Академический Документы
Профессиональный Документы
Культура Документы
1 s2.0 S0926860X00008516 Main
Загружено:
Izack Silva SИсходное описание:
Оригинальное название
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
1 s2.0 S0926860X00008516 Main
Загружено:
Izack Silva SАвторское право:
Доступные форматы
Applied Catalysis A: General 212 (2001) 129151
Consequences of catalyst deactivation for process design and operation
S.T. Sie
Laboratory for Chemical Technology, Delft University of Technology, Julianalaan 136, 2628 BL, Delft, The Netherlands
Abstract Catalyst deactivation has important consequences for the design of a process and the way it is operated. The nature of the deactivation, and in particular the question whether it can be reversed under conditions which are compatible with the normal operation or whether a separate regeneration treatment of the catalyst is required to restore its activity, as well as the time-scale of the deactivation determine the type of technology that is feasible and process options like reactor type and process conguration. This relationship between deactivation behaviour and process lay-out forms the subject matter of the present paper. The general principles that guide the choices of process type and parameters are illustrated in more detail with examples from the elds of catalytic reforming of petroleum naphtha and hydroprocessing of petroleum residues. In these elds, different catalyst deactivation mechanisms are operative and catalyst deactivation rates can vary widely depending upon feedstock and process parameters. Consequently, different reactor technologies and process congurational choices are possible. The relation between catalyst deactivation behaviour and process design and operation can be viewed from two sides: on the one hand, the deactivation behaviour may dictate the choice between viable process options and may provide an incentive for the development of novel technology that can cope optimally with the demands set by the deactivation of the catalyst. On the other hand, the introduction of novel technological options may widen the scope of a process, e.g. by opening the possibilities to apply novel catalysts or to operate under unconventional conditions that lead to a more economic or otherwise better process, possibilities that were previously barred by catalyst deactivation. 2001 Elsevier Science B.V. All rights reserved.
Keywords: Catalyst deactivation; Regeneration; Process design; Process conguration; Reactors; Catalytic reforming; Residue hydroprocessing
1. Introduction Deactivation of the catalyst during the course of a process is often unavoidable and has to be reckoned with in the design of a process. Catalyst deactivation represents a technical as well as an economic factor in the process and its effect on the performance of a given type of reactor or the economics of a certain process has been the subject of a number of quantitative analyses, e.g. [1,2]. However, catalyst deactivation may affect the process design in an even more principal way: the selection of process options such as the process conguration, reactor type, and mode of operation of
an industrial process can be inuenced or even dictated by the deactivation of the catalyst [3,4]. The nature of catalyst deactivation, the possibility of regaining lost catalyst activity either during the operation of the process or in a separate regeneration step, and the speed of deactivation are factors that determine these process options. The present article reviews these factors and seeks to provide a link between deactivation behaviour and the choice of the appropriate process technology. The general principles set out will be illustrated with some examples, viz. from the elds of catalytic reforming of petroleum naphtha and the hydroprocessing of residual oils. In both processes, different
0926-860X/01/$ see front matter 2001 Elsevier Science B.V. All rights reserved. PII: S 0 9 2 6 - 8 6 0 X ( 0 0 ) 0 0 8 5 1 - 6
130
S.T. Sie / Applied Catalysis A: General 212 (2001) 129151
mechanisms of catalyst deactivation are operative and these are coped with in different ways. Moreover, depending upon reaction conditions chosen or characteristics of the feedstock to be processed, the speed of catalyst deactivation can vary widely and consequently lead to different process congurational and operational choices.
2. Nature of catalyst deactivation and remedial actions Catalysts can lose their activity during a process for a variety of reasons. Most common reasons are poisoning or reaction inhibition by impurities in the feed or by reaction byproducts, deposition of polymeric material including coke on the catalyst as a result of side or consecutive reactions, and loss of catalyst dispersion by sintering of small particles of the active material. In addition, catalysts may become deactivated by loss of active components by leaching or vaporisation, or by changes in their porous texture.
Changes in porous texture that can affect the performance of a catalyst are, for instance, loss of specic surface area through sintering of the carrier or loss of permeability through plugging of pores. An important distinction between the deactivation processes is whether they are reversible or irreversible under the conditions of the process. A reversible deactivation caused by leaching of active material from the catalyst in a continuous process can be coped with by adding the leached product to the feed. At the correct dosing rate, the supply and leaching rates are in balance so that the net loss of active material from the catalyst is zero. An example of such a process is the isomerisation of parafns with a catalyst based on chlorided alumina. Loss of chlorine from the catalyst as a result from some hydrolysis by moisture is counteracted by feeding hydrogen chloride or a hydrogen chloride generating compound to the feed to make up for the hydrogen chloride lost, see Fig. 1. An irreversibly deactivated catalyst has either to be discarded or to be subjected to a reactivation treatment to restore its performance. The latter treatment
Fig. 1. Flow scheme of BPs parafn isomerisation process, showing the dosing of organic chloride to compensate for catalyst deactivation by stripping of hydrogen chloride [5].
S.T. Sie / Applied Catalysis A: General 212 (2001) 129151
131
Fig. 2. Periodic catalyst reactivation during catalytic hydrodewaxing of Arabian heavy way distillate of 5565 F pour point [6].
Fig. 3. Viable process technologies as determined by rapidity of catalyst deactivation.
132
S.T. Sie / Applied Catalysis A: General 212 (2001) 129151
is termed regeneration or rejuvenation. Rejuvenation is a term commonly used for a treatment which can be carried out under conditions that are rather similar or not principally different from the operating conditions. For instance, polymeric material that had been deposited on a catalyst under relatively mild conditions of hydroprocessing may still be sufciently reactive to be hydrocracked under somewhat more severe conditions. Thus, the deposited polymeric material may be removed in the form of cracked fragments at somewhat increased temperature and/or increased hydrogen rate (hydrogen stripping). An example of such a catalyst rejuvenation is shown in Fig. 2. When the deactivation process is irreversible and reactivation requires conditions that are incompatible with those of the main process, the catalyst has either to be regenerated in a separate step or it must be disposed off. Common examples of such irreversible deactivation processes are deposition of coke on the catalyst and sintering of metals. Coke deposited on the catalysts at high temperatures in the form of a carbon-rich polyaromatic material is generally too unreactive to be hydrocracked in a hydrogen stripping step and has to be removed by oxidation to carbon oxides. Sintering of active materials can be undone by redispersing, e.g. sintered platinum on alumina may be redispersed by treatment with chlorine. These decoking as well as redispersion steps are carried out in an oxidative atmosphere, which is incompatible with the reductive atmosphere of a hydrocarbon conversion process. If the regeneration option is not chosen, spent catalyst has to be disposed off. In catalyst disposal, the choice between dumping and working up of spent catalysts for recovery of valuable materials is dictated by the economics of materials recovery and does not affect the main process other than via the catalyst replacement costs. Regeneration of a catalyst can be carried out either ex-situ or in-situ. In the former case, the regeneration may be carried out in a separate facility at a different location from the main process and may even be performed as a service by an outside company. In the case of in-situ regeneration, the regeneration facilities are much more an integral part of the process installation. The regeneration procedure may even be carried out in the same reactor as used in the main process, but with the latter temporarily out of regular service during the regeneration campaign (this
mode of operation is often termed semi-regenerative operation). Another possibility is to install more than one reactor, with each reactor being alternately operated in a process mode or in a regeneration mode (swing operation). Yet another possibility is not to keep a batch of catalyst in the same vessel all of the time, but to circulate catalysts between reaction and regeneration vessels (continuous regeneration). This implies that the catalysts must be able to move in and out of a reactor, which is made possible by moving-bed or uid-bed technology.
3. Rate of catalyst deactivation In batch processes, the rate of catalyst deactivation determines catalyst consumption and is in general only a factor of economic importance. Aside from the
Fig. 4. Compensating for catalyst deactivation in catalytic reforming by increasing the reactor temperature during the run [7].
S.T. Sie / Applied Catalysis A: General 212 (2001) 129151
133
Fig. 5. (A) Progress of deactivation front through a xed-bed of Mobils methanol-to-gasoline (MTG) process, as indicated by the movement of the exothermic reaction zone [8]. Note the time-scale of the deactivation. (B) Flow scheme of the xed-bed MTG process, showing the arrangement to switch individual reactions from the operating to the regeneration mode [8]. (C) Cumulative gasoline yield of individual reactors as a function of time in the xed-bed MTG process [9].
134
S.T. Sie / Applied Catalysis A: General 212 (2001) 129151
Fig. 5 (Continued).
fact that it may have an impact on the sizing of catalyst storage and dosing vessels and the capacity of separating equipment to remove spent catalyst from the products, the rate of catalyst deactivation hardly affects the process conguration and reactor choice in batch processes. The situation is quite different, however, for continuous processes where catalyst deactivation rates may determine the choice of process options in a more principal way. This is especially true for processes in which the conditions of operation and regeneration are incompatible, so that the regeneration has to be carried out as a separate step. Fig. 3 shows the relation between possible reactor technologies and rapidity of catalyst deactivation for these cases. If the deactivation rate in a xed-bed reactor is sufciently low, no special on-site facilities for regeneration are required, and after an operating period spent catalyst can be discarded or regenerated elsewhere for reuse. This situation pertains if the life of the catalyst is about a year or longer. Assuming an average of 10 days downtime in 1 year for a catalyst change in a process operating with oxidisable material under pressure (which change involves cooling down, depressurising, and opening of the reactor, removal of spent catalyst, reloading with fresh catalyst, closing the reactor, repressurising and reheating), the
availability of the unit is reduced by about 3%, which is generally considered acceptable. The loss of availability can be further reduced if the time of catalyst change can be made to coincide with the generally required periodic safety inspection of the unit. When catalyst life becomes shorter, e.g. about half a year, dedicated facilities for on-site regeneration become of interest, particularly when it concerns an expensive catalyst. In the so-called semi-regenerative mode of operation, the catalyst remains in the reactor, which is taken out of service during the regeneration campaign. The required facilities for carrying out the regeneration, such as compressors for inert gas circulation and dosing of air may be a permanent part of the unit or be installed on a temporary basis. With the relatively slow rate of catalyst deactivation in the above non- and semi-regenerative process modes, the effect of deactivation during a catalyst life cycle is generally counteracted by raising the reactor temperature during operation so as to maintain the desired level of conversion or quality of the product. An example of such a temperature programme is shown in Fig. 4. With still shorter catalyst lives, i.e. less than a few weeks, the required frequency of regeneration dictates the installation of dedicated permanent facilities for
S.T. Sie / Applied Catalysis A: General 212 (2001) 129151
135
Fig. 6. Fluid-bed variant of the methanol-to-gasoline process [10].
regeneration which are an integral part of the process installation. The catalyst can either remain within the same reactor, which is switched between normal operation and regeneration modes (swing operation) or be transported as a more or less continuous ow of solids to a separate regeneration vessel and back to the reactor after having been regenerated (continuous regeneration). In the swing-type of operation two or more reactors are generally installed to ensure an almost uninterrupted product ow. With multiple reactors installed, it is customary to operate them in a staggered fashion so that at any moment in time a mix of products from differently aged catalyst-beds is obtained. This merry-go-round way of operation ensures an almost constant product quality, notwithstanding the aging of the catalyst. An example of such an operation is shown in Fig. 5AC, pertaining to Mobils methanol-to-gasoline (MTG) process. In this process, the catalyst (ZSM-5 zeolite) deactivates as a result of deposition of carbonaceous material in the course of a few hundred hours and is regenerated by an oxidative treatment [8,9]. The movability of catalyst in continuous regeneration systems sets a limit to the achievable catalyst circulation rate in practice and, therefore, determines a minimum catalyst lifecycle. Fluidised catalyst can be moved much faster than catalyst particles in a moving-bed. Another important limiting factor is the operating pressure: when the process operates in a
Fig. 7. Scheme of a uid catalytic cracking unit with cracking in a dilute uidised phase riser and catalyst stripping and regeneration in a dense uidised-bed.
136
S.T. Sie / Applied Catalysis A: General 212 (2001) 129151
reducing atmosphere at elevated pressure, the required positive sealing against the oxidative atmosphere in the regenerator requires the use of sluice vessels or lock-hoppers. This is in contrast to processes operating at (near) atmospheric pressure, where pressure differences over standpipes or seal legs are sufcient to ensure effective sealing between spaces with different atmospheres. Hence, much higher catalyst circulation rates can be achieved in low pressure processes, and much shorter catalyst lives are allowed. Fig. 6 depicts a process scheme for a process at elevated pressure, viz. the uidised-bed version of the previously discussed MTG process [10]. A widely applied process operating in the uidised mode at near atmospheric pressure with a very short-lived catalyst circulating at a high rate between a riser reactor and a bubbling uidised-bed regenerator is the modern uid catalytic cracking process (FCC process, see Fig. 7).
4. Catalytic reforming of naphtha 4.1. Basic reactions and deactivation processes Catalytic reforming of naphtha, a petroleum fraction boiling between about 80 and 180 C is a very
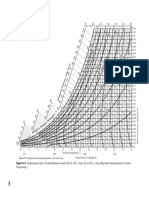
Fig. 9. Effect of operating pressure on the stability of a reforming catalyst [11].
Fig. 8. Relation between catalyst chloride content and the water/ hydrogen chloride ratio in the gas for a typical reforming catalyst.
Fig. 10. Effect of operating pressure on yields in reforming of naphtha with the Rheniforming process [7].
S.T. Sie / Applied Catalysis A: General 212 (2001) 129151
137
important renery process in the production of high-octane gasoline. The process produces aromatic hydrocarbons by dehydrogenation of cyclohexanes, by dehydro-isomerisation of cyclopentanes, and by dehydrocyclisation of alkanes. These reactions are catalysed by a bifunctional (metal + acid) catalyst. The metal function is generally represented by nely dispersed platinum or an alloy of platinum with a second metal (mostly Sn, Re, Ge, Ir), while the acid function is provided by chloriding the alumina carrier. In addition to the formation of aromatics, the production of hydrogen is important since for many reners the catalytic reformers are the main and often only source of the hydrogen needed for hydrotreating processes. A generally undesired side reaction is the formation of gaseous hydrocarbons by hydrocracking, which lowers the reformate yield and adversely affects the yield and purity of the hydrogen produced. The catalytic reforming process is carried out at elevated temperatures and moderately high pressures
in the presence of circulating hydrogen. Catalyst deactivation is generally caused by stripping of hydrogen chloride from the catalyst under operating conditions, and by deposition of carbonaceous material on the catalyst. In addition, the dispersion of the active metal may be negatively affected, e.g. by high temperatures especially during carbon burn-off. The loss of acidity by stripping of hydrogen chloride is a reversible deactivation and is generally counteracted by dosing of a chlorine-containing compound to the feed, e.g. hydrogen chloride or an organic compound that is easily converted to hydrogen chloride in the reactor, such as dichloro-ethane. The dosing rate depends on the (actual + potential) water content of the feed and the desired steady-state chloride level on the catalyst, see Fig. 8. The rate of carbon deposition and, therefore, the speed of the irreversible deactivation for a given catalyst and feedstock depends on the operating conditions, in particular on the operating pressure. At
Fig. 11. Flow scheme of a typical semi-regenerative reformer [12].
138
S.T. Sie / Applied Catalysis A: General 212 (2001) 129151
relatively high pressures (e.g. 2540 bar) less carbon is deposited and consequently long catalyst lives are obtained. However, the high pressures are unfavourable for the dehydrogenation reactions that produce the desired aromatic hydrocarbons and hydrogen. Moreover, due to an increased degree of hydrocracking, liquid yields are lowered and the hydrogen purity is decreased. At low pressures (e.g. 515 bar) the formation of aromatics is enhanced, and liquid yield, hydrogen yield and hydrogen purity are all improved as compared with the operation at higher pressures. However, these advantages of low pressure operation are obtained at the cost of a decreased catalyst stability. Fig. 9 compares the stabilities of the reforming catalyst at different pressures, while Fig. 10 shows the effect of pressure on yields. 4.2. Technological options The relatively low catalyst deactivation rates at high pressures makes a semi-regenerative type of operation a logical choice. In the semi-regenerative
catalytic reformer, the catalyst is placed in xed-bed reactors which are operated adiabatically. Because of the strong endothermicity of the process, three to four reactors are generally applied in series, and the process stream is reheated between the reactors, as is shown in Figs. 11 and 12. In the course of the run, temperatures are gradually increased so as to maintain the octane quality of the liquid product on target (see Fig. 4). The run ends at a point in time where the required temperature reaches the design limit of the unit, or when the yields have become uneconomically low. The unit is taken out of normal service and with the required safety precautions (such as using blind anges for isolating certain parts) the catalyst in the respective beds is subjected to a carbon-burn regeneration. This step may, if required, be followed by an oxidative chlorination treatment to redisperse agglomerated platinum or to re-establish the required interaction between platinum and the other alloying metals. Thereafter, the unit can be brought back to the normal operating mode and after catalyst reduction, optional presulding and adjustment of the chloride level on the catalyst a new operating cycle is started.
Fig. 12. Flow scheme of a fully-regenerative reformer [12].
S.T. Sie / Applied Catalysis A: General 212 (2001) 129151
139
Fig. 13. Flow scheme of U.O.P.s continuous regenerative reforming process [12].
The semi-regenerative catalytic reformer has the advantage of simplicity of plant hardware, but the relative long interruption of the production and the laborious character of the regeneration are clear disadvantages. For operation at the yieldwise attractive low pressures, the required frequency of regeneration renders this type of operation no longer viable, and either the xed-bed swing reactor type of operation or the moving-bed type of operation with a separate, dedicated reactor for regeneration becomes a logical choice. In both types of operation the regeneration facilities are an integral part of the process installation. Fig. 12 is a simplied ow scheme of a swing-type fully regenerative reformer. The unit features a number of reactors, each of which can be operated in a regeneration mode during a certain period. The other reactors operate in the main processing mode during that same period. The reactors are switched between the two modes by means of values that are
actuated automatically according to predetermined sequence. At a relatively low operating pressure with larger gas volumes to be circulated, the pressure drop over the catalyst-beds becomes a more constraining factor and the reactors are, therefore, designed for low pressure drop accross the beds. Designs that feature a low pressure drop are shallow, large diameter xed-beds that are accomodated in spherically shaped pressure vessels, or radial-ow reactors in which the catalyst occupies the annular space between concentric cylindrical screens through which the process stream ows in a radial direction. Fig. 13 shows a simplied ow scheme of the continuous regenerative catalytic reformer (CCR) of Universal Oil Products Co. (UOP) [13]. The reactors are radial-ow reactors through which the catalyst moves downward by gravity. In the reaction section, successive reactors are stacked above each other. Catalyst ows by gravity from the top of the stack to
140
S.T. Sie / Applied Catalysis A: General 212 (2001) 129151
Fig. 14. Comparison of catalyst activity and stability in processing of a distillate and residual feed from the same Middle East crude oil over a conventional hydrodesulfurisation catalyst. Note the much more severe operating conditions for processing the residual feed.
the bottom. Discrete portions of the spent catalyst are removed out from the bottom of the last reactor via a lock-hopper system and are transferred pneumatically to the top of the regenerator reactor which is likewise a radial-ow reactor. The regenerated catalyst is taken out from the bottom of the regenerator via a lock-hopper and transported pneumatically to the top of the rst reactor where it is reduced before being reused in the reforming process. The continuous regenerative catalytic reforming process can be applied under conditions that lead to
Table 1 Characteristics of some heavy distillates and residues Feedstock Kuwait vacuum gasoil Arabian heavy vacuum gasoil Kuwait atmospheric residue Arabian heavy atmospheric residue Iranian heavy atmospheric residue Maya atmospheric residue Kuwait vacuum residue Arabian heavy vacuum residue Iranian heavy vacuum residue Maya vacuum residue Tia Juana Pesado vacuum residue Sulfur content (wt.%) 2.9 2.9 4.2 4.3 2.7 4.1 5.3 5.5 3.6 5.6 3.4
relatively fast deactivation and short cycle times, e.g. a few days. Since the regeneration is carried out in a separate reactor, the capacity of this reactor can be designed to cope with the needs of such a fast deactivation. Thus, in contrast to a fully-regenerative reformer, the time required for a complete regeneration cycle does not pose a lower limit to the length of the operating cycle. An additional advantage is that the process is really continuous in that under steady-state conditions the yield and quality of the products are truly constant in time.
Asphaltenes C5 , (wt.%) Nil Nil 6 10 5 20 15 23 11 33 25
Nickel (ppm, weight) <1 0.7 13 27 34 68 28 49 84 130 89
Vanadium (ppm, weight) <1 0.5 53 88 145 330 105 152 275 630 675
S.T. Sie / Applied Catalysis A: General 212 (2001) 129151
141
Fig. 15. (A) Carbon on catalyst as a function of catalyst age. The data were obtained from xed-bed experiments with a large liquid recycle, terminated after processing different quantities of feed over a batch of catalyst. The gradientless reactor in these experiments is equivalent to a continuous stirred tank reactor. (B) Metals on catalyst as a function of catalyst age, from the same experiments as in Fig. 15A. Residue B contains about four times as much metals as residue A.
142
S.T. Sie / Applied Catalysis A: General 212 (2001) 129151
Fig. 16. Steady-state level of carbon on a catalyst as a function of hydrogen pressure.
5. Hydroconversion of residual oils 5.1. Deactivation mechanisms Catalyst deactivation is a major problem in the catalytic treatment with hydrogen of the residuals from atmospheric or vacuum distillation of petroleum, for the purpose of sulfur removal (hydrodesulfurisation) or cracking to distillates. Whereas the established Co/ Mo/alumina or Ni/Mo/alumina catalysts can remain active for several years in hydrotreatment of distillates, their activity decays in a matter of weeks or months
Fig. 18. Correlation between maximum uptake capacity for vanadium and utilised pore volume of catalysts. PV: specic pore volume; F is an effectiveness factor as determined by electron microprobe analysis.
when used in the processing of long (atmospheric) or short (vacuum) residues, see Fig. 14. Residual oils differ from distillate oils in that they contain much more material with condensed polyaromatic structures and organically bound metals such as nickel and vanadium, see Table 1. These two factors
Fig. 17. Radial concentration proles of vanadium as determined from electron microprobe scans along the diameter of catalysts used in hydroprocessing of residues. The proles A, B and C are typical for the catalyst types distinguished by the same letters in Table 2.
S.T. Sie / Applied Catalysis A: General 212 (2001) 129151
143
are responsible for the much lower activity and stability of the catalyst in residue procesing. The condensed polyaromatics as present in so-called asphaltenes (material insoluble in apolar solvents like n-pentane or n-heptane) can act as precursors for coke, and catalyst coking in residue processing is, therefore, much more severe than in processing of distillates. However, the deactivation by coking is reversible. This can be seen from Fig. 15A, which shows that after an intitial build-up period a steady-state coke level is established on the catalyst. For a given feed and catalyst, this steady-state coke level is inversely proportional to the hydrogen pressure, as is shown in Fig. 16. The important consequence of the reversibility of deactivation by coke is that one can cope with it by operating at a sufciently high hydrogen pressure. Thus, the amount of coke on the catalyst is controlled at a level where the residual activity of the catalyst is still sufcient for the intended duty. However, this remedy is not effective for the deactivation caused by
the presence of metals. Even though the absolute concentratios of metals in the residues are much lower than that of carbonaceous matter, their role in catalyst deactivation is much more serious. The metal-organic bonds by which these metals are attached to organic structures such as porphyrin groups that are also part of the asphaltenes are broken in the catalytic hydrotreatment so that in the presence of hydrogen sulde the metals deposit on the catalyst surface as metal suldes such as Ni3 S2 or V2 S3 . Catalyst deactivation by the deposition of nickel and vanadium suldes is irreversible in contrast to coking. An important feature of the deactivation by metals is that the process of deposition of metal suldes is not stopped when the original active sites of the catalyst are covered with deposited metal suldes. The reason for this is that the process is autocatalytic in that it is also catalysed by the deposited metal suldes themselves [14]. Hence, metals from the feed continue accumulating on the catalyst as is shown in Fig. 15B.
Fig. 19. Effect of pore size on useful life and hydrodesulfurisation activity of a set of catalysts with narrow monomodal pore size distributions, tested under standard conditions.
144
S.T. Sie / Applied Catalysis A: General 212 (2001) 129151
Table 2 Types of catalysts for hydroprocessing of residuesa Catalyst type Pore size Metals penetration Metal storage capacity Stability HC/HDS activity Application Location in reactor train
a
A Wide Deep High Very good Low HDM catalyst Front end
B Intermediate Intermediate Medium Fair Fair HC/HDS catalyst Middle
C Narrow Shallow Low Poor High HC/HDS catalyst Tail end
HDM: hydrodemetallisation; HC: hydroconversion; HDS: hydrodesulfurization.
Another feature of the feed demetallisation reaction is that it is subject to pore diffusion limitation, as is understandable considering the bulkiness of the asphaltene structures. As a consequence, the metals are generally deposited in an outer zone of the catalyst particle, as is demonstrated by electron microprobe analyses of used catalysts, see Fig. 17. Depending upon the degree of pore diffusion limitation, the metals deposition zone within the catalyst particle may be shallower or broader.
The end of the catalyst life is reached when the catalyst pores in the deposition zone are completely blocked by deposited metal suldes. Hence, the ultimate life of the catalyst will be determined by the pore volume available for storage of suldic metal deposits. Fig. 18 shows that for a given feed processed under the same conditions, the lives of different catalysts correlate well with the pore volume that is available for accomodating the deposited metals from the feed.
Fig. 20. Quick catalyst replacement (QCR) system according to Shell design [15]. Left: loading of reactor with fresh catalyst. Right: unloading spent catalyst.
S.T. Sie / Applied Catalysis A: General 212 (2001) 129151
145
5.2. Technological consequences: catalyst aspects The mechanism of catalyst deactivation by pore plugging as described above has important consequences for catalyst selection and design. Conventional distillate hydrotreating catalysts generally have relatively narrow pores so as to maximise their specic surface area and thereby their activity. With such narrow-pore catalyst, the demetallisation reaction is strongly limited by pore-diffusion, and the shallowness of the metal sulde deposition zone in the catalyst particles causes the catalyst to be very shortlived. Catalyst life can be extended by allowing deeper penetration of metals containing species by increasing the effective pore size of the catalyst. However, this goes at the expense of catalyst activity, since the non-fouled inner part of the catalyst particle becomes relatively smaller. The opposite effects of pore size variation on catalyst stability and activity are illustrated by Fig. 19. It follows that a compromise has to be reached between catalyst life and activity for the hydroconversion reaction such that neither of them are maximal, but both sufcient for the intended duty. In a xed-bed reactor as generally applied for distillate hydrotreating the removal of metals from the feed causes a decrease of the metals concentration in the process stream. This descending axial metals concentration prole implies that no single catalyst can be optimal in the whole reactor, but that in theory the metal suldes accomodating capacity and the residual activity have to be tailored to the local conditions within the reactor. This leads to the concept of using a combination of different catalysts rather than a single one in the reactor or reactor train. A highly active catalyst (with necessarily limited metals tolerance) may be applied at the downstream part of the reactor, where the metals content is low due to the demetallisation action of the preceding catalysts. This tail-end catalyst may be preceded by a catalyst which has a greater tolerance towards metal sulde deposition obtained at some cost of activity. In the front end, a highly metal tolerant catalyst capable of coping with the full metal content of the feed, but necessarily with a limited activity for the main hydroprocessing reaction can be used. Since its main function would be the removal of metals from the feed so as to protect the downstream catalyst-beds, this type of catalyst can
be termed a demetallisation catalyst. These different types of catalysts are distinguished in Table 2. 5.3. Technological implications: reactor aspects Fixed-bed reactors operated in the trickle-ow regime as used in hydrotreating or hydrocracking of heavy distillates are attractive on account of their relative simplicity. The use of combinations of suitably tailored catalysts as described above allows sufciently long run lengths (e.g. 0.51 year) with xed-bed reactors except for very demanding cases of residual oil hydroprocessing. The demands on minimum run length in practice can be lessened by reducing the downtime needed for catalyst change. A quick catalyst replacement (QCR) design by Shell [15] that avoids the opening and closing of the reactors and that shortens the cooling and heating-up periods as well as the catalyst loading and
Fig. 21. Ebullated-bed reactor [16] (reprinted from Fuel Processing Technology, 35 (1993), p. 22, with permission from Elsevier Science).
146
S.T. Sie / Applied Catalysis A: General 212 (2001) 129151
[16], depicted in Fig. 21. The catalyst inventory is kept in a uidised state by strong upward liquid and gas streams. With this reactor, catalyst life is no longer an issue as far as achievable run length is concerned but it has become an economic factor relating to catalyst consumption rate. With the ebullated-bed reactor, conventional catalysts as used in distillate hydrotreating may be used which can have the advantage of high activity (cf Table 2, catalyst type C). A disadvantage is the backmixed character of the reactor. The large liquid recycle causes the reactor to behave as a continuous stirred tank reactor, which is a less ideal reactor for reactions of positive order. The uidisation of the solid also results in solids backmixing, which implies that the catalyst removed also contains fresh catalyst particles. The latter disadvantage is absent in the so-called bunkerow reactor depicted in Fig. 22, which is a moving-bed reactor operated in the trickle-ow mode with the possibility to remove or add portions of catalyst during operation by means of valves and lock-hoppers. A special design of the reactor internals ensures that the catalyst can move down in plug ow. The narrow residence time distribution achievable with this type of reactor is shown in Fig. 23. The bunkerow reactor has been specically designed for application as front-end demetallisation
Fig. 22. Bunkerow reactor. Adapted from [17].
unloading time is shown in Fig. 20. Catalyst transport into the reactors occurs as a slurry in a stream of gasoil. A reactor that allows portions of catalyst to be added to or taken out of the reactor during normal operation is the so-called ebullated or ebulliating-bed reactor
Fig. 23. Residence time distribution of solid particles in a bunkerow reactor. The experimental distribution is about the same as the theoretical distribution in a series of about 200 mixers, which indicates a good approach to ideal plug-ow.
S.T. Sie / Applied Catalysis A: General 212 (2001) 129151
147
Fig. 24. Axial deactivation proles at end-of-run conditions in xed-beds and during operation in a continuous stirred tank or bunkerow reactor. F: feed, P: product, C(f): fresh catalyst, C(s): spent catalyst. The hatched area denotes the deactivated part.
Fig. 25. Relative catalyst life vs. metals content of the feed [18].
148
S.T. Sie / Applied Catalysis A: General 212 (2001) 129151
reactor. Operating conditions and catalyst addition/ withdrawal rate can be chosen so as to ensure that the catalyst taken out is completely spent, whilst still retaining an acceptable average activity in the reactor. Thus, better use is made of the metals sulde accomodating capacity of the catalyst as compared with other reactor systems, see Fig. 24.
5.4. Technological consequences: process conguration Since the metals content of the residual feedstock largely determines the catalyst deactivation rate, different feedstocks with widely differing metals content may require different catalyst combinations and
Fig. 26. (A) General process scheme for Shells residue hydroprocessing technology [19]. (B) Conguration of the reactor section in Shells residue conversion technology for feeds of different metals content. Adapted from [19].
S.T. Sie / Applied Catalysis A: General 212 (2001) 129151
149
Fig. 27. Simplied scheme of Shells demetallisation catalyst regeneration process [19].
reactor technologies. This can be seen from Fig. 25, which shows the relation between feed metals content and catalyst life. The wide range of metal contents in residual feeds and consequently the large differences in deactivation rates lead to the adoption of different process congurations in which different catalysts and reactors are applied. This is borne out by Fig. 26A and B pertai-
ning to Shells residue hydroconversion technology. Fig. 26B shows the different catalyst and reactor combinations in relation to the feeds metal content, while the general process scheme is depicted in Fig. 26A. The scheme of Fig. 26B also includes the option of regenerating the spent demetallisation catalyst by removal of the deposited metal suldes. With a specially developed carrier on the basis of silica, removal
Fig. 28. Reactor conguration in IFPs Hyvahl-S process with swing reactors in the hydrodemetallisation section [20].
150
S.T. Sie / Applied Catalysis A: General 212 (2001) 129151
Fig. 29. Reactor conguration for hydrotreating of resids according to Chevron, featuring the onstream catalyst replacement (OCR) system for removal of metals [18].
by acid leaching becomes a realistic option, and a demetallisation catalyst regeneration (DCR) process has been developed by Shell on this basis, see Fig. 27. This regeneration option makes the processing of feedstock of very high metals content feasible and more economic compared to the use of demetallisation catalysts on a once-through basis. An alternative solution for the processing of feedstocks with moderately high metals content is to use a swing reactor system in the demetallisation stage instead of the bunkerow reactor. Fig. 28 shows the swing reactor system as used in IFPs Hyvahl-S process [20]. Yet another alternative for Shells bunkerow reactor where the gas/liquid feed and the catalyst move in the same, i.e. downward, direction is an operation with gas/liquid upow and catalyst downow. The latter feed/catalyst countercurrent operation features in the Hyvahl-M process of IFP/Asvahl [20,21] and in the online catalyst replacement (OCR) system of Chevron [18], see Fig. 29.
6. Conclusion Catalyst deactivation is often inherent in the reaction mechanisms underlying a process and while it can be
minimised by proper choice of catalyst and process variables, it can in many cases not be entirely avoided. In such cases, the process technology has to be able to cope with the deactivation at hand. The nature of catalyst deactivation, in particular the question whether the deactivation mechanism is reversible or not under normal operating conditions, and the rapidity of catalyst performance decay have a large bearing on the choice of process options. Aside from catalyst parameters, these include the type of reactor and its hydrodynamic regime (xed-, moving-, uidised-bed, co- or counter-current, plug ow or mixed) as well as the conguration of the process. The relation between catalyst deactivation and process technology can be viewed in two ways: while on the one hand, the deactivation behaviour of a given type of catalyst in a certain conversion reaction may dictate the process technology to be applied, on the other hand, it is possible that the development of a novel specic technology widens the scope of the conversion process. The widening of the scope of a process may, for instance, encompass the feasibility to operate under conditions that are more desirable in terms of yields but inherently lead to faster catalyst deactivation. Or it may allow using alternative catalysts or the processing of more contaminated feeds
S.T. Sie / Applied Catalysis A: General 212 (2001) 129151
151
posing more severe deactivation problems. The developments of the continuous regeneration technology in catalytic reforming and the bunker-ow reactor in residue hydroprocessing are cases in point. References
[1] A. Sadana, L.K. Doraiswamy, Effect of catalyst fouling in xed-, moving-, and uid-bed reactors, J. Catal. 23 (1971) 147157. [2] J.M. Douglas, E.K. Reiff Jr., J.R. Kittrell, Process design with catalyst deactivation, Chem. Eng. Sci. 35 (1980) 322329. [3] W.H.J. Stork, Molecules, catalysts and reactors in hydroprocessing of oil fractions, in: G.F. Froment, B. Delmon, P. Grange (Eds.), Hydrotreatment and Hydrocracking of Oil Fractions, Elsevier, Amsterdam, 1997, pp. 4167. [4] J.W. Gosselink, J.A.R. Van Veen, Coping with catalyst deactivation in hydrocarbon processing, in: Proceedings of the 8th International Symposium on Catalyst Deactivation, Brugge, Belgium, 1013 October 1999. [5] B.W. Burbidge, P. Watson, A.H. Richardson, BPs isomerisation process takes on a new look, in: Proceedings of the NPRA Annual Meeting, San Antonio, Texas, 2325 March 1975. [6] N.Y. Chen, R.L Gorring, H.R. Ireland, T.R. Stein, New process cuts pour point of distillates, Oil Gas J. June, 6 (1977) 165170. [7] R.C. Robinson, L.A. Fredrickson, R.L. Jacobson, Cat-reforming pressure drops again, Oil Gas J. May, 15 (1972) 124136. [8] W. Lee, S. Yurchak, N. Daviduk, J. Maziuk, A xed-bed process for the conversion of methanol-to-gasoline, in: Proceedings of the NPRA Annual Meeting, New Orleans, LA, 2325 March 1980. [9] D.E. Krohn, M.G. Melconian, in: D.M. Bibby, C.D. Chang, R.F. Howe, S. Yurchak (Eds.), Methane Conversion, Elsevier, Amsterdam, 1988, p. 679.
[10] J.E. Penick, W. Lee, J. Maziuk, Development of the methanolto-gasoline process, in: Proceedings of the Inter national Symposium on Chemical Reactor Engineering (ISCRE-7), Boston, MA, 4 October 1982. [11] J. Barbier, E. Churin, P. Marecot, J.C. Menezo, Appl. Catal. 36 (1988) 277. [12] Royal Dutch/Shell Group of Companies, The Petroleum Handbook, 6th Edition, Elsevier, Amsterdam, 1983. [13] E.A. Sutton, A.R. Greenwood, F.H. Adams, A new promising concept: continuous platforming, Oil Gas J. May, 22 (1972) 5256. [14] S.T. Sie, Catalyst deactivation by poisoning and pore plugging in petroleum processing, in: B. Delmon, G.F. Froment (Eds.), Catalyst Deactivation, Elsevier, Amsterdam, 1980, pp. 545569. [15] W.C. Van Zijll Langhout, C. Ouwerkerk, K.M.A. Pronk, New process hydrotreats metal-rich feedstocks, Oil Gas J. December, 1 (1980) 120126. [16] R.M. Eccles, Residue processing using ebullated-bed reactors, Fuel Process. Technol. 35 (1993) 2138. [17] A.J.J. Van Ginneken, M.M. Van Kessel, K.M.A. Pronk, G. Renstrom, Shell process desulfurizes resids, Oil Gas J. April, 28 (1975) 5963. [18] G.L. Scheuerman, D.R. Johnson, B.E. Reynolds, R.W. Bachtel, R.S. Threlkel, Advances in Chevron RDS technology for heavy oil upgrading exibility, Fuel Process. Technol. 35 (1993) 3954. [19] P.B. Kwant, W.C. Van Zijll Langhout, The development of Shells residue hydroconversion process, in: Proceedings of the Conference on the Complete Upgrading of Crude Oil, Siofok, 2527 September 1985. [20] A. Hennico, G. Cohen, T. Des Courires, M. Espeillac, Hydroconversion process for high metal residues, Hydrocarbon Int. (1993) 3337. [21] J.P. Euzen, Moving-bed process for residue hydrotreating, Rev. Inst. Franc. du Ptrole 46 (1991) 517527.
Вам также может понравиться
- Teaching Chemical Reaction Engineering Using EMSO SimulatorДокумент13 страницTeaching Chemical Reaction Engineering Using EMSO SimulatorIzack Silva SОценок пока нет
- EMSO: A new environment for modelling, simulation and optimisationДокумент15 страницEMSO: A new environment for modelling, simulation and optimisationIzack Silva SОценок пока нет
- Em SoДокумент84 страницыEm Soalice.medeirosОценок пока нет
- Process OptimizationДокумент70 страницProcess OptimizationplanketОценок пока нет
- MyELT - Your ResourcesДокумент1 страницаMyELT - Your ResourcesIzack Silva SОценок пока нет
- Emso-Opc Link User Manual: Tiago Fiorenzano Finkler Rafael de Pelegrini SoaresДокумент18 страницEmso-Opc Link User Manual: Tiago Fiorenzano Finkler Rafael de Pelegrini SoaresIzack Silva SОценок пока нет
- Em So Quick Ref ThermДокумент2 страницыEm So Quick Ref ThermIzack Silva SОценок пока нет
- Solving Cubic Equation of StateДокумент73 страницыSolving Cubic Equation of StateIzack Silva SОценок пока нет
- EMSOQuickRef PDFДокумент6 страницEMSOQuickRef PDFHRC8055Оценок пока нет
- Install GuideДокумент189 страницInstall GuideAdnan RajkotwalaОценок пока нет
- SugarcaneДокумент1 страницаSugarcaneIzack Silva SОценок пока нет
- Diesel HydrotreatingДокумент21 страницаDiesel HydrotreatingIzack Silva S100% (1)
- Solution Manual-Chemical Engineering Thermodynamics - Smith Van NessДокумент621 страницаSolution Manual-Chemical Engineering Thermodynamics - Smith Van NessSurya Budi Widagdo87% (184)
- Kinetic Study and H2S Effect On Refractory DBTs Desulfurization in A Heavy GasoilДокумент33 страницыKinetic Study and H2S Effect On Refractory DBTs Desulfurization in A Heavy GasoilIzack Silva SОценок пока нет
- HDT of DieselДокумент17 страницHDT of DieselIzack Silva S100% (1)
- Yamada&Gunn RackettEquationДокумент3 страницыYamada&Gunn RackettEquationIzack Silva SОценок пока нет
- MyELT - Online English Language LearningДокумент1 страницаMyELT - Online English Language LearningIzack Silva SОценок пока нет
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeОт EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeРейтинг: 4 из 5 звезд4/5 (5784)
- The Yellow House: A Memoir (2019 National Book Award Winner)От EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Рейтинг: 4 из 5 звезд4/5 (98)
- Never Split the Difference: Negotiating As If Your Life Depended On ItОт EverandNever Split the Difference: Negotiating As If Your Life Depended On ItРейтинг: 4.5 из 5 звезд4.5/5 (838)
- The Emperor of All Maladies: A Biography of CancerОт EverandThe Emperor of All Maladies: A Biography of CancerРейтинг: 4.5 из 5 звезд4.5/5 (271)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceОт EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceРейтинг: 4 из 5 звезд4/5 (890)
- The Little Book of Hygge: Danish Secrets to Happy LivingОт EverandThe Little Book of Hygge: Danish Secrets to Happy LivingРейтинг: 3.5 из 5 звезд3.5/5 (399)
- Team of Rivals: The Political Genius of Abraham LincolnОт EverandTeam of Rivals: The Political Genius of Abraham LincolnРейтинг: 4.5 из 5 звезд4.5/5 (234)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaОт EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaРейтинг: 4.5 из 5 звезд4.5/5 (265)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryОт EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryРейтинг: 3.5 из 5 звезд3.5/5 (231)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureОт EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureРейтинг: 4.5 из 5 звезд4.5/5 (474)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersОт EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersРейтинг: 4.5 из 5 звезд4.5/5 (344)
- The Unwinding: An Inner History of the New AmericaОт EverandThe Unwinding: An Inner History of the New AmericaРейтинг: 4 из 5 звезд4/5 (45)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyОт EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyРейтинг: 3.5 из 5 звезд3.5/5 (2219)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreОт EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreРейтинг: 4 из 5 звезд4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)От EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Рейтинг: 4.5 из 5 звезд4.5/5 (119)
- Nest Installation GuideДокумент8 страницNest Installation GuideOzzyОценок пока нет
- Makalah Bahasa Inggris - Narrative TextДокумент21 страницаMakalah Bahasa Inggris - Narrative TextFenny KartikaОценок пока нет
- Raspberry PiДокумент19 страницRaspberry PiAnonymous E4Rbo2s100% (1)
- LTE Speech Traffic Dimenshioning For VoipДокумент6 страницLTE Speech Traffic Dimenshioning For VoipRahul GuptaОценок пока нет
- Answer Section B and C and Paper 3Документ21 страницаAnswer Section B and C and Paper 3Adnan ShamsudinОценок пока нет
- Blues, Rock and Americana MixДокумент4 страницыBlues, Rock and Americana MixLuis CrownОценок пока нет
- MACRO-ETCHING SOLUTIONS FOR ALUMINIUM ALLOYSДокумент1 страницаMACRO-ETCHING SOLUTIONS FOR ALUMINIUM ALLOYSsensoham03Оценок пока нет
- Instrument To Be CalibratedДокумент3 страницыInstrument To Be Calibratedsumit chauhanОценок пока нет
- 2nd - Science-Second-Quarter-Week-1Документ37 страниц2nd - Science-Second-Quarter-Week-1Arlene AranzasoОценок пока нет
- Margot's Cafe MenuДокумент1 страницаMargot's Cafe Menumichael_burns_24Оценок пока нет
- 322439480MVR Single Page Single Page Booklet - OPTДокумент12 страниц322439480MVR Single Page Single Page Booklet - OPTlarry vargas bautistaОценок пока нет
- Data Sheet ID FanДокумент5 страницData Sheet ID FanrudiawanОценок пока нет
- Particle Technology Che Calculations Separation Processes Heat and Mass TransferДокумент1 страницаParticle Technology Che Calculations Separation Processes Heat and Mass TransferAduchelab AdamsonuniversityОценок пока нет
- JCB 532-120 PDFДокумент4 страницыJCB 532-120 PDFSyazrur Syazmir0% (1)
- Operating and Installation Guide For The Digital Instrument: Motoscope Tiny / Speedster / VintageДокумент12 страницOperating and Installation Guide For The Digital Instrument: Motoscope Tiny / Speedster / Vintagepeter timmermansОценок пока нет
- Purification of Morphologically and Functionally Intact Human Basophils To Near HomogeneityДокумент9 страницPurification of Morphologically and Functionally Intact Human Basophils To Near HomogeneitySinaí GutierrezОценок пока нет
- English Test 6Документ87 страницEnglish Test 6Ha PhanОценок пока нет
- Kirloskar-Oil-Engines DescriptionsДокумент8 страницKirloskar-Oil-Engines Descriptionssinghhardeep760Оценок пока нет
- Pump Characteristics ExperimentДокумент7 страницPump Characteristics ExperimentJam JoОценок пока нет
- 3.1 From Algae To Terrestrial Plants-Student SheetДокумент2 страницы3.1 From Algae To Terrestrial Plants-Student Sheeteshaaljamal27Оценок пока нет
- Motor Cat 924HZДокумент6 страницMotor Cat 924HZAdemilson Rangelvieira100% (1)
- Turbine Stress EvaluatorДокумент14 страницTurbine Stress EvaluatorsumitОценок пока нет
- KTS - Sarao.bakus Temple of Eternal FiireДокумент176 страницKTS - Sarao.bakus Temple of Eternal FiireK.T.S. SaraoОценок пока нет
- Diesel HatchbackДокумент14 страницDiesel HatchbackloganathprasannaОценок пока нет
- Carta Psicrometrica PDFДокумент2 страницыCarta Psicrometrica PDFJuliethОценок пока нет
- 1B Cosmos-Standard - Technical - Guide - v40Документ45 страниц1B Cosmos-Standard - Technical - Guide - v40carla deiddaОценок пока нет
- Chapter 3 (CHM 127)Документ105 страницChapter 3 (CHM 127)FiqajasmeОценок пока нет
- Ebook Scientific InstrumentsДокумент9 страницEbook Scientific InstrumentsPavans LuckyОценок пока нет
- Large and Medium Manufacturing Industry Survey Report 2018Документ778 страницLarge and Medium Manufacturing Industry Survey Report 2018melakuОценок пока нет
- Cardiopulmonary System: Relevant Anatomy & Physiology: HeartДокумент12 страницCardiopulmonary System: Relevant Anatomy & Physiology: HeartJulia SalvioОценок пока нет