Академический Документы
Профессиональный Документы
Культура Документы
4.1 Exam Questions Ms
Загружено:
Rohini SelvarajahАвторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
4.1 Exam Questions Ms
Загружено:
Rohini SelvarajahАвторское право:
Доступные форматы
4.
1 EXAM QUESTIONS MS
1.
(a)
Increased surface area (1)
more collisions (1)
(b)
(i)
Experiment 2 = 96 10
(1)
Experiment 3 = 0010 (1)
Experiment 4 = 81 10
(1)
Experiment 5 = 0035 (1)
(ii)
k=
1.2 10 4
6 1
(1) = 15 (1) mol dm s (1)
( 0 020)( 0 020) 2
7
[9]
2.
(a)
order wrt A = 1;
order wrt NaOH = 1;
1
3
Initial rate in Exp 4 = 2.4 10 ;
(b)
(i)
r(ate) = k[A]
OR
0
r(ate) = k[A][NaOH] ;
(penalise missing [ ] but mark on)
(penalise missing [ ] once per paper)
(if wrong order, allow only units mark conseq
on their rate eqs)
(penalise ka or kw etc)
(ii)
k=
9.0 10 3
;
0.02
= 0.45;
s ;
(iii)
(large) excess of OH or [OH ] is large/high;
[OH ] is (effectively) constant
OR
[A] is the limiting factor
(Q of L mark)
1
[9]
3.
(a)
Power (or index or shown as x in [ ] ) of concentration term
(in rate equation) (1)
(b)
2 (1)
(c)
(i)
1
Order with respect to A: 2 (1)
Order with respect to B: 0 (1)
2
(ii)
Rate equation: (rate =) k [A] (1)
Allow conseq on c(i)
1
3 1
Units for rate constant: mol dm s (1)
conseq on rate equation
4
[6]
4.
(a)
(b)
Order with respect to A
Order with respect to B
Order with respect to C
Value of k
Units of k
Initial rate
(c)
1 (1)
1 (1)
2 (1)
8.0 10 5
= 0.1
(0.1)(0.2)(0.2) 2
(1)
(1)
3
9 1
mol dm s (1)
5
3 1
1.0 10 (mol dm s )
(1)
K=
increases (1)
1
[8]
5.
(a)
Substances added to an excess of zinc
3
and 100 cm of 0.2 M hydrochloric acid
Volume of
3
hydrogen/cm
Effect on initial rate of
reaction
100cm water
240 (1)
decreased (1)
l0g zinc
240 (1)
no change (1)
360 (1)
no change (1)
50 cm 0.2 M hydrochloric acid
6
(b)
Order with respect to A
1 (1)
Order with respect to B
1 (1)
Initial rate
2.8 10 (mol dm s ) (1)
3 1
either via k = 1.56 10 (1)
or via table eg expts 2 4: rate 12 34 = 83 (1)
(c)
(i)
Calculation
k=
7.5 10 3
(1) = 0.48 (1)
(0.25) 2 (0.50) 2
mol dm 3 s 1
Units
(mol dm 3 ) 2 (mol dm 3 ) 2
1
= mol dm s
(1)
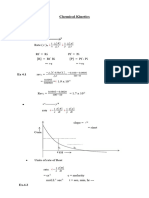
R a te
c o n s ta n t,
k
(ii)
4
(1 )
T e m p e r a tu r e
[14]
6.
(a)
(b)
exp2
4.0 10
exp3
0.45 10
exp4
9.0 10
1
1
1
1.8 10 5
(3.0 10 3 ) 2 (1.0 10 3
2000
6 1
mol dm s
1
[6]
7.
(a)
(b)
(i)
2 (1)
(ii)
0 (1)
(i)
Value of k: k =
rate
[ NO] 2 [ O 2 ]
2
6.5 10 4
(5.012 10 ) ( 2.0 10 )
2 2
= 13
6 1
Units of k: mol dm s (1)
(ii)
2 2
rate = 13 (6.5 10 ) (3.4 10 )
3
3 1
= 1.9 10
(mol dm s ) (1)
If k wrong, the mark in (ii) may be gained
4
conseq for their k 1.437 10
[6]
8.
(a)
(i)
Experiment 2: 0.4(0) 10 (1)
Experiment 3: 0.15 (1)
Experiment 4: 0.28 (1)
(ii)
k=
4.8 10 3
2
6 1
= 0.4(0) mol dm s
(0.20) 2 (0.30)
(1)
(b)
(1)
(1)
(change in) temperature (1)
6
1
[7]
9.
(a)
(i)
(Experiment 1 2) [A] doubled, ([B] constant,)
rate doubled (1)
stated or shown numerically
(ii)
(b)
(i)
2 (1)
2
or shown as ... [B]
k=
9.30 10 5
4
= 1.1(0) 10
(0.75) 2 (1.50)
(1)
(1)
2
6 1
units of k: mol dm s (1)
(ii)
rate = (1.10 10 ) (0.20) (0.10)
7
3 1
= 4.4(1) 10 (mol dm s )
(1) for the answer
Ignore units
Conseq on (i)
Upside down expression for k scores zero in (i) for 9073
2
but rate = 9073 (0.2) (0.1) = 36(.3)
conseq scores (1) in (ii)
4
[6]
10.
(a)
(i)
Expt
Initial
3
[A]/mol dm
Initial
3
[B]/mol dm
0.30
0.30
1.5 10
0.60 (1) (0.58 to 0.63)
0.60
6.0 10
0.45
1.20 (1) (1.17 to 1.25)
9.0 10
0.90
0.60
9.0 10 (1)
2
(8.6 to 9.2 10 )
K=
(ii)
rate
1.5 10 2
=
(1) = 0.166 (1) (or 0.17 or 0.16 )
[A][B] 0.3 0.3
(1)
(1)
1
3 1
units: mol dm s
(b)
Initial
3 1
rate/mol dm s
(1)
surface area more (than doubled) (1)
many more collisions (1)
2
[8]
11.
(a)
2 (1)
0
(1)
2
(b)
rate = k[J] (1)
4 10 4
k=
(1) = 20 (1)
(2 10 2 ) 2 (5 10 2 )
6 1
mol dm s
(c)
rate = k [ ]
units:
[ ] =
mol dm 3 s 1
s
greater/increase (1)
rate
k
= mol dm
n = 1 (2)
3
[9]
Вам также может понравиться
- Total Moles: 4.2 Test MsДокумент3 страницыTotal Moles: 4.2 Test MsridithaОценок пока нет
- 1.2 Test Mark SchemeДокумент5 страниц1.2 Test Mark Scheme:)Оценок пока нет
- Kinetics Independent HW MSДокумент3 страницыKinetics Independent HW MSIqbal WahyuОценок пока нет
- AS Chemistry Answer Sheet 02 AnsДокумент10 страницAS Chemistry Answer Sheet 02 Ansthegreatwardini0% (1)
- Answers: TEST - 1 (Paper-I)Документ10 страницAnswers: TEST - 1 (Paper-I)Vishal DaniОценок пока нет
- Physical Chemistry: Answer KeyДокумент15 страницPhysical Chemistry: Answer Keyvishal110085Оценок пока нет
- 1.2 Test Mark Scheme 1.: M MassДокумент5 страниц1.2 Test Mark Scheme 1.: M MassSuhail Alam KhanОценок пока нет
- 1.2 Assessed Homework MsДокумент4 страницы1.2 Assessed Homework MsSuhail Alam KhanОценок пока нет
- 1.2 Assessed Homework MsДокумент4 страницы1.2 Assessed Homework MsqwdqwedewdОценок пока нет
- SolutionsДокумент6 страницSolutionsVisal Ayse ArslanОценок пока нет
- Molar Solutions: Questionsheet 1Документ10 страницMolar Solutions: Questionsheet 1thegreatwardiniОценок пока нет
- MAA SL 1.1-1.2 NUMBERS - METHODS OF PROOF - SolutionsДокумент3 страницыMAA SL 1.1-1.2 NUMBERS - METHODS OF PROOF - SolutionsShivSantosh JhaОценок пока нет
- Pyramidal: Not Number of Electrons' or Number of Protons in An Element'Документ67 страницPyramidal: Not Number of Electrons' or Number of Protons in An Element'Darly SivanathanОценок пока нет
- Paper-1: Hints & SolutionsДокумент8 страницPaper-1: Hints & SolutionsAnudeex ShettyОценок пока нет
- 1.2 Test Mark SchemeДокумент5 страниц1.2 Test Mark SchemeEAОценок пока нет
- 1.2 Test Mark SchemeДокумент5 страниц1.2 Test Mark SchemeEAОценок пока нет
- Mark Scheme 29 Oct 2021Документ5 страницMark Scheme 29 Oct 2021Physics ProjectОценок пока нет
- AtkinsДокумент55 страницAtkinsJetco LawОценок пока нет
- Companion-Soln bk5Документ6 страницCompanion-Soln bk5samthegofОценок пока нет
- Unit 4 Rate of Reaction AnswersДокумент38 страницUnit 4 Rate of Reaction Answersareyouthere92Оценок пока нет
- AIATS For Medical - 2013 Test-1 SolutionДокумент9 страницAIATS For Medical - 2013 Test-1 SolutiondollulalОценок пока нет
- Mole Exam Mark SchemeДокумент17 страницMole Exam Mark Schemepaulcampbell37Оценок пока нет
- 46 International Chemistry Olympiad 2014 UK Round One Mark SchemeДокумент10 страниц46 International Chemistry Olympiad 2014 UK Round One Mark SchemedennysrochaОценок пока нет
- Answer Key: 13 VXY (Date: 18-12-2011) Review Test-5 Paper-2Документ15 страницAnswer Key: 13 VXY (Date: 18-12-2011) Review Test-5 Paper-2vishal110085Оценок пока нет
- Moles Practice Question MSДокумент3 страницыMoles Practice Question MSLayal AlyОценок пока нет
- Chemical Kinetics Exercise SolutionsДокумент98 страницChemical Kinetics Exercise SolutionsKivilia EduventuresОценок пока нет
- NQE 2008 Chemistry SolutionsДокумент6 страницNQE 2008 Chemistry SolutionsxargahОценок пока нет
- Komputasi Numerik Ujian Akhir Semester: Muhammad Zaki Zahirsyah 1406574522Документ22 страницыKomputasi Numerik Ujian Akhir Semester: Muhammad Zaki Zahirsyah 1406574522zakizahirsyahОценок пока нет
- JEE-MAIN 2013: Leader & Enthusiast CourseДокумент4 страницыJEE-MAIN 2013: Leader & Enthusiast Coursesanskarid94Оценок пока нет
- Answers: TEST - 3 (Paper-I)Документ13 страницAnswers: TEST - 3 (Paper-I)pachuОценок пока нет
- Derive The Integrated Rate Equation Half-LifeДокумент7 страницDerive The Integrated Rate Equation Half-Lifeumut2000Оценок пока нет
- RT Solutions-25!09!2011 XII ABCD Paper II Code AДокумент17 страницRT Solutions-25!09!2011 XII ABCD Paper II Code Avishal110085Оценок пока нет
- ch13 OddДокумент26 страницch13 OddCarolina GorzaОценок пока нет
- Nyjc - 2007 Jc1 h2 Promo p3 - AnswerДокумент4 страницыNyjc - 2007 Jc1 h2 Promo p3 - AnswerSudibyo GunawanОценок пока нет
- Mark Scheme (Results) January 2007: GCE Mathematics Mechanics M1 (6677)Документ5 страницMark Scheme (Results) January 2007: GCE Mathematics Mechanics M1 (6677)Sharmeen HelalОценок пока нет
- Multiplicacion Entre MatricesДокумент2 страницыMultiplicacion Entre MatricesdeyОценок пока нет
- RT Solutions-25!09!2011 XII ABCD Paper II Code BДокумент17 страницRT Solutions-25!09!2011 XII ABCD Paper II Code Bvishal110085Оценок пока нет
- Chapter 14Документ95 страницChapter 14Tanisha SenОценок пока нет
- © Science Exam Papers: Worked Solutions Edexcel C4 Paper KДокумент3 страницы© Science Exam Papers: Worked Solutions Edexcel C4 Paper KPa GesОценок пока нет
- Chemical Kinetics - 2Документ20 страницChemical Kinetics - 2bhawnam.1995Оценок пока нет
- Answer Key: Paper-1Документ16 страницAnswer Key: Paper-1vishal110085Оценок пока нет
- JEE Main Online Test 12-04-19 EveningДокумент26 страницJEE Main Online Test 12-04-19 EveningKRISHAN KUMARОценок пока нет
- 09 (2) PhysChem Exam-AnswersДокумент10 страниц09 (2) PhysChem Exam-Answerstiffanyyy00Оценок пока нет
- Question 1 (20 Marks) : Mathematics For Technology II Sample Test - Marking Scheme Page 1 of 5Документ5 страницQuestion 1 (20 Marks) : Mathematics For Technology II Sample Test - Marking Scheme Page 1 of 5Mohammed ABDO ALBAOMОценок пока нет
- 2 Chemistry - Chemical Kinetics - 2 60 SolutionsДокумент10 страниц2 Chemistry - Chemical Kinetics - 2 60 SolutionsPRUTHVIОценок пока нет
- Answer Key: Full Syllabus TestДокумент12 страницAnswer Key: Full Syllabus TestchakshuishanОценок пока нет
- Name - Mr. Perfect - Date - SP 17Документ4 страницыName - Mr. Perfect - Date - SP 17Sangram SahooОценок пока нет
- RT Solutions-25!09!2011 XII ABCD Paper I Code BДокумент17 страницRT Solutions-25!09!2011 XII ABCD Paper I Code Bvishal27042233Оценок пока нет
- SM ch01Документ43 страницыSM ch01Bibah GeeОценок пока нет
- Assignment (Chemical Kinetics and Chemical Equilibrium)Документ4 страницыAssignment (Chemical Kinetics and Chemical Equilibrium)Mapalo faith ChamaОценок пока нет
- Report HóaДокумент14 страницReport Hóatranbaohan0412Оценок пока нет
- Calculation Experiment 2a chm213Документ4 страницыCalculation Experiment 2a chm213Nurin FalihahОценок пока нет
- Answers to Selected Problems in Multivariable Calculus with Linear Algebra and SeriesОт EverandAnswers to Selected Problems in Multivariable Calculus with Linear Algebra and SeriesРейтинг: 1.5 из 5 звезд1.5/5 (2)
- Analytic Geometry: Graphic Solutions Using Matlab LanguageОт EverandAnalytic Geometry: Graphic Solutions Using Matlab LanguageОценок пока нет
- Analytical Modeling of Solute Transport in Groundwater: Using Models to Understand the Effect of Natural Processes on Contaminant Fate and TransportОт EverandAnalytical Modeling of Solute Transport in Groundwater: Using Models to Understand the Effect of Natural Processes on Contaminant Fate and TransportОценок пока нет
- Ten-Decimal Tables of the Logarithms of Complex Numbers and for the Transformation from Cartesian to Polar Coordinates: Volume 33 in Mathematical Tables SeriesОт EverandTen-Decimal Tables of the Logarithms of Complex Numbers and for the Transformation from Cartesian to Polar Coordinates: Volume 33 in Mathematical Tables SeriesОценок пока нет
- Trigonometric Ratios to Transformations (Trigonometry) Mathematics E-Book For Public ExamsОт EverandTrigonometric Ratios to Transformations (Trigonometry) Mathematics E-Book For Public ExamsРейтинг: 5 из 5 звезд5/5 (1)
- FY2 Post QuestionsДокумент5 страницFY2 Post QuestionsRohini Selvarajah67% (3)
- Heamophilia ResearchДокумент21 страницаHeamophilia ResearchRohini SelvarajahОценок пока нет
- WWW - Medicalppt.: For More Free Medical Powerpoint Presentations Visit WebsiteДокумент27 страницWWW - Medicalppt.: For More Free Medical Powerpoint Presentations Visit Websitevanstar7Оценок пока нет
- Mental State ExaminationДокумент5 страницMental State ExaminationRohini SelvarajahОценок пока нет
- Basal Cell Carcinoma Squamous Cell Carcinoma Guide Nov 2008-Final With CorrigendumsДокумент183 страницыBasal Cell Carcinoma Squamous Cell Carcinoma Guide Nov 2008-Final With CorrigendumsBoofy27Оценок пока нет
- Advanced 12-Lead InterpretationДокумент30 страницAdvanced 12-Lead InterpretationRohini SelvarajahОценок пока нет
- Depersonalisation Is Associated With - A. PTSD - B. Schizophrenia - C. Agoraphobia - D. Ecstatic Religious Experience - E. All of The AboveДокумент41 страницаDepersonalisation Is Associated With - A. PTSD - B. Schizophrenia - C. Agoraphobia - D. Ecstatic Religious Experience - E. All of The AboveRohini SelvarajahОценок пока нет
- 15 Feb 2014.doc HinaДокумент12 страниц15 Feb 2014.doc HinaRohini SelvarajahОценок пока нет
- Sleep DisordersДокумент32 страницыSleep DisordersRohini Selvarajah100% (1)
- 15 March 2014Документ14 страниц15 March 2014Rohini Selvarajah100% (2)
- Drug KardexДокумент1 страницаDrug KardexRohini SelvarajahОценок пока нет
- Tehreem - Recalls MODIFIED BY Me & AARAVДокумент58 страницTehreem - Recalls MODIFIED BY Me & AARAVRohini SelvarajahОценок пока нет
- Basal Cell Carcinoma Squamous Cell Carcinoma Guide Nov 2008-Final With CorrigendumsДокумент183 страницыBasal Cell Carcinoma Squamous Cell Carcinoma Guide Nov 2008-Final With CorrigendumsBoofy27Оценок пока нет
- ScabiesДокумент14 страницScabiesRohini SelvarajahОценок пока нет
- Topical Issues in DermatologyДокумент8 страницTopical Issues in DermatologyRohini SelvarajahОценок пока нет
- Community Resource MobilizationДокумент17 страницCommunity Resource Mobilizationerikka june forosueloОценок пока нет
- Mindray PM 9000 User ID10240 PDFДокумент378 страницMindray PM 9000 User ID10240 PDFJuan FernandoОценок пока нет
- SahanaДокумент1 страницаSahanamurthyarun1993Оценок пока нет
- What Is Product Management?Документ37 страницWhat Is Product Management?Jeffrey De VeraОценок пока нет
- SAED90DR Rev1 2 21.01.2011Документ24 страницыSAED90DR Rev1 2 21.01.2011Cherry AbhiОценок пока нет
- Jurnal 1 Ieevee LPF PDFДокумент4 страницыJurnal 1 Ieevee LPF PDFNanda SalsabilaОценок пока нет
- MQXUSBDEVAPIДокумент32 страницыMQXUSBDEVAPIwonderxОценок пока нет
- BA 4722 Marketing Strategy SyllabusДокумент6 страницBA 4722 Marketing Strategy SyllabusSri GunawanОценок пока нет
- Revised Corporation Code - Non Stock Close and Special CorporationsДокумент19 страницRevised Corporation Code - Non Stock Close and Special CorporationsVenziel PedrosaОценок пока нет
- P1 Chp12 DifferentiationДокумент56 страницP1 Chp12 DifferentiationbobОценок пока нет
- Atlascopco XAHS 175 DD ASL Parts ListДокумент141 страницаAtlascopco XAHS 175 DD ASL Parts ListMoataz SamiОценок пока нет
- 2 - Sample Kids Can Read and Write 2 and 3 Letter Words - Step 2 Final Downloadable Version For Website PDFДокумент18 страниц2 - Sample Kids Can Read and Write 2 and 3 Letter Words - Step 2 Final Downloadable Version For Website PDFsantoshiОценок пока нет
- Compact 1.8" Height Standardized Installation 9 Months To Flight Powerful and LightweightДокумент2 страницыCompact 1.8" Height Standardized Installation 9 Months To Flight Powerful and LightweightStanley Ochieng' OumaОценок пока нет
- 2.1 DRH Literary Translation-An IntroductionДокумент21 страница2.1 DRH Literary Translation-An IntroductionHassane DarirОценок пока нет
- Beer Pilkhani DistilleryДокумент44 страницыBeer Pilkhani DistillerySunil Vicky VohraОценок пока нет
- Thesis On Retail Management of The Brand 'Sleepwell'Документ62 страницыThesis On Retail Management of The Brand 'Sleepwell'Sajid Lodha100% (1)
- Astm D 1196 PDFДокумент3 страницыAstm D 1196 PDFSetyawan Chill Gates0% (1)
- JCIPДокумент5 страницJCIPdinesh.nayak.bbsrОценок пока нет
- Uts Cmo Module 5Документ31 страницаUts Cmo Module 5Ceelinah EsparazОценок пока нет
- Biscotti: Notes: The Sugar I Use in France, Is CalledДокумент2 страницыBiscotti: Notes: The Sugar I Use in France, Is CalledMonica CreangaОценок пока нет
- Using Visual Rating To Diagnose DementiaДокумент10 страницUsing Visual Rating To Diagnose DementiaImágenes Rosendo GarcíaОценок пока нет
- Trading Journal TDA Branded.v3.5 - W - Total - Transaction - Cost - BlankДокумент49 страницTrading Journal TDA Branded.v3.5 - W - Total - Transaction - Cost - BlankChristyann LojaОценок пока нет
- Strategic Capital Management: Group - 4 Jahnvi Jethanandini Shreyasi Halder Siddhartha Bayye Sweta SarojДокумент5 страницStrategic Capital Management: Group - 4 Jahnvi Jethanandini Shreyasi Halder Siddhartha Bayye Sweta SarojSwetaSarojОценок пока нет
- Navi-Planner User ManualДокумент331 страницаNavi-Planner User ManualRichard KershawОценок пока нет
- Channel & Lomolino 2000 Ranges and ExtinctionДокумент3 страницыChannel & Lomolino 2000 Ranges and ExtinctionKellyta RodriguezОценок пока нет
- Controlador DanfossДокумент2 страницыControlador Danfossfrank.marcondes2416Оценок пока нет
- Remedy MidTier Guide 7-5Документ170 страницRemedy MidTier Guide 7-5martin_wiedmeyerОценок пока нет
- Windows Intrusion Detection ChecklistДокумент10 страницWindows Intrusion Detection ChecklistJosé Tomás García CáceresОценок пока нет
- Rocker ScientificДокумент10 страницRocker ScientificRody JHОценок пока нет
- Application of Contemporary Fibers in Apparel - LyocellДокумент5 страницApplication of Contemporary Fibers in Apparel - LyocellVasant Kothari100% (1)