301
SSEA-1
309 Counts Counts 372
Oct-4
206 R2 103
248 R2 124
0 10 0 258
10 1
10 2 FL2 Log
10 3
10 4
0 10 0 217
10 1
10 2 FL2 Log
10 3
10 4
SSEA-4
193 Counts Counts 162
TRA-180
129 R2 64
108 R2 54
0 10 0 724
10 1
10 2 FL2 Log
10 3
10 4
0 10 0
10 1
10 2 FL2 Log
10 3
10 4
Unstained
543 Counts
TRA-1-80 Oct-4
362 R2 181
SSEA4 SSEA1 Unstained 0 20 40 60 80 100
0 10 0
10 1
10 2 FL2 Log
10 3
10 4
% Cells Positive
FIG. 2. Human embryonic stem (hES) cells cultured in feeder-cell-free and serum-free conditions retain expression of pluripotent cell surface and intracellular markers. hES cells that had been propagated for at least 30 passages in serum-free and feeder-cell-free conditions were analyzed for expression of cell surface (SSEA-4, SSEA-1, and TRA-180) and intracellular (Oct-4) markers using fluorescence-activated cell sorter (FACS). Unstained cells are also shown. The graph shows the percentage of cells that were positive for undifferentiated (Oct-4, SSEA-4, TRA-180) and differentiated (SSEA-1) hES markers.
potential, we performed in vitro differentiation experiments. hES cells that had been cultured for up to 30 passages were successfully differentiated into ectoderm (nestin) and mesoderm (troponin, smooth muscle actin) tissue lineages, as shown by positive immunofluorescence (Fig. 5, top row). We also performed real-time PCR for markers of differentiation using RNA from hES cells that had undergone differentiation and compared them with RNA from hES cells that were maintained in an undifferentiated state. Real-time PCR revealed hES cells differentiated into mesoderm (HAND1, Troponin), ectoderm (nestin, MAP2), and endoderm (AFP, GATA) lineages (Fig. 5, bottom table) compared with undifferentiated hES.
Discussion
Most of the current conditions for culturing hES cells utilize animal-derived feeder cells and other poorly defined components in the culture systems. The possibility that these xeno-derived or nonsynthetic components can contaminate the hES culture process makes these cells unviable for safe and effective human clinical use. Thus, there is a global push to develop hES culture conditions that are free from xeno-derived feeder layers and components and that are instead composed of synthetic, fully defined formulations. Previously 2 key articles were published, which investigated ways to minimize the use of animal-derived
Released by QUT under Right to Information Act
1302 MANTON ET AL.
FIG. 3. Human embryonic stem (hES) cells cultured in feeder-cell-free and serum-free conditions retain expression of pluripotent genes. Real-time polymerase chain reaction (PCR) analysis was used to quantitate RNA expression of pluripotent genes in hES cells grown in feeder-cell-free and serum-free conditions after 14, 17, 24, and 35 passages. Relative expression unit (REU) values are normalized to the GAPDH housekeeping gene. Each RT-PCR analysis was performed on pooled duplicate hES samples. Error bars depict the standard deviation of triplicate real-time reactions. ***P < 0.001; **P < 0.01; *P < 0.05. components in hES culture. Ludwig and colleagues (2006) reported the development of what is now the commercially available mTESR1 media (Stem Cell Technologies, Vancouver, BC). This media utilizes BSA, insulin, purified HA, and Matrigel as replacements for the fetal calf serum and the animal-derived feeder cell layer [5,6]. Lu and colleagues (2006) published the HESCO media (not yet commercially available) that incorporates bFGF, insulin, transferrin, Wnt3a, April, and Matrigel [9]. Notably, both the mTESR1 and HESCO media still contain animal-derived substrates for coating the tissue culture plastic and large concentrations of poorly defined and/or animal-derived
Released by QUT under Right to Information Act
SERUM-FREE AND FEEDER-CELL-FREE HES CULTURE 1303 of the literature and a preliminary proteomic analysis of the culture medium (Luke Cormack, personal communication), we elected to include several proteins and metabolites in the serum-free media formulationcholesterol lipid concentrate, lithium chloride, and trace elements B and C. Interestingly, the removal of LIF did not effect the serum-free and feeder-cell-free culture of the hES cells. However, higher amounts of bFGF (100 ng/mL) were required in our current formulation compared with 20 ng/mL of bFGF, which we reported previously for the serum-free formulation in Richards (2008). This suggests that MEF feeder cells may be involved in the paracrine production of this mitogen. We also observed that growth of hES cells in the feeder-cell-free, serum-free culture system had an absolute requirement for 4 mg/mL of recombinant HA following the removal of the irradiated iMEF cell feeder layer. In addition, our new media formulation included the use of a novel VN:IGF-I chimeric protein [14]. This single chimeric protein was able to replace the IGF-I, IGFBP3, and VN that was previously used in our original serum-free media formulation [16]. This is significant as the chimeric protein offers significant manufacturing and cost advantages as only a single protein is required, rather than 3. Morphological analysis, as well as RNA and protein-based characterization studies, demonstrated that hES cells could be grown for up to 30 passages in the serum-free and feedercell-free culture system while still maintaining a phenotype equivalent to hES cells grown in serum-containing media. While acknowledging that the culture of the hES cells in the serum-free, feeder-cell-free media still requires the use of HA, we have employed recombinant HA, hence the medium we used is fully defined. Furthermore, in the main nanogram doses of proteins and growth factors are required. In addition, significantly lower amounts of recombinant HA (4 mg/mL) compared with that reported by Ludwig et al. (13 mg/mL) were incorporated into the formulation [6]. It is also interesting to note that recombinant human vitronectin has been utilized by others as a substrate for coating tissue culture plates and has been shown to support hES culture in mTeSR1 media. The hES cell growth obtained in this situation is equivalent to that of hES cells in MEF-conditioned
10
11
12
13
14
15
16
17
18
19
08-BRESA 4~A
20
032 A 1260/8896
21
49,XXY,+12,+17 QA 3
22
Y
49
FIG. 4. Feeder-cell-free and serum-free culture of the BG01V human embryonic stem (hES) cells does not produce any additional chromosome abnormalities. Karyotype analysis on the hES cells grown serum-free and feeder-cell-free conditions for 40 passages was conducted by the GTG banding method. The BG01V hES cells were found to be 49, XXY, +12, +17 (ISCN Nomenclature 2009). components. In addition, the large concentrations of proteins in these media suggest that they are not commercially viable from a cost perspective nor are they sustainable in the emerging regulatory environment. Our previous success in propagating hES cells in serumfree media [16] required the hES cells to be co-cultivated with inactivated MEF cells. We anticipated, however, that removal of the MEF cells from the culture system would require additional factors to substitute for those produced from the MEF cells in an autocrine and paracrine manner. Through analysis
Actin Smooth Muscle
Nestin
Troponin
Mesoderm Endoderm Ectoderm
HAND1 Troponin AFP GATA Nestin MAP2
P = 20 17.91 10.16 1.05 4.81 18.78 475.44
P = 25 20.74 10.85 0.83 3.25 9.25 169.93
FIG. 5. Human embryonic stem (hES) cells cultured for up to 30 passages in feeder-cell-free and serum-free conditions retain the ability to differentiate down the germ lineages. Differentiated hES cells were probed for markers of ectoderm (nestin) and mesoderm (smooth muscle actin, troponin) using immunofluorescence (top row). Real-time polymerase chain reaction (PCR) was used to determine fold increase in expression of markers of the 3 germ lineages as compared with undifferentiated hES (bottom table); p = passage number.
Released by QUT under Right to Information Act
1304 medium [7]. These results, combined with the studies we report herein demonstrating that our VN:IGF-I chimera in combination with other factors allows serum-free and feeder-cell-free culture of hES cells, indicate that vitronectin is an essential component for hES culture in either adherent [7] or soluble form (described herein). An important issue to acknowledge is that our hES culture methodology still required laminin-coated culture ware. We did conduct preliminary studies investigating other substrates for coating (ie, collagens I and IV), but found laminin to be the most desirable. Unfortunately, the current high cost of recombinant human laminin necessitated the use of purified mouse laminin for our hES studies. The next step would clearly incorporate recombinant human laminin, together with good manufacturing process (GMP)-compliant chimeric VN:IGF-I protein (currently in manufacture) to arrive at the end point of a fully defined, synthetic, recombinant GMP grade hES culture system. Clearly GMP-compliant products are required for the safe culture of hES cells for human clinical and therapeutic applications. Our characterization studies revealed that hES cells that were cultured serum-free and feeder-cell-free showed consistently low levels of REX-1 expression compared with hES cells cultured in serum-containing media on iMEF feeders. This suggests that: (1) the serum-free, feeder-cell-free culture affects the expression of REX-1 and the phenotype of the hES cells; or that (2) REX-1 is not an essential gene product required to maintain hES pluripotency. Recently, iPS cells generated by Sommer and colleagues [23] showed very low/ undetectable expression of REX-1, thus indicating that perhaps REX-1 is not an essential gene for the pluripotent phenotype in hES cells. Conversely, work by Chan and colleagues [24] showed that REX-1 expression is correlated with pluripotent, fully reprogrammed iPS cells. Hence, the true function of REX-1 in ES cells remains unclear. In addition, the expression of Dppa and UTF1 remained stable in the feeder-cellfree and serum-free hES culture while, conversely increasing in the hES cells cultured in KSR medium on iMEF feeders. However, despite these changes in gene expression, several key pluripotent genes (Nanog, TERT, and Oct-4) showed no significant changes in gene expression throughout the culture period, indicating that the hES cells cultured in feedercell-free and serum-free conditions still retain pluripotent potential and validating the media described herein. The BG01V hES cells cultured in feeder-cell-free and serum-free conditions were capable of differentiating into cells of the mesoderm lineage, as demonstrated by expression of troponin protein and the up-regulation of HAND1 and Troponin RNA expression. However, the BG01V hES showed limited potential to differentiate into cells of the endoderm lineage. Immunofluorescent reactivity for AFP was negative (data not shown) and the gene expression of AFP and GATA was only slightly increased in the differentiated hES cells. Previously, the BG01V hES cell line has been shown to transcribe relatively low AFP mRNA in differentiating hES cells, while maintaining the expression of other markers of mesoderm and pluripotency [25]. The data presented herein reflect the previously published neural (ectoderm) dominant differentiation tendency of the BGO1V hES cell line [22]. All the same, the BG01V cells have been shown by others to form teratomas containing all 3 germ lineages in SCID mice, suggesting they do in fact have pluripotent potential [22]. MANTON ET AL. The ability to culture hES cells in serum-free and feedercell-free conditions using defined synthetic formulations is clearly the preferred culture approach for toxicity testing of pharmaceutical reagents and for testing of cellular therapies [26]. This ensures that consistent results are generated as there is limited batch-to-batch or culture-to-culture variation. Although the BG01V hES cell line contains an abnormal karyotype, this cell line has been utilized as a useful experimental tool for cell therapy, developmental biology, and genetic research [22], thereby explaining our initial focus on this cell line in our evaluation of the feeder-cellfree and serum-free culture system. A further benefit of this serum-free and feeder-cell-free culture method is that it will enable proteomic analyses of hES cell-conditioned media without the complications that arise from the presence of highly abundant proteins, such as those present in animal serum. Indeed, we have recently established that the recombinant HA that is used in this media can be removed for 48 h prior to the collection of conditioned media, thus removing all highly abundant large molecular mass species to facilitate more efficient, accurate, and informative proteomic analyses. In summary, we have demonstrated the synthetic VN:IGF-I chimera and the new media formulation holds potential in supporting serum-free and feeder-cell-free culture of hES cells, and this may have profound implications in facilitating clinical translation of hES cells. For example, human epidermal cells for clinical use in skin reconstruction have recently been successfully derived from hES [27]. This technique used animal protein (ie, serum) and feeder cells for both the hES cell culture and the epidermal differentiation processes. The hES culture methodology we report here, combined with our previously published methods for serum-free culture of human keratinocytes and dermal fibroblasts [16,17], suggests it will be possible to achieve completely xeno-free derivation of such epidermal cells from hES for safe effective clinical use. This culture methodology, incorporating our chimeric VN:IGF-I protein, not only holds the potential to deliver benefits to the hES research community, but also provides a clear way forward toward the generation of safe, reliable culture of sufficient quantities of hES cells for human clinical therapies. Moreover, this may well be achievable at lower cost than has been reported previously by others.
Acknowledgments
We thank Dr. Leonore De Bore for assistance with the FACS analysis. This work was funded by a Queensland Government Smart State Research Industries Partnership Program (RIPP) Grant and the Queensland University of Technology (QUT) Tissue Repair and Regeneration Program. ZU was supported by a Queensland State Government Smart State Senior Fellowship.
Author Disclosure Statement
No competing financial interests exist.
References
1. Thomson JA, J Itskovitz-Eldor, SS Shapiro, MA Waknitz, JJ Swiergiel, VS Marshall and JM Jones. (1998). Embryonic stem cell lines derived from human blastocysts. Science 282:1145 1147.
Released by QUT under Right to Information Act
SERUM-FREE AND FEEDER-CELL-FREE HES CULTURE
2. Martin MJ, A Muotri, F Gage and A Varki. (2005). Human embryonic stem cells express an immunogenic nonhuman sialic acid. Nat Med 11:228 232. 3. Heiskanen A, T Satomaa, S Tiitinen, A Laitinen, S Mannelin, U Impola, M Mikkola, C Olsson, H Miller-Podraza, M Blomqvist, A Olonen, H Salo, P Lehenkari, T Tuuri, T Otonkoski, J Natunen, J Saarinen and J Laine. (2007). N-glycolylneuraminic acid xenoantigen contamination of human embryonic and mesenchymal stem cells is substantially reversible. Stem Cells 25:197202. 4. Xu C, MS Inokuma, J Denham, K Golds, P Kundu, JD Gold and MK Carpenter. (2001). Feeder-free growth of undifferentiated human embryonic stem cells. Nat Biotechnol 19:971974. 5. Ludwig TE, V Bergendahl, ME Levenstein, J Yu, MD Probasco and JA Thomson. (2006). Feeder-independent culture of human embryonic stem cells. Nat Methods 3:637 646. 6. Ludwig TE, ME Levenstein, JM Jones, WT Berggren, ER Mitchen, JL Frane, LJ Crandall, CA Daigh, KR Conard, MS Piekarczyk, RA Llanas and JA Thomson. (2006). Derivation of human embryonic stem cells in defined conditions. Nat Biotechnol 24:185 187. 7. Braam SR, L Zeinstra, S Litjens, D Ward-van Oostwaard, S van den Brink, L van Laake, F Lebrin, P Kats, R Hochstenbach, R Passier, A Sonnenberg and CL Mummery. (2008). Recombinant vitronectin is a functionally defi ned substrate that supports human embryonic stem cell self-renewal via alpha V beta 5 integrin. Stem Cells 26:22572265. 8. Derda R MS, BP Orner, JR Klim, L Li and LL Kiessling. (2010). High-throughput discovery of synthetic surfaces that support proliferation of pluripotent cells. J Am Chem Soc. [Epub ahead of print] 9. Lu J, R Hou, CJ Booth, SH Yang and M Snyder. (2006). Defined culture conditions of human embryonic stem cells. Proc Natl Acad Sci USA 103:5688 5693. 10. Kricker JA, CL Towne, SM Firth, AC Herington and Z Upton. (2003). Structural and functional evidence for the interaction of insulin-like growth factors (IGFs) and IGF binding proteins with vitronectin. Endocrinology 144:28072815. 11. Upton Z, H Webb, K Hale, CA Yandell, JP McMurtry, GL Francis and FJ Ballard. (1999). Identification of vitronectin as a novel insulin-like growth factor-II binding protein. Endocrinology 140:2928 2931. 12. Ainscough SL, Z Barnard, Z Upton and DG Harkin. (2006). Vitronectin supports migratory responses of corneal epithelial cells to substrate bound IGF-I and HGF, and facilitates serumfree cultivation. Exp Eye Res 83:1505 1514. 13. Hollier BG, JA Kricker, DR Van Lonkhuyzen, DI Leavesley and Z Upton. (2008). Substrate-bound insulin-like growth factor (IGF)-I-IGF binding protein-vitronectin-stimulated breast cell migration is enhanced by coactivation of the phosphatidylinositide 3-Kinase/AKT pathway by alphav-integrins and the IGF-I receptor. Endocrinology 149:1075 1090. 14. Van Lonkhuyzen DR, GK Shooter and Z Upton. (2005). Production and characterization of a Vitronectin-insulin-like growth factor-I chimeric molecule. FEBS J 272:278 279. 15. Van Lonkhuyzen DR, BG Hollier, GK Shooter, DI Leavesley and Z Upton. (2007). Chimeric vitronectin:insulin-like growth factor proteins enhance cell growth and migration through coactivation of receptors. Growth Factors 25:295 308. 16. Richards S, D Leavesley, G Topping and Z Upton. (2008). Development of defined media for the serum-free expansion of primary keratinocytes and human embryonic stem cells. Tissue Eng Part C Methods 14:221232.
1305
17. Mujaj S, K Manton, Z Upton and S Richards. (2010). Serum-Free Primary Human Fibroblast and Keratinocyte Coculture. Tissue Eng Part A. 18. DAmour KA, AD Agulnick, S Eliazer, OG Kelly, E Kroon and EE Baetge. (2005). Efficient differentiation of human embryonic stem cells to defi nitive endoderm. Nat Biotechnol 23:1534 1541. 19. Chen HF, HC Kuo, CL Chien, CT Shun, YL Yao, PL Ip, CY Chuang, CC Wang, YS Yang and HN Ho. (2007). Derivation, characterization and differentiation of human embryonic stem cells: comparing serum-containing versus serum-free media and evidence of germ cell differentiation. Hum Reprod 22:567577. 20. Seabright M. (1971). A rapid banding technique for human chromosomes. Lancet 2:971972. 21. Wang L, L Li, P Menendez, C Cerdan and M Bhatia. (2005). Human embryonic stem cells maintained in the absence of mouse embryonic fibroblasts or conditioned media are capable of hematopoietic development. Blood 105:4598 4603. 22. Plaia TW, R Josephson, Y Liu, X Zeng, C Ording, A Toumadje, SN Brimble, ES Sherrer, EW Uhl, WJ Freed, TC Schulz, A Maitra, MS Rao and JM Auerbach. (2006). Characterization of a new NIH-registered variant human embryonic stem cell line, BG01V: a tool for human embryonic stem cell research. Stem Cells 24:531 546. 23. Sommer CA, M Stadtfeld, GJ Murphy, K Hochedlinger, DN Kotton and G Mostoslavsky. (2009). Induced pluripotent stem cell generation using a single lentiviral stem cell cassette. Stem Cells 27:543 549. 24. Chan EM, S Ratanasirintrawoot, IH Park, PD Manos, YH Loh, H Huo, JD Miller, O Hartung, J Rho, TA Ince, GQ Daley and TM Schlaeger. (2009). Live cell imaging distinguishes bona fide human iPS cells from partially reprogrammed cells. Nat Biotechnol 27:1033 1037. 25. Tavakoli T, XR Xu, E Derby, Y Serebryakova, Y Reid, MS Rao, MP Mattson and W Ma. (2009). Self-renewal and differentiation capabilities are variable between human embryonic stem cell lines 13, 16 and BG01V. BMC Cell Biol 10:44. 26. Amen C, R Strehl, P Bjrquist, A Lindahl, J Hyllner and P Sartipy. (2008). Human embryonic stem cells: current technologies and emerging industrial applications. Crit Rev Oncol Hematol 65:54 80. 27. Guenou H, X Nissan, F Larcher, J Feteira, G Lemaitre, M Saidani, M Del Rio, CC Barrault, FX Bernard, M Peschanski, C Baldeschi and G Waksman. (2009). Human embryonic stem-cell derivatives for full reconstruction of the pluristratified epidermis: a preclinical study. Lancet 374:1745 1753.
Address correspondence to: Dr. Kerry J. Manton Tissue Repair and Regeneration Program Institute of Health and Biomedical Innovation Queensland University of Technology 60 Musk Avenue Kelvin Grove, QLD 4059 Australia E-mail: kerry.manton@qut.edu.au Received for publication December 15, 2009 Accepted after revision February 2, 2010 Prepublished on Liebert Instant Online February 3, 2010
Released by QUT under Right to Information Act
Вам также может понравиться
- Comparative Proteomic Analysis of Extracellular Proteins of Edwardsiella TardaДокумент43 страницыComparative Proteomic Analysis of Extracellular Proteins of Edwardsiella TardaArif SetiawanОценок пока нет
- 1 s2.0 S1934590907000677 MainДокумент7 страниц1 s2.0 S1934590907000677 MainsuperherosavesОценок пока нет
- A Nature20810Документ17 страницA Nature20810pnom43582Оценок пока нет
- C-FRC Hippo 2020 NatComДокумент15 страницC-FRC Hippo 2020 NatCompnom43582Оценок пока нет
- 10.1038@s41588 018 0315 5Документ20 страниц10.1038@s41588 018 0315 5Omar FernándezОценок пока нет
- Science - Adf1226 SMДокумент23 страницыScience - Adf1226 SMbenqtenОценок пока нет
- Ronda2019 SupДокумент31 страницаRonda2019 SupEmna BouhajjaОценок пока нет
- Centromere Protein A Dynamics in Human Pluripotent Stem Cell Self-Renewal, Differentiation and DNA DamageДокумент13 страницCentromere Protein A Dynamics in Human Pluripotent Stem Cell Self-Renewal, Differentiation and DNA Damage10sgОценок пока нет
- Resource: A TALEN Genome-Editing System For Generating Human Stem Cell-Based Disease ModelsДокумент14 страницResource: A TALEN Genome-Editing System For Generating Human Stem Cell-Based Disease ModelsfitkaОценок пока нет
- Partial Atle Sequencing of Staphylococcus Epidermidis Strains From Prosthetic Joint InfectionsДокумент4 страницыPartial Atle Sequencing of Staphylococcus Epidermidis Strains From Prosthetic Joint InfectionsDarshan MarjadiОценок пока нет
- JC I 97119492Документ8 страницJC I 97119492fransiskaОценок пока нет
- Albert Et Al., 2014 PDFДокумент19 страницAlbert Et Al., 2014 PDFIndrani BhattacharyaОценок пока нет
- Chromosomal Aberrations Induced by 5-Azacytidine Combined With VP-16 (Etoposide) in CHO-K1 and XRS-5 Cell LinesДокумент16 страницChromosomal Aberrations Induced by 5-Azacytidine Combined With VP-16 (Etoposide) in CHO-K1 and XRS-5 Cell LinesAry AguiarОценок пока нет
- Plate I Xvii: Figure 2A.2 Figure 2A.3Документ12 страницPlate I Xvii: Figure 2A.2 Figure 2A.3Xing WeiОценок пока нет
- Analysis On Protein Expression MiceДокумент11 страницAnalysis On Protein Expression MiceShonil DabreoОценок пока нет
- Evidence For Two Types of Brown Adipose Tissue in Humans: LettersДокумент5 страницEvidence For Two Types of Brown Adipose Tissue in Humans: LettersgotokoreaОценок пока нет
- J. Biol. Chem.-1992-Sadoshima-10551-60Документ10 страницJ. Biol. Chem.-1992-Sadoshima-10551-60Yusa ResaОценок пока нет
- Production and Control of Coagulation Proteins For Factor X Activation in Human Endothelial Cells and FibroblastsДокумент19 страницProduction and Control of Coagulation Proteins For Factor X Activation in Human Endothelial Cells and FibroblastsEsteban TapiaОценок пока нет
- Yilmaz 2006Документ8 страницYilmaz 2006haemophilicОценок пока нет
- Stem Cell ResearchДокумент3 страницыStem Cell Researchjhanel bacquianОценок пока нет
- Clinical Proteomics NM23Документ9 страницClinical Proteomics NM23suryasantoshОценок пока нет
- Microduplicación de xp22.31Документ14 страницMicroduplicación de xp22.31Roberth SanchezОценок пока нет
- PARODI - Not Proper ROC Curves As New Tool For The Analysis of Differentially Expressed Genes in Microarray ExperimentsДокумент30 страницPARODI - Not Proper ROC Curves As New Tool For The Analysis of Differentially Expressed Genes in Microarray ExperimentsAnonymous PsEz5kGVaeОценок пока нет
- Targeting LIPA Independent of Its Lipase Activity Is A Therapeutic Strategy in Solid Tumors Via Induction of Endoplasmic Reticulum StressДокумент40 страницTargeting LIPA Independent of Its Lipase Activity Is A Therapeutic Strategy in Solid Tumors Via Induction of Endoplasmic Reticulum Stressender000Оценок пока нет
- Takahashi - Yamanaka 2006Документ14 страницTakahashi - Yamanaka 2006Andrea VelaОценок пока нет
- AutophagyДокумент7 страницAutophagyMartga Bella RahimiОценок пока нет
- Stem Cell Research: Lab Resource: Multiple Cell LinesДокумент5 страницStem Cell Research: Lab Resource: Multiple Cell LinesGeos KarОценок пока нет
- 200 TTT A Systematic Survey of Loss of Function Variants in Human Protein Coding GenesДокумент8 страниц200 TTT A Systematic Survey of Loss of Function Variants in Human Protein Coding GenesFlorence FlorahОценок пока нет
- Brief Definitive ReportДокумент6 страницBrief Definitive ReportenilОценок пока нет
- Human Embryonic Stem Cells and Gene TherapyДокумент17 страницHuman Embryonic Stem Cells and Gene Therapyhiazz17Оценок пока нет
- Arti IngleДокумент6 страницArti Ingleshadow darkОценок пока нет
- Evidence for Human Lung Stem Cells: Identification and CharacterizationДокумент11 страницEvidence for Human Lung Stem Cells: Identification and CharacterizationChairunisa AnggrainiОценок пока нет
- Hybrid Embryonic Stem Cell-Derived Tetraploid Mice Show Apparently Normal Morphological, Physiological, and Neurological CharacteristicsДокумент8 страницHybrid Embryonic Stem Cell-Derived Tetraploid Mice Show Apparently Normal Morphological, Physiological, and Neurological CharacteristicsGmewop30mОценок пока нет
- Letter: Genome-Wide Analysis of A Long-Term Evolution Experiment With DrosophilaДокумент6 страницLetter: Genome-Wide Analysis of A Long-Term Evolution Experiment With DrosophilaPeteMossОценок пока нет
- Significance of The Patched Gene in Developmental Biology: Evan Zhou T05 CMMB 403Документ9 страницSignificance of The Patched Gene in Developmental Biology: Evan Zhou T05 CMMB 403EvanОценок пока нет
- Chromatin State Maps New Technologies, New InsightsДокумент12 страницChromatin State Maps New Technologies, New InsightsRebecca PotterОценок пока нет
- Xun Xu The Genomic Sequence Chinese HamsterДокумент8 страницXun Xu The Genomic Sequence Chinese HamsterAbhishek NaikОценок пока нет
- Bone Marrow Mesenchymal Stem Cells For Improving Hematopoietic Function An in Vitro and in Vivo Model On Bone Marrow MicroenvironmentДокумент9 страницBone Marrow Mesenchymal Stem Cells For Improving Hematopoietic Function An in Vitro and in Vivo Model On Bone Marrow Microenvironmentyanyan wangОценок пока нет
- 1 - Immunofluorescent StainingДокумент5 страниц1 - Immunofluorescent StainingNini CioconОценок пока нет
- 2020 - Article - 18180 11Документ13 страниц2020 - Article - 18180 11PabloОценок пока нет
- Process-related impurities found in ChAdOx1 nCov-19 vaccine including human proteinsДокумент29 страницProcess-related impurities found in ChAdOx1 nCov-19 vaccine including human proteinsGerardo de Gyves AvilaОценок пока нет
- CTM2 12 E823Документ7 страницCTM2 12 E823Emanuel Eduardo Lopez LopezОценок пока нет
- Material Suplementario Art. 7 PDFДокумент11 страницMaterial Suplementario Art. 7 PDFmaria alejandra parada aguilarОценок пока нет
- MS 1-1 MS 1-3: Christine Sers Burkhard Scharm, Reinhold SCH FerДокумент47 страницMS 1-1 MS 1-3: Christine Sers Burkhard Scharm, Reinhold SCH FerindilaОценок пока нет
- 0203 Fin MarkДокумент5 страниц0203 Fin Markapi-3839553Оценок пока нет
- Loems Ziegler - MonocytesДокумент10 страницLoems Ziegler - MonocytesRaluca ChiruОценок пока нет
- Neuronal Migration Genes and A Familial Translocation T (3 17) : Candidate Genes Implicated in The PhenotypeДокумент11 страницNeuronal Migration Genes and A Familial Translocation T (3 17) : Candidate Genes Implicated in The PhenotypeTiago ChavesОценок пока нет
- PLOS One-6-E21250-2011Документ16 страницPLOS One-6-E21250-2011Sandi Head McAdamsОценок пока нет
- Counting Live and Dead Bull SpermatozoaДокумент6 страницCounting Live and Dead Bull SpermatozoaSadam IrshadОценок пока нет
- MHC Restricted Cytotoxic T Cell ResponsesДокумент6 страницMHC Restricted Cytotoxic T Cell ResponsesAnthony NguyenОценок пока нет
- 4 UploadДокумент12 страниц4 Uploadlsaranya308Оценок пока нет
- Takahashi Yamanaka Cell06Документ14 страницTakahashi Yamanaka Cell06abcdefgОценок пока нет
- A three-state model for integrating multidimensional genomic dataДокумент5 страницA three-state model for integrating multidimensional genomic dataRodrigo Mendoza SmithОценок пока нет
- Forensic Science International Genetics 2016 NovroskiДокумент13 страницForensic Science International Genetics 2016 NovroskiMukul SuryawanshiОценок пока нет
- Utilizado - JoenjeДокумент4 страницыUtilizado - JoenjedeliveryworkОценок пока нет
- 2012 Plosone Meaf Phf1Документ6 страниц2012 Plosone Meaf Phf1Ioannis PanagopoulosОценок пока нет
- 14 2019 流式诊断陷阱Документ15 страниц14 2019 流式诊断陷阱huangzz1016Оценок пока нет
- Nature 04559Документ4 страницыNature 04559Benjan't PujadasОценок пока нет
- DNA Methods in Food Safety: Molecular Typing of Foodborne and Waterborne Bacterial PathogensОт EverandDNA Methods in Food Safety: Molecular Typing of Foodborne and Waterborne Bacterial PathogensОценок пока нет
- Mechanism of Tissue RepairДокумент22 страницыMechanism of Tissue RepairWalidad AarbiОценок пока нет
- CapixylДокумент6 страницCapixylZoè NoyolaОценок пока нет
- Jimenez, 2004Документ9 страницJimenez, 2004wanda oktariaОценок пока нет
- Pathophysiology of Varicose Vein - Chronic Venous InsufficiencyДокумент31 страницаPathophysiology of Varicose Vein - Chronic Venous InsufficiencyDavid Christian100% (1)
- Connective TissueДокумент26 страницConnective TissueooiszehuiОценок пока нет
- Granactive Acne BrochureДокумент20 страницGranactive Acne Brochureapi-291771056100% (1)
- Sunekos Clinical-StudiesДокумент6 страницSunekos Clinical-StudiesGustavo Henrique MüllerОценок пока нет
- Enhancing Skin Health: by Oral Administration of Natural Compounds and Minerals With Implications To The Dermal MicrobiomeДокумент35 страницEnhancing Skin Health: by Oral Administration of Natural Compounds and Minerals With Implications To The Dermal MicrobiomeMiruna Deleanu MariaОценок пока нет
- Review Article: Amnion and Chorion Membranes: Potential Stem Cell Reservoir With Wide Applications in PeriodonticsДокумент10 страницReview Article: Amnion and Chorion Membranes: Potential Stem Cell Reservoir With Wide Applications in PeriodonticsElissa IsdasariОценок пока нет
- Path NotesДокумент111 страницPath NotesNirav Patel100% (1)
- Fascia Science and Clinical Applications ReviewДокумент17 страницFascia Science and Clinical Applications ReviewWanniely KussОценок пока нет
- Cobalt-Chromium Alloys Fabricated With Four Different Techniques: Ion Release, Toxicity of Released Elements and Surface RoughnessДокумент12 страницCobalt-Chromium Alloys Fabricated With Four Different Techniques: Ion Release, Toxicity of Released Elements and Surface RoughnessUntuk TugasОценок пока нет
- Primer: Frozen ShoulderДокумент16 страницPrimer: Frozen ShoulderJose PerezОценок пока нет
- Cryptogenic Organizing Pneumonia: Review ArticleДокумент12 страницCryptogenic Organizing Pneumonia: Review ArticleMatías Jesús Flamm ZamoranoОценок пока нет
- Morphogenetic Fields in Embryogenesis, Regeneration, and Cancer - Non-Local Control of Complex PatterningДокумент41 страницаMorphogenetic Fields in Embryogenesis, Regeneration, and Cancer - Non-Local Control of Complex PatterningritaОценок пока нет
- (Psychology of Emotions, Motivations and Actions) Leandro Cavalcanti (Ed.), Sofia Azevedo (Ed.) - Psychology of Stress - New Research-Nova Science Publishers (2013)Документ214 страниц(Psychology of Emotions, Motivations and Actions) Leandro Cavalcanti (Ed.), Sofia Azevedo (Ed.) - Psychology of Stress - New Research-Nova Science Publishers (2013)Jesus Jimenez100% (1)
- Acupuncture Connective Tissue and Peripheral Sensory ModulationДокумент6 страницAcupuncture Connective Tissue and Peripheral Sensory ModulationClaudia de MenesesОценок пока нет
- Tissue Repair: Regeneration, Healing, Fibrosis & Growth FactorsДокумент13 страницTissue Repair: Regeneration, Healing, Fibrosis & Growth Factorsمحسن حدوان عليخانОценок пока нет
- AP Biology Webquest Cell SignalingДокумент7 страницAP Biology Webquest Cell Signalingtori100% (1)
- Connective Tissue Histology LectureДокумент19 страницConnective Tissue Histology LectureLOUISSE ANNE MONIQUE L. CAYLOОценок пока нет
- Gingival Enlargement Causes, Classification and GradingДокумент49 страницGingival Enlargement Causes, Classification and Gradingdhwanit31100% (1)
- Granulation Tissue 13102015Документ33 страницыGranulation Tissue 13102015Widi Widurai100% (1)
- HistologyДокумент116 страницHistologyAshwani KumarОценок пока нет
- GRC 2023 PCTSДокумент1 страницаGRC 2023 PCTS김현수Оценок пока нет
- Owth Factors Profile in Conditioned MediumДокумент11 страницOwth Factors Profile in Conditioned MediumApriyanti SembiringОценок пока нет
- CollagenДокумент39 страницCollagenSaaraAlleyahAlAnazi100% (1)
- Yy Yyy Y Yy Y YYYДокумент64 страницыYy Yyy Y Yy Y YYYsreedam100% (1)
- M.Phil Thesis of Asma SohailДокумент64 страницыM.Phil Thesis of Asma SohailRehan Sadiq ShaikhОценок пока нет
- 2014 - MesenchymalДокумент18 страниц2014 - MesenchymalMiguel ÁngelОценок пока нет
- Mitochondrial Energy Metabolism in Baby Hamster Kidney (BHK-21/C13) Cells Treated With Karnozin EXTRA®Документ7 страницMitochondrial Energy Metabolism in Baby Hamster Kidney (BHK-21/C13) Cells Treated With Karnozin EXTRA®Garbuz ElenaОценок пока нет