Академический Документы
Профессиональный Документы
Культура Документы
1938 Egan Dissociation Pressure Carbamate
Загружено:
Vinh Do ThanhАвторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
1938 Egan Dissociation Pressure Carbamate
Загружено:
Vinh Do ThanhАвторское право:
Доступные форматы
454
INDUSTRIAL A N D ENGINEERING CHEMISTRY
Vol. 38, No. 4
acids occurs a t t,he outer region and increases as it approachcs the heartwood. When the heartwood is reached, a material increase in resin acid is noted, but whether the inner or outer heartwood has the larger or smaller percentage of resin acids is dcpendent upon their respective cxtractivc contents. FREE FATTY ACIDS. This constituent iiicreases in per (wit as it passes from the newly formed sapwood to the inner sapwood where it reaches a maximum; then the percentage of fatty acids decreases as it approachcs the pith. VOLATILES. This fraction usually iiicreases in per cent as it approaches the pith of the tree. ESTERS. I n heartwood the percentage of esters decrenses froni t,he outer heartwood to the pith. I n some instances the percentage continues t o increase as it approachcs the out,er sapwood; in others it tends to decrease. UXSAPONIFIABLES. I n general, the perccntage of unsaponifiable is the least a t the outer sapwood and iiicreases, in some < maximum in the outer heartwood and then decreases as it nilproaches the center of the trce. I t is interesting to note that the coniposit.ion of thc outrr niitltll(~ hear,tnood in stand I11 indicates that it is apparently in the trailsition stage from sapwood to heartwood. The relatively lo\\rwin acid content, together withihe slight increase in unsaponifiable material in this outer heartwood region, suggests that this transitional deposition of extractives was t,aking place.
APPLICATION OF RESULTS
I11 seeking means for effectively utilizing the potential c.lieriiicw1 products from ponderosa pine, it was found that this wood coiitained a sufficient quantity of extractives to warrant the possible removal and recovery of these materials from the lumber and from forest and mill wood waste. Preliminary investigation\ have shown that it is possible to extract all or a large portion 01 the extractives from lumber; the result is 8 further improveiiicnt in the lumber offered by manufacturers; in addition, a commercial volume of extractive products may become available from this wood. The amount of recoverable extractables is not uniformly distributed throughout the trunk of the tree. The average extractive content in sapwood is usually within tlie limit. 2.0 to 9.8%.of the weight of the dry wood, whilc the heartwood extractables are usually within the limits 3.5 t o 31.5% of tho weight of the dry wood. Tho greater quantities of extractivci
are obtained from the lumbci antl wood waste originating from the butt portion of the trunk and from that portion of the trcl containing massed pitch arcs?. The acetone extractives, R Iictlier from the heartwood or \ u p wood, contain, in addition to rebin acids and terpenes, free fatly acids, fats, and unsaponifiablc msteiial. Thus the extractive. differ from gum oleoresin foiIncd by wounding the tree by bhe presence of these aliphatic and un5aponifiable substances. T h e percentage of each of thesc erilitics is not uniformly distributed throughout the tree but depcntls from which part of the log thc. extractives arc obtained. I n tlic case of heartwood extractive<, the products found in approxiinaic order of quantity preicnt are resin acids, free fatty acids, unLaponifiable, esters, volatile, wntri soluble, and water and ether insoluble. In sapwood extractive. these entitios are found in thc following older: free fatty acidi, r e h acids, water soluble, estcrs, uninponifiable, mater and eth6.r iiisoluble, and volstile. Since thr conimercial value of t h e extractives is contingent in part upon the quantity and exact r i i tule of its entities, the identificdtion o f ~xc*ki of thesc produvt iindo1 inviLhtigatiori by thi- lahlr i ( o r r
ACK \ 0 \% J,LI)( ;Rf E N 1
The author is indebted to Gcorgc Schroeder and C. V. Zaayeu for collecting the wood sections antl the advice of Albert H c r r n ~ n ~ I > greatly appreciated.
LITEHA7'UL{ 1 . : c;t'rl?;l)
(1) Adariis, J. INU. ENO.C m x . , 7, 957 (1913), ( 2 ) A4nderson,Ibid., 36, 662-3 (1944).
( 3 ) Assoc. of Official Agr. Chein., Methods of Analysis, pp. 469-71 (1940). 141 Benson and Jones. .J. ISD. $hi;. CHEM.. , 9. , 1096 (191'7i Dore, I t ~ i d . , 11, 556-63 (1919). (6) Hihbert and Phillips, Can. J . licssnrch, 4 , 1-34 (1931) (7) Koch and Kricg, Chem.-ZtN., 15, 1 4 0 - 1 (1938). (8) Kiajriiiovic, Ibid., 55, 894 (1931). (9) Kurth, IXD. ENG. CHEM., 23, 1156 (1931). (10) Schorger, U. S. Forest Service, Bull. 119 (1913). (11) Trendelertburg and Schailc, Papier-Fnhr., 35, 221-30 (1937). (12) Tiertelak and Garbaczowiia, lsn. RSG. CHBM., ANAL. ED., a , 110-11 (1935). (13) JTise, "Wood Chemistry", pp. X i 3 - 4 , A.C.S. Monograph 87 New York, Reinhold Pub. Gorp., 1944. (14) \TOM and Scholae, Chern,-Zlg., 38, 3G!)-70 (1914).
issociation Pressure of Ammonium Car
k.
P. EG-kN, JR., J. E. POTI'S, JR., AND GEQRGETTE 1). POn'S
Tennessee Valley A u t h o r i t y , Wilson D u m , Ala.
The present paper cover5 .t study of the dissociation preism c of solid ammonium carbamate over the temperature range 35' $ 0 83' C. and in the absence of 'til excess of either gaseous reactvnf From the vapor pressure data tlie lree energy of dissociatioii and the heat of dissociation h a w bwn derived.
PREPARATION O F SOLID A3tMOh'IUR.I CARBAMATE
EMPERATURE-pressure relations for the dissociation of solid ammonium carbamate into gaseous ammonia and carbon dioxide have been measured by several investigators. (1-5). The reported values are divcrgeiit; at a total pressure of 40 atmospheres the divergence is as much as 17 atmospheres. Briggs and Migrdichian (1)-measured the dissociation pressure of ammonium carbamate over temperature range 10" to 49" C. and obtained very consistent data. They also studied the effect of excess ammonia or carbon dioxide and found excellent agreement mith the mass Ian accoiding to the equation:
KII&OZ1\"2
(solid)
BTH,(gas)
+ COz (gas)
(1)
Solid ammonium carbam i t e ~ v n sprepared directly in a 50-c sample bulb (Figure 1) which later was connected to the pre?siirci measuring system. Stoichiometric proportioning of the r('ii< tants, as was employed by Briggs and Migrdichian ( f ) , proved ta br unnecessary. Thc carbamatc n a s deposited in the bulb fron,
April, 1946
INDUSTRIAL AND ENGINEERING CHEMISTRY
455
system was evacuated t o 10-4 mm. of mercury. After three such cycles of degasification, the sample was assumed t o be free of foreign gases. On completion of the purification step, all the tubes joining the sample bulb were sealed off, except a short control manometer. The purified sample vas riot weighed but was estimated t o be 0.2 to 0.4 gram. Each preparation was checked for quality by comparison of its dissociation pressure at an arbitrarily selected temperature of 34.5 O C. with the value interpolated from the d a t a of Briggs and Migrdichian (1). About one third of the preparations were discarded because their dissociation pressures exceeded the adopted tolerance of 1 mm. of mercury deviation from the interpolated value of 170 mm. of mercury. None of the preparations yielded a pressure of less than 170 mm. a t 34.5" C.
Figure 1. Apparatus for Preparing Ammonium Carbamate 1 IP Dissociation Pressure
it
r i d hleasuring
MEASUREMENT OF DISSOCIATION PRESSURE
roughly equimolar mixture of ammonia and carbon dioxide which was charged a t a rate of 1 liter per minute. Deposition of carbamate in the inlet and outtet tubes was prevented by maintaining the temperature of the tubes a t 100" C. The gases, which were of commercial grade, were dried thoroughly t o prevent the formation of ammonium carbonate and bicarbonate. The carbon dioxide first was washed with acid permanganate and then was dried successively with sulfuric acid and Dehydrite. The ammonia was dried with freshly pulverized fused potassium hydroxide. I n preliminary trials in which the sample bulb waa cooled with dry ice, expansion of the product on warming t o room temperature shattered the bulb. This indicated either a high coefficient of expansion or a phase change in solid ammonium carbamate between -78" C. and room temperature. In subsequent work, therefore, the bulb was cooled in an ice-salt bath. The ammonium carbamate was purified by alternate partial vaporization and evacuation through a vacuum line that contained a trap cooled with dry ice. The sample was first warmed until the dissociation pressure reached 700 t o 800 mm. of mercury, then wm cooled w<th a mixture of dry ice and acetone, and the
ti
T h e pressure of dissociation of solid ammonium carbamate into gaseous ammonia and carbon dioxide was measured over the temperature range 35' to 83' C. The results, when plotted as log P against 1/T, fall o n the same straight line as the data of Briggs and Migrdichian for the range 10' to 45' C. The combined data of the two studies are represented by, the equation: log P (mm. Hg) = -2741.9/T
4-11.1448 (283' to 355" K.)
The slope of the curve defined by this equation indicates that the heat of dissociation of ammonium carbamate, if assumed to be constant over the experimental range of temperature, is 37.6 kg.-cal. per mole. This value agrees with calorimetrically determined values reported previously. The equation is applied also in the derivation of the free energy of dissociation.
In the technique used for the pressure measurements. the autogeiious dissociation pressure was allowed to come to equilibrium at a given temperature with the gases exposed only t o the sample bulb and the short control manometer. The control manometer was a null-point instrument with sealed-in electrical contacts in a circuit that automatically balanced the dissociation pressure with nitrogen pressure. The measuring manometer was in the nitrogen system, as Figure 1 shows. The control-manometer used for pressures above 1000 mm was a glass Bourdon gage t h a t established electrical contact at the tip of the free end of the elastic element. This gage had the advantage that it presented only glass surface t o the products of dissociation, but its sensitivity t o temperature introduced a significant correction factor a t pressures below 1000 mm. At these lower pressures, therefore, a mercurial control manometer was used. A correction factor representing the pressure required t o establish electrical contact was determined for each type of gage and was added algebraically t o the observed dissociation pressures. The balancing pressure was obtained from a nitrogen cylinder through a reducing valve. The dissociation pressure was balanced by continuously bleeding a small amount of nitrogen from the system t o a vacuum and intermittently introducing nitrogen under pressure through a solenoid valve t h a t would pass a slow stream of gas under a differential pressure of about 10 cm. The valve consisted of an 8-mm. tube t h a t terminated with a fritted glass disk slightly above a pool of mercury in a n integral jacket t o which the nitrogen source was connected. A cylindrical iron plunger surrounded the 8-mm. tube and floated on the mercury. A solenoid 'surrounding the jacket pulled down the plunger and thereby raised the mercury surface sufficiently t o seal the fritted disk and prevent the passage of nitrogen. The solenoid w a a actuated through a vacuum tube relay and the contacts in the null-point manometer. At equilibrium the intermittent action of the solenoid caused fluctuations of less than 0.2 mm. in the level of mercury in the measuring manometer. Pressures up t o 1000 mm. were measured on a mercurial manometer with B 12-mm. bore. One leg of the manom6ter waa evacuated t o < 10-4 mm., which made the manometer absolute within the accuracy of the readings. T o eliminate parallax, a glass mirror scale and a sliding hairline index were employed for
456
INDUSTRIAL AND ENGINEERING CHEMISTRY
Vol. 38, No. 4
approaching equilibrium from both the high- and the lorn-pressure sides. Thr pressure was read at half-hour interval5 until three surressive readings agreed within 0.5 mm.
DISCUSSION O F R E S U L T S
Table I gives the measured dissociation pressures. Of the d a t a previouslj reported, only those of Rriggs and Rligrdichian ( 1 ) fall on the same straight line with the present measurements when plotted as the logarithm of the pressure in millimeters of mercury against the reciprocal of the absolute temperat,urr Application of the method of least squarca to the combined data of Briggs and RIigrdichiaii and of the present work yields the following equation or thc dissociation pressure of solid ammoniiirri carbamate:
log P
Figure 2.
=
Dissociation Pressure of Solid
Amrnotiiiiiri
(:arbamate
-2741.9/T $11.1448 (283" to 355" K.)
= =
(2)
whwe P T
pressure, mm. of I l g absolute temperature
reading the manometer. The pressurc reading5 u t'w c7orrertPd to 0 " C. for the expansion of glass and mercury. Pressures above 1000 mm. Kere read on a three-stage compound mercurial manometer in which the interstage spaces were filled with freshly boiled distilled water. Ss with t h e qimple manometer, the final leg of the compound manometer was evacuated The manometer was mounted on a heavy paper scale that differed from the glass scale by less than 1 part in 1000. The pressure differentials in the three stages of the manometer, corrected for the head of water in each stage, were added t o give the total pressure. The total pressure as corrected t o 0" C. for the expansion of mercury. The sample was heated in a thermoregulated oil bath. The temperature of the bath was estimated to the nearest 0.01 O C. with a calibrated thermometer; the over-all accurary probably was +=0.1"C. The attainment of equilibrium x-as ensured by
The average deviation of the present measuremrnts from Equation 2 is *0.47, and the maximum deviation is 1.4Cr0; the corresponding values for the data of Briggs and htigrdichian are ~ 0 . and 3 1.3%, which are of the same magnitude as for an equation representing Briggs and hIigrdichian's data alone Figure 2 compares calculatrd and rnpnwred dissociation presqui-es. If it is assumed that d i d ammonium carbamate dissociateq according to Equation 1 and that the vapor is a perfect gas w e tem,
TABLE I.
Temp.,
OC.
DISSOCIATION PPESSrRY: O F d l l M o N I U M
4RB 4\lATE
P, \Im. H g
T-dues of the dissociation constant, K p , as calculated frum smoothed dissociation pressures derived from Equation 2, are presented in Table I1 together v i t h values for the free encrgy of dissociation as calculated from the relation:
AFo = - R T l n K ,
P,
h i m . Hg 170.1 171.2 170.9 294.2 295.1 366.8 388.9 479.2 517.2 603.2 610.2 641.6
Typ.,
C.
P,
Mm. Hg
'Temp., C. 68.91 70.43 71.74 73.18 74.40 76.47 77.30 78.82 80.27 81.81 83.33 83.38
OF
34.49 34.55 34.59 42.99 43.18 46.48 47,53 50,92 52.19 54.73 55 00 55.78
56 98 58 26 59.31 59 8 3 61 24 62 09 62 09 62.13 65 20 66 19 67 20 67 56
688 7 743.7 790.1 812.5 881.9 924 2 925 7 927.2 1096 1158 1223 1248
1327 1450 1551 1673 180.5 1998 2093 2264 2456 2659 2856 2864
TABLE 11. FREE EXERGYOF DIssocraTrox A\rvon-~r-arCARBAMATE
I.
SOLID
I errip.,
0" 20 40 60 80 1OOb 120b 140b
P , Aim.
Xp,
Atm."
Ak' ', Cal./hIole
7700 5500
3310
The heat of dissociation corresporiding to Equation 1, as c-ulculated from the slope of the vapor pressure line in Figure 2 on the assumption of constancy of the heat of dissociation, is 37.6 kg.-cal. per mole of solid ammonium carbamate. This value agrees with calorimetrically determined values (3) but is lower than the heat of dissociation calculated by Krase ( 3 ) from the dissociation pressure data of Briner (9). I n this paper no correction of measured pressure to fugacity has been made. A trial calculation, assuming the absence of mixture effect, indicates that, a t the melting point, the fugacity of the mixed gases mould be about lOyolower than the pressiire. n-hich is within t.he error int,roduced by extrapolation.
LlTERATUHE CITED
0 0167 0,0811 0.3212 1,078 3.153 8,223 19.45 42.33
10-7 7 . 9 0 X 10-6 4 91 x 10-3 1 . 8 6 X 10-1 4.64 8 . 2 3 X 10 10.90 x 1 0 2 1 1 . 2 3 X lo3
6.90
1110
- 1080
-3270 - 5460 - 7660
K p = A P s , where P is dissociation pressure in atmospheres. 27 Extrapolated.
R., and Migrdirhian, V., J . Phus. Chem.. 28, 1121-35 (1924). 121 Briner. E.. J. chim.nhws.. 4. 267-84 (1906'1. {3j Curtis,' H.'A., "Fixed Nitrogen", Chap. XIII, New York, Chernical Catalog Co., 1932. (4) Xlatignon, C., and Frejacques, M., Bull. S O C . chim., 31, 307-16 (1922). ( 5 ) Tokuoka, M., J . Agr. f'hmri. .SOC. Japan, 10, 1333-44 (1034).
( I ) Bnggs, T.
Вам также может понравиться
- Himeno JChemEngData 2005 PDFДокумент8 страницHimeno JChemEngData 2005 PDFEduardo Enrique Choto AguirreОценок пока нет
- Hydrogen Peroxide Oxidation of Tertiary AminesДокумент4 страницыHydrogen Peroxide Oxidation of Tertiary AminesRadja LintangОценок пока нет
- Flammability Characteristics of Methylene Chloride (DichloromethaneДокумент5 страницFlammability Characteristics of Methylene Chloride (DichloromethaneBigbearBigbearОценок пока нет
- Dinosaur Body Temperatures Determined From Isotopic (C-O) Ordering in Fossil BiomineralsДокумент48 страницDinosaur Body Temperatures Determined From Isotopic (C-O) Ordering in Fossil BiomineralsVera MillerОценок пока нет
- Vapor Pressures of Phlegmatic Liquids. I. Simple and Mixed TriglyceridesДокумент7 страницVapor Pressures of Phlegmatic Liquids. I. Simple and Mixed TriglyceridesShayane MagalhãesОценок пока нет
- Use of Liquid Smoke Flavouring As An AlternativeДокумент5 страницUse of Liquid Smoke Flavouring As An AlternativePondokaceh FaizalalbaqarohОценок пока нет
- Articulo Comparacion ResiduosДокумент8 страницArticulo Comparacion ResiduoswisangwidyarsaОценок пока нет
- CHM170L Exp6 Heat of CombustionДокумент5 страницCHM170L Exp6 Heat of CombustionKaiser SaltoОценок пока нет
- Decompositions of Di-t-Alkyl Peroxides. I. Kinetics: Frederick F. EДокумент7 страницDecompositions of Di-t-Alkyl Peroxides. I. Kinetics: Frederick F. Emartinml_1191Оценок пока нет
- Intrinsic and Global Reaction Rate of Methanol Dehydration Over 7-A1203 PelletsДокумент6 страницIntrinsic and Global Reaction Rate of Methanol Dehydration Over 7-A1203 PelletsHectorОценок пока нет
- Lang MuirДокумент7 страницLang MuirLao ZhuОценок пока нет
- Anjuarve - Solubilidad de Caffeina en CO2Документ18 страницAnjuarve - Solubilidad de Caffeina en CO2Karina MesaОценок пока нет
- Statement of Assignment 4Документ2 страницыStatement of Assignment 4Bilal ArifОценок пока нет
- Bioresources: Estimation of Hardwood Lignin Concentrations by Uv Spectroscopy and Chlorine DemethylationДокумент13 страницBioresources: Estimation of Hardwood Lignin Concentrations by Uv Spectroscopy and Chlorine DemethylationThiago SantosОценок пока нет
- Emissions of Volatile Organic Compounds by Coal-Fired P O W E R StationsДокумент9 страницEmissions of Volatile Organic Compounds by Coal-Fired P O W E R StationssauravОценок пока нет
- High-Temperature Continuous Bulk Copolymerization of Styrene and Acrylic Acid Determination of Monomer ConversionsДокумент11 страницHigh-Temperature Continuous Bulk Copolymerization of Styrene and Acrylic Acid Determination of Monomer ConversionsKumaranОценок пока нет
- Thermal Degradation of RosinДокумент14 страницThermal Degradation of RosinDmitry TarakanovОценок пока нет
- Recommended Reference Materials for Realization of Physicochemical Properties: Pressure–Volume–Temperature RelationshipsОт EverandRecommended Reference Materials for Realization of Physicochemical Properties: Pressure–Volume–Temperature RelationshipsE. F. G. HeringtonОценок пока нет
- Kinetics of The Thermal Isomerization Bicyclo) Hexane1Документ3 страницыKinetics of The Thermal Isomerization Bicyclo) Hexane1Adelmo FilhoОценок пока нет
- Adsorption of Co2Документ6 страницAdsorption of Co2Anila TasaddaqОценок пока нет
- Analysis of Liquid Smoke and Smoked Meat Volatiles by Headspace Gas ChromatogrДокумент10 страницAnalysis of Liquid Smoke and Smoked Meat Volatiles by Headspace Gas ChromatogrMuhammad AzzamОценок пока нет
- Paracetamol SolubilityДокумент6 страницParacetamol SolubilityJoao DinizОценок пока нет
- Report 1 - Exper 4Документ4 страницыReport 1 - Exper 4Walmiria LimaОценок пока нет
- Catalytic Oxidation of Benzene to Maleic AnhydrideДокумент7 страницCatalytic Oxidation of Benzene to Maleic AnhydrideMirko GraneseОценок пока нет
- Determination of Total Chromium in Sediments by FAASДокумент5 страницDetermination of Total Chromium in Sediments by FAASngobaochanОценок пока нет
- Measurement of Biocarbon in Flue Gases Using CДокумент6 страницMeasurement of Biocarbon in Flue Gases Using CBeta AnalyticОценок пока нет
- Reaction Products of Aquatic Humic Substances With ChlorineДокумент9 страницReaction Products of Aquatic Humic Substances With ChlorinefrtklauОценок пока нет
- 13 E. Bicchim - Bio@Ys., J.Biocksm., J. Chews., Biol. Chew., Biochim - Bio$Hys. Acta, Proc - Nate.Acad - Sci. U.S., 55 (1966)Документ4 страницы13 E. Bicchim - Bio@Ys., J.Biocksm., J. Chews., Biol. Chew., Biochim - Bio$Hys. Acta, Proc - Nate.Acad - Sci. U.S., 55 (1966)SanelaОценок пока нет
- Advanced Inorganic Lab ExperimentДокумент4 страницыAdvanced Inorganic Lab ExperimentThanhThao TranОценок пока нет
- Oxygen From Hydrogen Peroxide: A Safe Molar Volume-Molar Mass ExperimentДокумент2 страницыOxygen From Hydrogen Peroxide: A Safe Molar Volume-Molar Mass ExperimentManuel Curitol PiutrinОценок пока нет
- Purification of Nitric Acid at Trace Metal LevelsДокумент2 страницыPurification of Nitric Acid at Trace Metal LevelsBo-Shian WangОценок пока нет
- 1939-The N-Fatty Acids and Certain of Their DerivativesДокумент28 страниц1939-The N-Fatty Acids and Certain of Their DerivativesSoodooNavindraОценок пока нет
- Activity Coefficients of The Tetramethyl Compounds of Group 14 Elements in N-Alkane Solutions From g.1.c. MeasurementsДокумент7 страницActivity Coefficients of The Tetramethyl Compounds of Group 14 Elements in N-Alkane Solutions From g.1.c. Measurementsm_adnane_dz3184Оценок пока нет
- Separation of Acetonitrile From Hazardous Waste: Honors Senior Thesis Pass With DistinctionДокумент42 страницыSeparation of Acetonitrile From Hazardous Waste: Honors Senior Thesis Pass With DistinctionJoshua JohnsonОценок пока нет
- 1 s2.0 S0304420396000643 MainДокумент13 страниц1 s2.0 S0304420396000643 MainasdasОценок пока нет
- Cromatografi e TextДокумент8 страницCromatografi e TextRaunciucGianinaОценок пока нет
- Thermodynamics of the Dissociation of Protonated Tris(hydroxymethy1)aminomethaneДокумент4 страницыThermodynamics of the Dissociation of Protonated Tris(hydroxymethy1)aminomethaneLuis F. OlguinОценок пока нет
- goldberg1947 (2)Документ8 страницgoldberg1947 (2)Matias Daniel LimaОценок пока нет
- Exp't 41: The Reaction of Maleic Anhydride and CycloheptatrieneДокумент5 страницExp't 41: The Reaction of Maleic Anhydride and CycloheptatrienelovehopeОценок пока нет
- Jcpsa6 24 3 559 1Документ12 страницJcpsa6 24 3 559 1eddyterryОценок пока нет
- Estimation of Viable Biomass in Wastewater A N D Activated Sludge by Determination of Atp, Oxygen Utilization Rate A N D Fda HydrolysisДокумент7 страницEstimation of Viable Biomass in Wastewater A N D Activated Sludge by Determination of Atp, Oxygen Utilization Rate A N D Fda HydrolysismpakzadehОценок пока нет
- Incineration of Styrene-Butadiene Rubber: The Influence of Heating Rate and Oxygen Content On Gas Products FormationДокумент14 страницIncineration of Styrene-Butadiene Rubber: The Influence of Heating Rate and Oxygen Content On Gas Products FormationPhilip ShihОценок пока нет
- Paracetamol Solubility in Pure SolventsДокумент6 страницParacetamol Solubility in Pure SolventsValentino DhiyuОценок пока нет
- Decomposition of 1,3,5-Trioxane at 700-800 KДокумент3 страницыDecomposition of 1,3,5-Trioxane at 700-800 KPilar MayaОценок пока нет
- Formal Lab Report-CarbethoxycoumarinДокумент6 страницFormal Lab Report-Carbethoxycoumarinyanet1408100% (1)
- OPTIMUM CONDITIONS FOR THE PREPARATION OF KETENE FROM ACETONE - J. Am. Chem. Soc., 1925, 47 (5), PP 1427-1430Документ4 страницыOPTIMUM CONDITIONS FOR THE PREPARATION OF KETENE FROM ACETONE - J. Am. Chem. Soc., 1925, 47 (5), PP 1427-1430muopioidreceptor100% (1)
- Industrial & Engineering Chemistry Research Volume 49 Issue 8 2010Документ6 страницIndustrial & Engineering Chemistry Research Volume 49 Issue 8 2010Rizaldi FirdausОценок пока нет
- Kinetics An Esterification Cation-Exchange Resin Catalyst: AcknowledgmentДокумент4 страницыKinetics An Esterification Cation-Exchange Resin Catalyst: AcknowledgmentChagua HernandezОценок пока нет
- Fast Pyrolysis of Agricultural Wastes: Characterization of Pyrolysis ProductsДокумент6 страницFast Pyrolysis of Agricultural Wastes: Characterization of Pyrolysis ProductsyemresimsekОценок пока нет
- A Study of Chlorobenzene PyrolysisДокумент9 страницA Study of Chlorobenzene PyrolysisWalter Ruiz AstoОценок пока нет
- A Study of Chlorobenzene PyrolysisДокумент9 страницA Study of Chlorobenzene PyrolysisWalter Ruiz AstoОценок пока нет
- Cellulose Acetate Recovery From Cigarette ButtsДокумент6 страницCellulose Acetate Recovery From Cigarette ButtsDaniele SabbatoОценок пока нет
- Cellulose Acetate Recovery From Cigarette Butts: ProceedingsДокумент6 страницCellulose Acetate Recovery From Cigarette Butts: ProceedingsrinochiroОценок пока нет
- Foreword:, Pndis)Документ2 страницыForeword:, Pndis)SajidAliKhanОценок пока нет
- METHANE AnalysisДокумент4 страницыMETHANE AnalysisAdeelRafiqОценок пока нет
- Thermal Decomposition of Tetrachloroethylene: Chemosphere April 1993Документ7 страницThermal Decomposition of Tetrachloroethylene: Chemosphere April 1993Alexander Quebra Madeira NemchinovОценок пока нет
- Detonation Characteristics Hydrogen-Oxygen Mixtures High Initial PressuresДокумент5 страницDetonation Characteristics Hydrogen-Oxygen Mixtures High Initial PressuresGustavo Gabriel JimenezОценок пока нет
- Holdup-Charge Ratio in Istillation: 1480 Industrial and Engineering Chemistry No. 6Документ7 страницHoldup-Charge Ratio in Istillation: 1480 Industrial and Engineering Chemistry No. 6Anonymous ee5dOjОценок пока нет
- Advances in Smoking of Foods: Plenary Lectures Presented at the International Symposium on Advances in Smoking of Foods, Warsaw, Poland, 8 - 10 September, 1976От EverandAdvances in Smoking of Foods: Plenary Lectures Presented at the International Symposium on Advances in Smoking of Foods, Warsaw, Poland, 8 - 10 September, 1976A. RutkowskiОценок пока нет
- The Total Synthesis of Natural ProductsОт EverandThe Total Synthesis of Natural ProductsJohn ApSimonОценок пока нет
- Studies On Drying Kinetics of Solids in A Rotary DryerДокумент6 страницStudies On Drying Kinetics of Solids in A Rotary DryerVinh Do ThanhОценок пока нет
- 4244 12672 1 PB PDFДокумент15 страниц4244 12672 1 PB PDFVinh Do ThanhОценок пока нет
- Proper Air-Fuel Ratios for Starting, Idling, Accelerating & MoreДокумент9 страницProper Air-Fuel Ratios for Starting, Idling, Accelerating & MoreVinh Do ThanhОценок пока нет
- Air-Fuel Ratio, Lambda and Engine Performance: AFR M MДокумент12 страницAir-Fuel Ratio, Lambda and Engine Performance: AFR M MVinh Do ThanhОценок пока нет
- 4244 12672 1 PB PDFДокумент15 страниц4244 12672 1 PB PDFVinh Do ThanhОценок пока нет
- NPK-15 8 15Документ5 страницNPK-15 8 15Vinh Do ThanhОценок пока нет
- Effects of Drying Parameters On Heat Transfer During DryingДокумент13 страницEffects of Drying Parameters On Heat Transfer During DryingVinh Do ThanhОценок пока нет
- Modeling and Simulation of A Co-Current Rotary Dryer Under Steady ConditionsДокумент8 страницModeling and Simulation of A Co-Current Rotary Dryer Under Steady ConditionsVinh Do ThanhОценок пока нет
- Modelling and Simulation of A Direct Contact Rotary DryerДокумент16 страницModelling and Simulation of A Direct Contact Rotary DryerVinh Do ThanhОценок пока нет
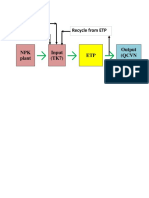
- Recycle From ETP Make Up H2O DAP, UreaДокумент1 страницаRecycle From ETP Make Up H2O DAP, UreaVinh Do ThanhОценок пока нет
- The Heart of Operations - World Cement - 02-2015Документ4 страницыThe Heart of Operations - World Cement - 02-2015fetniОценок пока нет
- Tinh Luong Nuoc Bay HoiДокумент22 страницыTinh Luong Nuoc Bay HoiVinh Do ThanhОценок пока нет
- Dryer CalculationsДокумент4 страницыDryer CalculationsVinh Do Thanh0% (1)
- Ansi B16-104Документ1 страницаAnsi B16-104Monica Suarez100% (1)
- Aoac - Methods.1.1990. MoistureДокумент2 страницыAoac - Methods.1.1990. MoistureVinh Do ThanhОценок пока нет
- Multi-Use Chair DesignДокумент7 страницMulti-Use Chair DesignVinh Do ThanhОценок пока нет
- Review On Development of Polypropylene Manufacturing ProcessДокумент11 страницReview On Development of Polypropylene Manufacturing ProcessShweta Yadav100% (1)
- PEP Report 267A: Ihs ChemicalДокумент8 страницPEP Report 267A: Ihs ChemicalVinh Do ThanhОценок пока нет
- 1 0ProjectManagementProceduresДокумент8 страниц1 0ProjectManagementProceduresRamiesRahmanОценок пока нет
- CRACKER A PC Based Simulator For Industr PDFДокумент6 страницCRACKER A PC Based Simulator For Industr PDFVinh Do ThanhОценок пока нет
- Equivalent Grades of Cast IronsДокумент2 страницыEquivalent Grades of Cast IronsVinh Do ThanhОценок пока нет
- Natural Evaporation RateДокумент16 страницNatural Evaporation RateVinh Do ThanhОценок пока нет
- Metal Price IndexДокумент1 страницаMetal Price IndexVinh Do ThanhОценок пока нет
- Mau Giay Uy Quyen Bang Tieng AnhДокумент3 страницыMau Giay Uy Quyen Bang Tieng AnhVinh Do ThanhОценок пока нет
- DRS 279-2015 Organic Fertilizer - SpecificationДокумент17 страницDRS 279-2015 Organic Fertilizer - SpecificationVinh Do ThanhОценок пока нет
- Estimating Evaporation From Water SurfacesДокумент27 страницEstimating Evaporation From Water SurfacesVinh Do ThanhОценок пока нет
- How To Calculate Heat Load - 5 StepsДокумент1 страницаHow To Calculate Heat Load - 5 StepsVinh Do ThanhОценок пока нет
- Optimization of Wall Thickness For Minimum Heat LossesДокумент9 страницOptimization of Wall Thickness For Minimum Heat LossesVinh Do ThanhОценок пока нет
- How To Calculate Heat Load - 5 StepsДокумент1 страницаHow To Calculate Heat Load - 5 StepsVinh Do ThanhОценок пока нет
- Investigation of Sensible Heat Storage and Heat Insulation in The Exploitation of Concentrated Solar EnergyДокумент5 страницInvestigation of Sensible Heat Storage and Heat Insulation in The Exploitation of Concentrated Solar EnergyradanpetricaОценок пока нет
- 21.6 Buffer Solutions: StarterДокумент37 страниц21.6 Buffer Solutions: StarterJackie HardakerОценок пока нет
- Bảng phổ IRДокумент5 страницBảng phổ IRĐan KhanhОценок пока нет
- IIT Chemistry: LeaderДокумент37 страницIIT Chemistry: LeaderNamanОценок пока нет
- Synthetic Bioplastics From Cassava SkinДокумент20 страницSynthetic Bioplastics From Cassava SkinlisaОценок пока нет
- ASTM C125 15aДокумент4 страницыASTM C125 15aOLOFINTUYI Ilesanmi OlanrewajuОценок пока нет
- I Gcse Chemistry The Periodic TableДокумент16 страницI Gcse Chemistry The Periodic TableAlex BoumanОценок пока нет
- Effect of Microbes On Drilling Fluid FormulationДокумент8 страницEffect of Microbes On Drilling Fluid FormulationIJAERS JOURNALОценок пока нет
- Benzene Structure and Reactions QuizДокумент65 страницBenzene Structure and Reactions QuizMoaz AzabОценок пока нет
- S22 - URAIAN Dan KONTROL PROSES PEMBUATANДокумент7 страницS22 - URAIAN Dan KONTROL PROSES PEMBUATANTrisnawati AmaliaОценок пока нет
- C146-94a (2014) Standard Test Methods For Chemical Analysis of Glass SandДокумент12 страницC146-94a (2014) Standard Test Methods For Chemical Analysis of Glass SandAhmed AlzubaidiОценок пока нет
- Polyoxometalate-Based Catalysts For CO2 ConversionДокумент26 страницPolyoxometalate-Based Catalysts For CO2 ConversionANDRESОценок пока нет
- Sulhuric Acid PDFДокумент2 страницыSulhuric Acid PDFBrilian Retna AmamuhtiОценок пока нет
- IBC Code Chapter 17 SummaryДокумент32 страницыIBC Code Chapter 17 SummaryakashawalkerОценок пока нет
- AQA A2 CHEMISTRY EQUILIBRIA REVISIONДокумент8 страницAQA A2 CHEMISTRY EQUILIBRIA REVISIONLeen JabbanОценок пока нет
- Safety Sign Course Note-EngДокумент15 страницSafety Sign Course Note-Engchoco pandapurpleОценок пока нет
- Topic-1 IntroductionДокумент30 страницTopic-1 IntroductionJOHNDOETОценок пока нет
- Ch-17 Solutions and Colligative Properties - CaabilДокумент46 страницCh-17 Solutions and Colligative Properties - CaabilAshish KumarОценок пока нет
- Stain ExitДокумент1 страницаStain ExitBenОценок пока нет
- CV of Srijan Sengupta, Assistant Professor at IIT JodhpurДокумент6 страницCV of Srijan Sengupta, Assistant Professor at IIT JodhpurSrijan SenguptaОценок пока нет
- Clariant Technical Article High-Performance Dispersing Agents For Carbon Blacks 201807 ENДокумент18 страницClariant Technical Article High-Performance Dispersing Agents For Carbon Blacks 201807 ENJose E Batista100% (1)
- Sulphur and its Compounds: Extraction and AllotropesДокумент16 страницSulphur and its Compounds: Extraction and AllotropesVaibhav TripathiОценок пока нет
- B.E. Poling, J.M. Prausnitz, J.P. O'Connell, 'The Properties of Gases and Liquids' 5ht Ed. Property Data Bank. Appendix AДокумент61 страницаB.E. Poling, J.M. Prausnitz, J.P. O'Connell, 'The Properties of Gases and Liquids' 5ht Ed. Property Data Bank. Appendix AIsaac A Vazquez MedranoОценок пока нет
- Biorenewable Polymers Based On Acrylated Epoxidized Soybean OilДокумент5 страницBiorenewable Polymers Based On Acrylated Epoxidized Soybean Oilaslı aslanОценок пока нет
- Casting DefectsДокумент21 страницаCasting DefectsImran KhanОценок пока нет
- Cell Biology Interview Questions and Answers Guide.: Global GuidelineДокумент19 страницCell Biology Interview Questions and Answers Guide.: Global Guidelineymir shoyoОценок пока нет
- PropranololДокумент6 страницPropranololDaniel LawsonОценок пока нет
- Properties of Manufactured Glasses PDFДокумент4 страницыProperties of Manufactured Glasses PDFEngr Ishfaque TunioОценок пока нет
- Module 9Документ13 страницModule 9michaelОценок пока нет
- Development of Antistatic Finish in Textiles: Usha Sayed, Kanchan SharmaДокумент6 страницDevelopment of Antistatic Finish in Textiles: Usha Sayed, Kanchan SharmaSadaf SweetОценок пока нет
- Compressive Strength and Durability Properties of Roller-Compacted Concrete Pavement Containing Electric Arc Furnace Slag Aggregate and Fly AshДокумент11 страницCompressive Strength and Durability Properties of Roller-Compacted Concrete Pavement Containing Electric Arc Furnace Slag Aggregate and Fly AshMJundiОценок пока нет