Академический Документы
Профессиональный Документы
Культура Документы
Develop More Accurate Prediction of Flash Points
Загружено:
NXVNИсходное описание:
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
Develop More Accurate Prediction of Flash Points
Загружено:
NXVNАвторское право:
Доступные форматы
PROCESS DEVELOPMENTS
Develop more accurate prediction of flash points
New calculation improves estimating minimum flash temperature for petroleum products
K"
ffi
,&V,&K&.&*i&ffi,
{,*rp.
l-lr:i", fr
rj*r*1,
flash point of a hydrocarbon mixture is a very imporrant parameter especially with regards to the safe handiing, storage r,, and transportation of hydrocarbons. By definition, the flash point is the minimum temperature at which vapors from the mixtue would produce a momentary flash when subjected to a standard test flame. The two widely accepted test methods for fiash-point determinations are Pens Martins and Abels flash apparatus. Flash points are extremely dependent on the lighter ends of any petroleum fraction.
'::""'"':'"'):':he
For fractions up to 500"F, Eq. 2 predicts flash poinrs with
reasonable accuracy, while Eq. 1 is applicable for higher boiling
,:
point fractions.
Methods. Riazi and Daubert determined that for petroleum fractions, ASTM 10% is more appropriate for calculating flash points. They proposed two equations for predicting flash points:
Better prediction method. A slight modification can predict the flash point value even more closer to the experimental value. Instead of taking ASTM 107o, if one replaces the value of ASTM 10% with the average of the initial boiling point (IBP), 5o/o and 10% in Eq. 1, the calculated flash point values obtained are very much closer to experimental values. Tble 1 summaizes the observations for diesel flash points ofvarious units from a refinery.
rf
tfr, = -o.ot+568+(2.84e4t lr,)*(,.r0: " ro-')r"
where
r,
rr:
-o.or+s6s +(z.a4t47lr^"0)*
(r.loe,
ro-3
(r
)ua"o
(3)
is the
Q and I1
are
in'R *O
ASTM 10% temperature of petroleum fraction.
where f,,, ir {uun .
5% + 10%)/31
in 'R and
is the fiash
point in "R.
Tf = -t24.72+0.707047,(+
are
in'F)
Error analysis.
Q)
Tble 2 lists the error analysis berween experimental and predicted values using Eq. 3.
Calculated flash points of diesel with varying ASTMs for different refineries
ASTM distillation
Samples
Diesel Diesel
Density
IBP
1 816.5 162 2 824.3 194 Diesel 3 853.9 230 Diesel4 sil.- - is) Diesel5 8058 152
flash point, 'C D1 = flash point estrmated from Eq. l pont D2 = f ash estrmated from Eq. 2
Exp =
5o/o 'l0o/o 50% 185 195 256 220 233 213 255 268 314 260 275 317 '168 176 263
90!o
JJ
I
95o/o FBP
352 346
381
Flash
55
Exp pt
b9
97 103 45
Flash,
330 367
360
D2 Mod (0,5,10) f Flash, "C "C 'C 66.96 63.38 180.67 57.32 g0.24 90.40 215.67 A.0
Flash2,
Dl
392
3g7
10e.1 112.59 54.10
lti,..lg
11g.34
zsi.o
roo.jg
3to
348
385 358
372
49.94
257.33 103.74 '165.33 46.56
sps,r..,,
25
Modfed=FlashpointestimtedfrommodifredEq. l,bysubstituting(1BP+5%+'T0%)/3
in place of ASTM 1 0%
20
Error analysis between experimental and
predicted values
r
t' o
Mod
2.32
15
Samples
Diesel
1
D1
1'l .96
D2
Elo
5 0
8.38 21.24 17.99
16.g4
1
Diesel 2 Diesei 3 Diesel 4 Diesel
5
21.40 12.16 9.59
1.09
i.s
0.7 4
Diesel
't
Diesel
Diesel
Diesel
Diesel 5
e.io
4.94
1.56
HyDRocARBoNpRocrss\c
ruavzooa
|,
PROCESS DEVELOPM ENTS
Comparison of predicted flash points with various dieselfractions using the f (0,5,10) method
160 140
I
Samples r
Sample
1
Sample 2 Sample 3 Sample 4 Sample 5 Sample 6 Sample 7 Sample 8 Sample 9 Sample 10 Sample
11
(0,5,10) 161 .3 162.6 163.6 164.6 166.3 169 110 177.3 17g 296.6 31 3.6
Dl
52
49.8 49.8
53.4
51 .2
57.5 76.5 88.6
66.29 124.1
13'r .6
D2 47.8 45.7 45.6 4g.2 41.1 53.4 88.1 62.6 139 51
'1
Mod
+31
44.6
Exp
41
120
O
l;;;pl
<*r*#l "L---rz_-\.-
'It-aroeatl
.^100
t
45
45.3
46.1
46
42 43
E o E
--v
360
40 20 0
,r'\
\d/
I
47.2 49.1 +:e.8
13.e
48 40 39 49 138
141
50.8
56.1
Samples
122.5
129.5
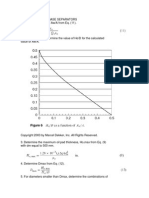
As is quite evident fromTable 2 and Fig. 1, using f (0, 5, 10)-Eq. 3-in place of only 10% yields a flash point value which is close to the experimentally determined value. AIso, from a safery viewpoint, predicting a lower flash value is always safer rvhen handling and transporting petroleum fractions. To further test its compatibiliry a comparison using different fractions rvas carried out. Table 3 and Figs. 2-4list the resulrs of this study. From Figs. 2-4, the modified Riazi and Daubertt eqi-ration is accurate for all fractions with minor deviations from the experimenta-l values in the mid range. Most modern simulation and chemical software packages take into account Daubert equation for predicting flash
160 140 --l
120
I rr-
+oz
fxp
.
lLaroeazI
"-100
'e
o
80
\,r
860
40
U
*-
,/\l
\/
,1
-*
t)
l0
12
Samples
r#K
fip- Iis"{r* #*rx
o
o t
160
140
_l+.No
120
l.c-
exp
l_
I
"-100
'6
iE
80
360
40 20
0
I
J
6 Samples
poir.rts of petroleum fractions. By considering the average of IB!,5o/o
and 10%, o.ne can predict flash points even more accurately. Since, at the IBB we get the first drop of the fraction, it is at this point that the flash is predominant. So, the average of the first three points will give a more accllrate prediction of the flash point. HP
LITERATURE CITED
r Riazi, M. R. and T.
F.. Dauberr, "Predicting flash ancl
1
pour points," Hylrocdrbon
Prorcsing,Scpternber
987, pp. 81-83.
K. R. Ramakumar is a product on engineer at Indian Orl Corp. Ltd., Gujarat, lnd a. He has a BS degree in chemical engrneering from Sardar Val abhbhai National lnstitute of Technology, Surat, Gularat, where he was a bachelor of chemica enqlneer nq
jj,ii,,t:.i
&*JJi*
f**++*s:**; i;*
.&;*,.;"f
Select 1 79 at www.HydrocarbonProcessing.com/RS
Вам также может понравиться
- Incropera - Dewitt Answers To Selected ExercisesДокумент29 страницIncropera - Dewitt Answers To Selected ExercisesMatthew Eyre100% (1)
- HRSG Understand The BasicsДокумент14 страницHRSG Understand The BasicsMazen Darwish100% (1)
- Predict Storage-Tank Heat Transfer: PreciselyДокумент6 страницPredict Storage-Tank Heat Transfer: PreciselyRuben LealОценок пока нет
- ENERGY STAR Guide Petroleum Refineries 20150330Документ7 страницENERGY STAR Guide Petroleum Refineries 20150330rameshkarthik810Оценок пока нет
- Ibp1325 12Документ7 страницIbp1325 12Marcelo Varejão CasarinОценок пока нет
- Comparison Between Rotary PD Pumps & Centrifugal PumpsДокумент2 страницыComparison Between Rotary PD Pumps & Centrifugal Pumpsrafli septiwanОценок пока нет
- VALVE TYPES AssignmentДокумент10 страницVALVE TYPES Assignmentbabe100% (2)
- Curve Char AДокумент3 страницыCurve Char AvantaОценок пока нет
- Hydro Processing Corrosion Wash WaterДокумент9 страницHydro Processing Corrosion Wash WaterNagendra H100% (1)
- Chemistry of Crudes - DR y K SharmaДокумент48 страницChemistry of Crudes - DR y K Sharmasuprateem100% (1)
- 112 Refinery Overview ChevronДокумент2 страницы112 Refinery Overview Chevronupender345Оценок пока нет
- Horizontal - THREE (Revisar, Petroleum and Gas Field ProcessingДокумент4 страницыHorizontal - THREE (Revisar, Petroleum and Gas Field Processingrene123456789eduardoОценок пока нет
- Reliance HPC Course 2009 - 01 - IntroductionДокумент8 страницReliance HPC Course 2009 - 01 - IntroductionsuprateemОценок пока нет
- Code of Practice forThermalOilHeaters PDFДокумент56 страницCode of Practice forThermalOilHeaters PDFarjmandquestОценок пока нет
- PHD Thesis Naveen BhutaniДокумент252 страницыPHD Thesis Naveen BhutaniSagar SrinivasОценок пока нет
- Designing Atmospheric Crude Distillation For Bitumen Service PDFДокумент6 страницDesigning Atmospheric Crude Distillation For Bitumen Service PDFfawmer61Оценок пока нет
- Integrating CDU, FCC and Product Blending Models Into Refinery Planning PDFДокумент19 страницIntegrating CDU, FCC and Product Blending Models Into Refinery Planning PDFahmed1581973Оценок пока нет
- 08 Hydrocracking Example PDFДокумент17 страниц08 Hydrocracking Example PDFAshwani KumarОценок пока нет
- Control Valve CoefficientsДокумент2 страницыControl Valve CoefficientsjroperОценок пока нет
- Repsol - Use of Simulation For HDS UnitДокумент16 страницRepsol - Use of Simulation For HDS UnitHoracio RodriguezОценок пока нет
- Troubleshooting Liquid Carryover in Gas Compression Systems MySep White PaperДокумент11 страницTroubleshooting Liquid Carryover in Gas Compression Systems MySep White Paperthlim19078656Оценок пока нет
- AspenHYSYSRefiningV7 3 OpsДокумент478 страницAspenHYSYSRefiningV7 3 OpsAnonymous w46Y2Qf84Оценок пока нет
- Oil Ref Walk ThroughДокумент7 страницOil Ref Walk ThroughSumedh SinghОценок пока нет
- Select the Best Fitting Pressure Loss CorrelationДокумент4 страницыSelect the Best Fitting Pressure Loss Correlationpal_stephenОценок пока нет
- Don Colley Presentation PDFДокумент38 страницDon Colley Presentation PDFHIPAPОценок пока нет
- Hot Oil SystemДокумент2 страницыHot Oil SystemAbdulmalik HakimОценок пока нет
- Refrig JT Plant Brochures - SNC - Valerus - Recd 20170623Документ4 страницыRefrig JT Plant Brochures - SNC - Valerus - Recd 20170623johnОценок пока нет
- Freeze Protection: Heat TracingДокумент4 страницыFreeze Protection: Heat TracingNaser JahangiriОценок пока нет
- Understanding CFPP: The Cold Filter Plugging PointДокумент2 страницыUnderstanding CFPP: The Cold Filter Plugging PointNenad PopovicОценок пока нет
- CHE Facts 1211Документ1 страницаCHE Facts 1211kumar_chemicalОценок пока нет
- D1740Документ7 страницD1740rpajaro75Оценок пока нет
- Increase Crude Unit Capacity Through Better IntegrationДокумент3 страницыIncrease Crude Unit Capacity Through Better IntegrationRuben LealОценок пока нет
- Chemsep Tutorial: Distillation With Hypothetical ComponentsДокумент25 страницChemsep Tutorial: Distillation With Hypothetical ComponentsErving MJОценок пока нет
- DynsimДокумент22 страницыDynsimAbdomatarОценок пока нет
- For Exchanger Tube Rupture PDFДокумент3 страницыFor Exchanger Tube Rupture PDFNikhil DivateОценок пока нет
- 2009-03 CleanDieselHydroPTQ MustangДокумент7 страниц2009-03 CleanDieselHydroPTQ Mustanganhchangleloi100% (2)
- Bitumen Processing: Crude Unit RevampsДокумент10 страницBitumen Processing: Crude Unit Revampszubair1951Оценок пока нет
- Constituent Pure Liquid - Capacity Certified (Pure Toluene) Pressure Relief Valve PSV 102Документ6 страницConstituent Pure Liquid - Capacity Certified (Pure Toluene) Pressure Relief Valve PSV 102CHONG HAN LIN ALLANОценок пока нет
- PUMPS ProblemsДокумент1 страницаPUMPS ProblemsGeorge Isaac McQuilesОценок пока нет
- Reducing Pressure - Increasing Efficiency: PanoramaДокумент4 страницыReducing Pressure - Increasing Efficiency: PanoramapsshnkrОценок пока нет
- Advanced Heat Exchanger Design TechniquesДокумент9 страницAdvanced Heat Exchanger Design Techniquesjdgh1986Оценок пока нет
- PC 2 2008 MohaddecyДокумент8 страницPC 2 2008 MohaddecyAnonymous 1FaavtОценок пока нет
- Gravity Separator Fundamentals and DesignДокумент29 страницGravity Separator Fundamentals and Designmatrix69Оценок пока нет
- Chemicalengineeringmagzinenov2012 PDFДокумент77 страницChemicalengineeringmagzinenov2012 PDF施君儒Оценок пока нет
- Crude Tower Simulation in HYSYSДокумент42 страницыCrude Tower Simulation in HYSYSEstrellaОценок пока нет
- Process Engineering for a Small Planet: How to Reuse, Re-Purpose, and Retrofit Existing Process EquipmentОт EverandProcess Engineering for a Small Planet: How to Reuse, Re-Purpose, and Retrofit Existing Process EquipmentОценок пока нет
- High-Pressure Fluid Phase Equilibria: Phenomenology and ComputationОт EverandHigh-Pressure Fluid Phase Equilibria: Phenomenology and ComputationОценок пока нет
- Handbook of Thermal Conductivity, Volume 2: Organic Compounds C5 to C7От EverandHandbook of Thermal Conductivity, Volume 2: Organic Compounds C5 to C7Оценок пока нет
- Chemical Process Retrofitting and Revamping: Techniques and ApplicationsОт EverandChemical Process Retrofitting and Revamping: Techniques and ApplicationsGade Pandu RangaiahОценок пока нет
- Energy and Process Optimization for the Process IndustriesОт EverandEnergy and Process Optimization for the Process IndustriesОценок пока нет
- The Data Industry: The Business and Economics of Information and Big DataОт EverandThe Data Industry: The Business and Economics of Information and Big DataОценок пока нет
- APITECH 03 DecryptedДокумент23 страницыAPITECH 03 Decryptedjokerveloz100% (2)
- Crude Oil Distillation SimulationДокумент17 страницCrude Oil Distillation SimulationMelitza Gonzalez100% (1)
- PREDICT RVP OF PETROLEUM FUELSДокумент2 страницыPREDICT RVP OF PETROLEUM FUELSHicoolguy RiqОценок пока нет
- PRP - Added 04 Practicals (30th Oct 2013)Документ6 страницPRP - Added 04 Practicals (30th Oct 2013)idyllic20Оценок пока нет
- Cloud& Pour Points in Fuel BlendsДокумент5 страницCloud& Pour Points in Fuel Blendsramy86Оценок пока нет
- Commonly Asked Petroleum QuestionsДокумент17 страницCommonly Asked Petroleum QuestionssuperjerixОценок пока нет
- Prediction of The Reid Vapor Pressure of Petroleum Fuels: M. R. Riazi, T. A. Albahri and A. H. AlqattanДокумент2 страницыPrediction of The Reid Vapor Pressure of Petroleum Fuels: M. R. Riazi, T. A. Albahri and A. H. AlqattanFernanda GuerreroОценок пока нет
- Crafts Ebook Kirigami Paper Japanese - Arts of Paper - OrigamiДокумент59 страницCrafts Ebook Kirigami Paper Japanese - Arts of Paper - Origamisusiblue63% (8)
- Don't Gamble With Physical Properties For SimulationsДокумент12 страницDon't Gamble With Physical Properties For Simulationslaiping_lum100% (1)
- Assess Crude Oil CorrosivityДокумент7 страницAssess Crude Oil CorrosivityNXVNОценок пока нет
- Assess Crude Oil CorrosivityДокумент7 страницAssess Crude Oil CorrosivityNXVNОценок пока нет
- Creating The Smart PlantДокумент0 страницCreating The Smart PlantNXVNОценок пока нет
- NASA NSS 1740-15 - Safety STD O2 and O2 SystemsДокумент116 страницNASA NSS 1740-15 - Safety STD O2 and O2 SystemsNXVNОценок пока нет
- Guidelines On Fluid Flow SystemsДокумент8 страницGuidelines On Fluid Flow SystemsNXVNОценок пока нет
- HCL Corrosion Under ControlДокумент4 страницыHCL Corrosion Under ControlNXVNОценок пока нет
- Assess Crude Oil CorrosivityДокумент7 страницAssess Crude Oil CorrosivityNXVNОценок пока нет
- Water DissociationДокумент3 страницыWater DissociationNXVNОценок пока нет
- SteamДокумент10 страницSteamarunava001Оценок пока нет
- Eliminate foam carryover to increase diesel and AGO yieldsДокумент8 страницEliminate foam carryover to increase diesel and AGO yieldsNXVNОценок пока нет
- IFQC - Lowering Sulfur in FuelsДокумент10 страницIFQC - Lowering Sulfur in FuelsNXVNОценок пока нет
- Naphthenic Acid Corrosion in Synthetic FuelsДокумент15 страницNaphthenic Acid Corrosion in Synthetic FuelsNXVNОценок пока нет
- Fluid Mechanics d203Документ302 страницыFluid Mechanics d203Vignesh SundaramОценок пока нет
- FCC CATALYST ANALYSES GUIDEДокумент11 страницFCC CATALYST ANALYSES GUIDEshanpyanОценок пока нет
- Reducing Carbon Emissions With AntifoulantsДокумент4 страницыReducing Carbon Emissions With AntifoulantsNXVNОценок пока нет
- Assessing Corrosion in Oil Refining and Petrochemical Processing John Pelton EtalДокумент12 страницAssessing Corrosion in Oil Refining and Petrochemical Processing John Pelton EtalNabil Al-Khirdaji100% (1)
- Spark TestingДокумент23 страницыSpark TestingJad MacintoshОценок пока нет
- MmeДокумент114 страницMmemanojОценок пока нет
- Masel Catalog - IndexДокумент6 страницMasel Catalog - IndexOrtho OrganizersОценок пока нет
- WollastoniteДокумент5 страницWollastonitetrdanoОценок пока нет
- Process DescriptionДокумент9 страницProcess DescriptionBhuneshwar ChelakОценок пока нет
- Proeceedings of Geotechnical Advancement in Hong Kong Since 1970s, 15 May 2007, Hong KongДокумент313 страницProeceedings of Geotechnical Advancement in Hong Kong Since 1970s, 15 May 2007, Hong KongcheewingyuenОценок пока нет
- Aws D1.1welding Qualification.Документ10 страницAws D1.1welding Qualification.idealparrotОценок пока нет
- Optimizing The Life of Ore Passes in A Deep-Level Gold MineДокумент6 страницOptimizing The Life of Ore Passes in A Deep-Level Gold MineMotlatjo RakgothoОценок пока нет
- Electrolysis CalculationsДокумент8 страницElectrolysis CalculationsPius OsiliОценок пока нет
- Micro MachiningДокумент302 страницыMicro Machiningapulavarty100% (2)
- CPT Sundaram and Pandian Faq Chief MateДокумент12 страницCPT Sundaram and Pandian Faq Chief MatePrashanth Arumugam100% (1)
- JPCL September 2014 - Topcoating Ethyl Silicate Inorganic Zinc-Rich Primers Too SoonДокумент8 страницJPCL September 2014 - Topcoating Ethyl Silicate Inorganic Zinc-Rich Primers Too SoonTamerGalhoum100% (1)
- Liquid Jet EductorДокумент2 страницыLiquid Jet EductorSanthosh Kumar0% (1)
- Materials Glossary 2Документ458 страницMaterials Glossary 2Lailanie BrionesОценок пока нет
- SKS Service - B 1-28-10 (426337 2nd Ed) PDFДокумент970 страницSKS Service - B 1-28-10 (426337 2nd Ed) PDFIsrael Miranda Zamarca100% (9)
- Chilled Water System PresentationДокумент119 страницChilled Water System Presentationceo123456100% (4)
- Manual Usua RioДокумент132 страницыManual Usua RioSDL ElectricistasОценок пока нет
- Vehicle: Unit-5 Body Materials, Trim and MechanismsДокумент6 страницVehicle: Unit-5 Body Materials, Trim and MechanismskismuganОценок пока нет
- Time Flow Basin Pillar Tap - Push ButtonДокумент6 страницTime Flow Basin Pillar Tap - Push ButtonDota NgОценок пока нет
- Eq Fermentor ValidationДокумент3 страницыEq Fermentor Validationbt_sachinОценок пока нет
- 002DP48 PDFДокумент2 страницы002DP48 PDFDiadam SharmaОценок пока нет
- P37 To P275 High Pressure Piston Air Compressor English Tcm795-3514985Документ12 страницP37 To P275 High Pressure Piston Air Compressor English Tcm795-3514985Jozsef MagyariОценок пока нет
- 03 04 Faradays Laws of Electrolysis and ApplicationsДокумент12 страниц03 04 Faradays Laws of Electrolysis and ApplicationsTeoh Ah NgohОценок пока нет
- LEATHER CHEMISTRY GUIDEДокумент16 страницLEATHER CHEMISTRY GUIDESaravana Vel50% (2)
- Ips Energy Available Relay Models 2013 07Документ27 страницIps Energy Available Relay Models 2013 07Mareeswaran RamasamyОценок пока нет
- List of Welding StandardsДокумент5 страницList of Welding StandardsSandip Das100% (1)
- Nibbelink - Capacitive Humidity Sensor CombinedДокумент14 страницNibbelink - Capacitive Humidity Sensor CombinedDan ZerupОценок пока нет
- Dheeraj Project Final PDFДокумент28 страницDheeraj Project Final PDFDrAmit Verma100% (1)
- 1A Study On The Work Hardening and The Effect of Triaxiality On The Fracturebehaviour of Some Cryorolled Aluminium AlloysДокумент13 страниц1A Study On The Work Hardening and The Effect of Triaxiality On The Fracturebehaviour of Some Cryorolled Aluminium AlloysAnonymous c14ePs5Оценок пока нет