Академический Документы
Профессиональный Документы
Культура Документы
Two-Component Phase Equilibria Solutions
Загружено:
sgybleeИсходное описание:
Оригинальное название
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
Two-Component Phase Equilibria Solutions
Загружено:
sgybleeАвторское право:
Доступные форматы
5.
60 Spring 2008 Lecture #22 page 1
Two-Component Phase Equilibria III
Ideal and Non-Ideal Solutions
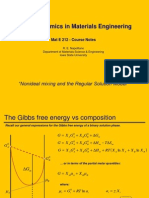
Free energy change AG
mix
in ideal solutions
A (liq)
B (liq)
A + B (liq)
AG
*
( )
*
G ( )
mix
( )
mix
G ( ) = n x + n x ( ) = n x + n x ( )
1 A A A B B B 2 A A A B B B
AG = G ( ) G ( )
mix 2 1
A
-
A
( )
A
(
B
B
-
( )
B
nx
A
A
-
nx
B
B
-
= nx + RTlnx + nx + RTlnx
(
( ) ( )
AG = nRT x lnx + x lnx
mix
(
A A B B
)
Purely entropic, as in gas mixture
G = Vdp SdT
| cAG |
mix
\
cT
mix
|
|
.
p
(
A A B B
)
AS = = nR x lnx + x lnx
AH
mix
= A G
mix
A
mix
+ T S = 0
No enthalpy change, all of AG is due to entropy of mixing
Change in volume AV
mix
AV
mix
=
|
cA
c
G
p
mix
|
|
|
= 0
\ .
T
No volume change, just like ideal gas.
5.60 Spring 2008 Lecture #22 page 2
Non-Ideal Solutions:
In reality, molecules interact:
A A A
A
B
B B B
u
AA
< 0 u
BB
< 0 u
AB
u
AB
PURE MIXED
Au = 2u
AB
- (u
AA
+ u
BB
)
This difference determines how solutions depart from ideality.
I. Positive Deviations: Au > 0 (most common)
Mixing is energetically not favorable in liquid phase.
AH = AU + A(PV) AU
AG = Au + nRT x lnx + x lnx > AG
(
ideal
)
mix
n
4
(
A A B B
)
mix
e.g. acetone & carbon disulphide
CH
3
C = O + S = C = S
CH
3
B
A
*
5.60 Spring 2008 Lecture #22 page 3
=
* *
acetone A
p p
=
2
*
B CS
p p
p = p
A
+ p
B
p
B
p
A
1
x
CS2
= x
B
0
p
Real
Raoult
*
p
CS
2
> x
CS
2
p
CS
2
*
p
acetone
> x
acetone
p
acetone
p
tot
(
real
)
> p
tot
(
Raoult
)
vapor pressure is higher than expected by Raoults Law
II. Negative Deviations: Au < 0
e.g. acetone & chloroform
CH
3
CH
3
C = O +
B
A
Cl
Cl
H - C - Cl
H-bonding
attraction
Mixing is energetically favorable in liquid phase.
3
*
CHCl
p
B
A B
5.60 Spring 2008 Lecture #22 page 4
Real
p
0 x
CHCl3
= x
=
p = p + p
p
B
p
A
*
p
B
* *
p
acetone
= p
A
Raoult
1
*
p
CS
2
< x
CS
2
p
CS
2
*
p
CHCl
3
< x
CHCl
3
p
CHCl
3
p
(
real
)
< p
(
Raoult
)
tot tot
Ideal Dilute Solutions and Henrys Law:
Non-ideal solutions are difficult to describe completely
Describe limits x
B
1 and x
B
0 Ideal Dilute Solution
I. x
CS2
= x
B
1 (B is the solvent)
Then Raoults Law applies for CS
2
CS
2
molecules see mostly other CS
2
molecules
*
p
CS
2
= x
CS
2
p
CS
2
0
5.60 Spring 2008 Lecture #22 page 5
II. x
B
0 (B is the solute)
Then Henrys Law applies: p
CS
2
= x
CS
2
K
CS
2
p
B
= x
B
K
B
Henrys Law constant
K
B
Henrys Law constant, depends on the solvent/solute mixture
and the temperature.
Labeled just K
B
, even though it depends on A also.
2
K
CS
=
B
K
Positive deviation
B
K
*
B
p >
Henry
* *
(Negative deviation would
2
p
CS
=
B
p
have
B
K
*
B
p < )
1 x
CS2
= x
B
p
Raoult
Real
Ideal dilute solution:
Solvent, e.g. A: x
A
~ 1 Raoults Law
A
p
*
A A
x p =
Solute, e.g. B: x
B
~ 0 Henrys Law p
B
= x
B
K
B
*
5.60 Spring 2008 Lecture #22 page 6
Total phase diagram:
Ideal
Real
p
Liquid
p
Liquid
* *
phase
p
B
phase
p
B
* *
p
A
p
A
gas
gas
phase
phase
0 x
B
,y
B
1
0 x
B
,y
B
1
Stronger non-ideality: Azeotropes
Liquid
p
phase
At this point
p
B
x
B
= y
B
*
p
A
gas
phase
0 x
B
,y
B
1
Similar for constant p phase diagram
Can have positive or negative azeotropes
T T
phase
gas
T
A
*
T
A
*
T
B
*
Liquid
Liquid
phase
phase
0 x
B
,y
B
1
0 x
B
,y
B
1
Cannot distill
past azeotrope
x
B
= y
B
gas
phase
T
B
*
Вам также может понравиться
- 2008 Physical Chemistry 3Документ52 страницы2008 Physical Chemistry 3julianodesouzaОценок пока нет
- October 25, 2001 Reading: Chapter IX Homework: 9.1, 9.3, 9.5, 9.7, 9.9, 9.10, 9.11 Theory of SolutionsДокумент6 страницOctober 25, 2001 Reading: Chapter IX Homework: 9.1, 9.3, 9.5, 9.7, 9.9, 9.10, 9.11 Theory of SolutionsclaudioОценок пока нет
- 4.6 Real solution: activity of solute and solvent: γ γ By R.L. The activity factorДокумент18 страниц4.6 Real solution: activity of solute and solvent: γ γ By R.L. The activity factordhika mtОценок пока нет
- PhyChem II Simple Mixture PDFДокумент39 страницPhyChem II Simple Mixture PDFJupert Jasser AbellanaОценок пока нет
- Chemical Equilibrium: Ideal GasesДокумент6 страницChemical Equilibrium: Ideal GasessgybleeОценок пока нет
- Equilibrium in Solution: TPC TPRTC TPRT A TPДокумент6 страницEquilibrium in Solution: TPC TPRTC TPRT A TPsgybleeОценок пока нет
- November 1, 2001 Reading: Chapter X Homework: 10.1, 10.3, 10.5, 10.6, 10.7 Free Energy Composition Diagram For A Homogeneous Solid SolutionДокумент4 страницыNovember 1, 2001 Reading: Chapter X Homework: 10.1, 10.3, 10.5, 10.6, 10.7 Free Energy Composition Diagram For A Homogeneous Solid SolutionclaudioОценок пока нет
- Chemical Engineering 301 Lecture Notes: (Revised 9/04)Документ9 страницChemical Engineering 301 Lecture Notes: (Revised 9/04)shiv kr dubeyОценок пока нет
- Mixture Problems PDFДокумент3 страницыMixture Problems PDFKnspeisОценок пока нет
- Polymer Solution ThermodynamicsДокумент18 страницPolymer Solution ThermodynamicsCarlos OliveiraОценок пока нет
- CHEMICAL EQUILIBRIUMДокумент0 страницCHEMICAL EQUILIBRIUMAlex Samuel SilvaОценок пока нет
- Isothermal ReactorДокумент58 страницIsothermal ReactorRoxanna LevineОценок пока нет
- Binary SolutionsДокумент25 страницBinary SolutionsLaila Ambar SariОценок пока нет
- PC 1 Formulae Sheet 3Документ2 страницыPC 1 Formulae Sheet 3Kaaya GodfreyОценок пока нет
- "Diffusive" Heat and Mass Transfer: NPTEL, IIT Kharagpur, Prof. Saikat Chakraborty, Department of Chemical EngineeringДокумент7 страниц"Diffusive" Heat and Mass Transfer: NPTEL, IIT Kharagpur, Prof. Saikat Chakraborty, Department of Chemical EngineeringShanmukShannuОценок пока нет
- Thermodynamic Relations & Equations of StateДокумент10 страницThermodynamic Relations & Equations of StateJamesBanglaganОценок пока нет
- Binary SolutionsДокумент23 страницыBinary SolutionsskluxОценок пока нет
- Chemical Equilibrium: 2.1. Some DefinitionsДокумент24 страницыChemical Equilibrium: 2.1. Some DefinitionsNguyễn Quốc HưngОценок пока нет
- Chem EquiДокумент8 страницChem EquiJeremy CОценок пока нет
- CH 11Документ22 страницыCH 11Ingenio MetalurgiaОценок пока нет
- Temperature Dependence: Change in G - (Change in T) X SДокумент5 страницTemperature Dependence: Change in G - (Change in T) X SShahday BayanОценок пока нет
- 5.60 Thermodynamics & Kinetics: Mit OpencoursewareДокумент7 страниц5.60 Thermodynamics & Kinetics: Mit OpencoursewarecaptainhassОценок пока нет
- 3.012 PS 7 Thermo Solutions 3.012 Fall 2003Документ11 страниц3.012 PS 7 Thermo Solutions 3.012 Fall 2003Sanjeev SahuОценок пока нет
- 5.60 Thermodynamics & Kinetics: Mit OpencoursewareДокумент7 страниц5.60 Thermodynamics & Kinetics: Mit OpencoursewarecaptainhassОценок пока нет
- 7.catalysis ProblemsДокумент21 страница7.catalysis ProblemsvamsiakellaОценок пока нет
- October 22, 2001 Reading: Chapter VIII Homework: 8.1, 8.3, 8.5, 8.6, 8.7 Mixing of Ideal Gas Under Various ConditionsДокумент6 страницOctober 22, 2001 Reading: Chapter VIII Homework: 8.1, 8.3, 8.5, 8.6, 8.7 Mixing of Ideal Gas Under Various ConditionsclaudioОценок пока нет
- Thermodynamics Workshop Problems Model Answers 2010-2011 1Документ9 страницThermodynamics Workshop Problems Model Answers 2010-2011 1djsmilie77Оценок пока нет
- Two-Component Phase Equilibria ExplainedДокумент5 страницTwo-Component Phase Equilibria ExplainedSiti Halimatus Sa'diahОценок пока нет
- Chemical Reaction Engineering (CRE)Документ29 страницChemical Reaction Engineering (CRE)Phuong PhamОценок пока нет
- Chapter 11 Equilibrium Lecture NotesДокумент40 страницChapter 11 Equilibrium Lecture NotesGabriel OkolОценок пока нет
- Reacting Mixtures and CombustionДокумент23 страницыReacting Mixtures and CombustionDeepti KanadeОценок пока нет
- Chemical+EquilibriumДокумент142 страницыChemical+Equilibriumlaxmikudva47007Оценок пока нет
- CH 17 Solutions ManualДокумент41 страницаCH 17 Solutions Manuallmbrn0415Оценок пока нет
- Short Notes On EquilibriumДокумент2 страницыShort Notes On EquilibriumRichard VincentОценок пока нет
- Principles of chemical equilibrium Atkins Chapter 7Документ6 страницPrinciples of chemical equilibrium Atkins Chapter 7JohnS.GallianoОценок пока нет
- Chap 06Документ25 страницChap 06echelon120% (1)
- Chem 241 Chapter 7Документ15 страницChem 241 Chapter 7Hamzah MahmoudОценок пока нет
- Concept of The Chemical Potential and The Activity of ElementsДокумент11 страницConcept of The Chemical Potential and The Activity of ElementssmrutiОценок пока нет
- Equations and TablesДокумент22 страницыEquations and TablesleandroniedbalskiОценок пока нет
- Solutions Manual Chapter13Документ42 страницыSolutions Manual Chapter13Daeyoung KimОценок пока нет
- Chemical Equilibrium in Ideal Gas MixturesДокумент6 страницChemical Equilibrium in Ideal Gas MixturesMia Nur AliaОценок пока нет
- Chapter 1. Thermodynamics and Phase DiagramsДокумент56 страницChapter 1. Thermodynamics and Phase DiagramsLuis Enrique Quispe HanccoОценок пока нет
- GC2 Chemical EquilibriumДокумент41 страницаGC2 Chemical EquilibriumAkisha FijoОценок пока нет
- General Chemistry Lecturer-2Документ34 страницыGeneral Chemistry Lecturer-2Bảo Long Trần LêОценок пока нет
- Principles of Chemical Equilibrium: BG BGДокумент30 страницPrinciples of Chemical Equilibrium: BG BGJudith Del Valle MorejonОценок пока нет
- Chemical EquilibriumДокумент6 страницChemical Equilibriumlmcristina5Оценок пока нет
- Homework 2 FinalpdfДокумент2 страницыHomework 2 FinalpdfAslıhan KayaОценок пока нет
- Chemical EquilibriumДокумент13 страницChemical EquilibriumAyodele AdeyonuОценок пока нет
- 5 60 Lecture23Документ4 страницы5 60 Lecture23sgybleeОценок пока нет
- Mid Term3 Review 15Документ32 страницыMid Term3 Review 15Jeremy SchneiderОценок пока нет
- Thermodynamics and Equilibrium Class NotesДокумент40 страницThermodynamics and Equilibrium Class NotesDotan NutodОценок пока нет
- 4-ITK-330 Steady State Nonisothermal Reactor DesignДокумент37 страниц4-ITK-330 Steady State Nonisothermal Reactor DesignadyckarockОценок пока нет
- Chemical Equilibria: Concept of EquilibriumДокумент6 страницChemical Equilibria: Concept of Equilibriumskywalker_handsomeОценок пока нет
- October 24, 2001 Reading: Chapter IX Homework: None Theory of SolutionsДокумент5 страницOctober 24, 2001 Reading: Chapter IX Homework: None Theory of SolutionsclaudioОценок пока нет
- Regular SolutionsДокумент19 страницRegular SolutionsRahul PandeyОценок пока нет
- Chemical EquilibriumДокумент18 страницChemical EquilibriumSohila A. MabroukОценок пока нет
- Solution Manual for an Introduction to Equilibrium ThermodynamicsОт EverandSolution Manual for an Introduction to Equilibrium ThermodynamicsОценок пока нет
- Critical Evaluation of Some Equilibrium Constants Involving Organophosphorus ExtractantsОт EverandCritical Evaluation of Some Equilibrium Constants Involving Organophosphorus ExtractantsОценок пока нет
- Instructions For The EssayДокумент1 страницаInstructions For The EssaysgybleeОценок пока нет
- MAPK Signaling PathwayДокумент2 страницыMAPK Signaling PathwaysgybleeОценок пока нет
- OPT STEM ExtensionGuidelinesДокумент1 страницаOPT STEM ExtensionGuidelinessgybleeОценок пока нет
- Pcast Nano Report20102Документ96 страницPcast Nano Report20102sgybleeОценок пока нет
- GRE BioChemДокумент33 страницыGRE BioChemsgybleeОценок пока нет
- Hmwk2 SolutionsДокумент9 страницHmwk2 SolutionssgybleeОценок пока нет
- Hmwk2 SolutionsДокумент9 страницHmwk2 SolutionssgybleeОценок пока нет
- Single Page Integral Table PDFДокумент2 страницыSingle Page Integral Table PDFMehmet Helva100% (2)
- Equilibrium in Solution: TPC TPRTC TPRT A TPДокумент6 страницEquilibrium in Solution: TPC TPRTC TPRT A TPsgybleeОценок пока нет
- 2 PDFДокумент3 страницы2 PDFsgybleeОценок пока нет
- UK Nanotechnologies Strategy: Small Technologies, Great OpportunitiesДокумент56 страницUK Nanotechnologies Strategy: Small Technologies, Great OpportunitiessgybleeОценок пока нет
- Equilibrium: Application To Drug Design: Nature BiotechnologyДокумент5 страницEquilibrium: Application To Drug Design: Nature BiotechnologysgybleeОценок пока нет
- DOM Research Day ScheduleДокумент1 страницаDOM Research Day SchedulesgybleeОценок пока нет
- Flow CytometryДокумент30 страницFlow CytometrysgybleeОценок пока нет
- QPCR Quant Protocol Guide 11322363 AДокумент22 страницыQPCR Quant Protocol Guide 11322363 AsgybleeОценок пока нет
- Kinetics: Reaction Rates, Orders, Half Lives: Aa + BB CC + DDДокумент8 страницKinetics: Reaction Rates, Orders, Half Lives: Aa + BB CC + DDsgyblee100% (1)
- Quantum Mechanics PostulatesДокумент6 страницQuantum Mechanics PostulatessgybleeОценок пока нет
- Thermochemistry Lec - 1Документ10 страницThermochemistry Lec - 1wolfofphysics08IPMP01Оценок пока нет
- Multicomponent Systems, Partial Molar Quantities, and The Chemical PotentialДокумент5 страницMulticomponent Systems, Partial Molar Quantities, and The Chemical PotentialsgybleeОценок пока нет
- Complex Reactions and Mechanisms (Continued)Документ7 страницComplex Reactions and Mechanisms (Continued)sgybleeОценок пока нет
- QPCR Quant Protocol Guide 11322363 AДокумент22 страницыQPCR Quant Protocol Guide 11322363 AsgybleeОценок пока нет
- 5 60 Lecture23Документ4 страницы5 60 Lecture23sgybleeОценок пока нет
- 5 60 Lecture31Документ6 страниц5 60 Lecture31sgybleeОценок пока нет
- Applications: Chemical and Phase Equilibria: 5.60 Spring 2008 Lecture #29Документ6 страницApplications: Chemical and Phase Equilibria: 5.60 Spring 2008 Lecture #29sgybleeОценок пока нет
- 5 60 Lecture11Документ7 страниц5 60 Lecture11sgybleeОценок пока нет
- Model Systems: Double-Stranded Polymer ModelДокумент7 страницModel Systems: Double-Stranded Polymer ModelsgybleeОценок пока нет
- Lecture 1Документ2 страницыLecture 1K Sandeep RaoОценок пока нет
- Kinetics: Reaction Rates, Orders, Half Lives: Aa + BB CC + DDДокумент8 страницKinetics: Reaction Rates, Orders, Half Lives: Aa + BB CC + DDsgyblee100% (1)
- Handouts PPE Day 3Документ4 страницыHandouts PPE Day 3terrence miguel balitaОценок пока нет
- Q and A Problem Solving ReliefДокумент3 страницыQ and A Problem Solving ReliefCharlotte Bacani SamilinОценок пока нет
- Chapter 5.T-102 (5.4)Документ51 страницаChapter 5.T-102 (5.4)hazmi_omar100% (1)
- The Kinetic Theory of GasesДокумент3 страницыThe Kinetic Theory of GaseszaedmohdОценок пока нет
- IWCF Well Control 4Документ13 страницIWCF Well Control 4Abdur Rahman100% (1)
- 13X DatasheetДокумент3 страницы13X Datasheetdrizzt299Оценок пока нет
- Erosion Due To FlowДокумент3 страницыErosion Due To FlownguyenОценок пока нет
- Bicos Spray SprayingДокумент40 страницBicos Spray SprayingAntônio MiguelОценок пока нет
- Is 8198 2004-1 PDFДокумент20 страницIs 8198 2004-1 PDFBorislav VulićОценок пока нет
- Dean StarkДокумент15 страницDean Starkfaizuanismail100% (1)
- Fluid Mechanics EssayДокумент2 страницыFluid Mechanics EssayKismaury Shamelle Maldonado PegueroОценок пока нет
- The Performance of Centrifugal Pumps When Pumping Ultra-Viscous Paste SlurriesДокумент6 страницThe Performance of Centrifugal Pumps When Pumping Ultra-Viscous Paste SlurriesPatricioECarrascoPerezОценок пока нет
- LNG An IntroductionДокумент60 страницLNG An Introductionsyafiq86% (7)
- Silk Solution Temperature and Mechanical EffectsДокумент1 страницаSilk Solution Temperature and Mechanical EffectsMuhammad Fadhila Ragil YogaОценок пока нет
- Tutorial 6sol2 PDFДокумент4 страницыTutorial 6sol2 PDFSohayb GattousОценок пока нет
- Vacuum Evaporators Mateo Torres.Документ11 страницVacuum Evaporators Mateo Torres.Jakson Mateo Torres GuerraОценок пока нет
- Primary Science: A2 Solid Liquid Gas, Notes From Bernard J Nebel Building Foundations of Scientific UnderstandingДокумент3 страницыPrimary Science: A2 Solid Liquid Gas, Notes From Bernard J Nebel Building Foundations of Scientific UnderstandingS SultanОценок пока нет
- CH ThermodynamicsДокумент16 страницCH Thermodynamicsmukeshg108Оценок пока нет
- Aim of The Experiment: To Set Up The ApparatusДокумент5 страницAim of The Experiment: To Set Up The ApparatusRajeev DuttОценок пока нет
- Calculation of The Saturation Pressure of A Gas/VaporДокумент6 страницCalculation of The Saturation Pressure of A Gas/VaporMarielle PerejonОценок пока нет
- Charge Neutrality FinalДокумент11 страницCharge Neutrality FinalBern Michael VillenaОценок пока нет
- EHB en 7.5 Sizing According To ISO 4126 1Документ10 страницEHB en 7.5 Sizing According To ISO 4126 1XpizmonОценок пока нет
- 2021 - Naturepho - YH KimДокумент10 страниц2021 - Naturepho - YH Kim이규형Оценок пока нет
- Thesis Presentation HollyДокумент41 страницаThesis Presentation HollyОльга Владимировна МинаковаОценок пока нет
- Q D 4 2 P 1 D D: Ideal 2 4Документ2 страницыQ D 4 2 P 1 D D: Ideal 2 4MuhammadAzrulОценок пока нет
- Introduction To Offshore Petroleum Production SystemДокумент55 страницIntroduction To Offshore Petroleum Production SystemJoaoCOS100% (1)
- Complete CV CalculationДокумент42 страницыComplete CV Calculationgalant_pp100% (1)
- Lecture13 Shell Momentum Balance 5Документ17 страницLecture13 Shell Momentum Balance 5shubhamОценок пока нет
- Selection and Design of CondensersДокумент27 страницSelection and Design of Condensersjdgh1986Оценок пока нет
- CompressorsДокумент43 страницыCompressorsyashvantОценок пока нет