Академический Документы
Профессиональный Документы
Культура Документы
Drug Therapy E T O: Editor
Загружено:
Chris WariakaОригинальное название
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
Drug Therapy E T O: Editor
Загружено:
Chris WariakaАвторское право:
Доступные форматы
Vol.
332
No. 2
DRUG THERAPY
99
DRUG THERAPY ALASTAIR J.J. WOOD, M.D., Editor
men with symptomatic benign prostatic hyperplasia are reviewed. EVALUATING TREATMENT OPTIONS The efcacy of any medical, minimally invasive, or surgical treatment for symptomatic benign prostatic hyperplasia is determined primarily on the basis of two factors: decrease in the patients symptoms and improvement in the rate of urinary ow. Other variables, less commonly used, include the residual volume of urine after voiding and the results of pressureow urodynamic studies. To assess the symptoms caused by an enlarging prostate gland and the improvement resulting from treatment, several different systems have been developed, including the MadsenIversen point system, the Boyarsky guidelines, and the Maine Medical Assessment Program score.33-35 None of these, however, have been formally evaluated with regard to the reliability and reproducibility of results, and all rely on clinical judgment and interviews with patients. The recently formulated and validated American Urological Association Symptom Index is becoming the standard test with which to assess symptoms of prostatism.36,37 It contains seven questions and yields a score corresponding to ratings from mild to severe; the patient administers the test to himself to eliminate any bias from interview technique (Table 2). Unlike prostatic size, which has no correlation with the degree of prostatism, the score obtained from the symptom index is a reliable indicator of symptoms. The other factor that provides objective information about a patients ability to urinate is the urinary-ow rate (Table 3).39,40 This is determined by electronically recording the velocity of urine expelled from the bladder during micturition; it is the single best test for assessing obstruction of the bladder outlet.41 The peak ow rate is a more specic indicator of benign prostatic hyperplasia than the mean rate. The rate decreases in all men with advancing age and decreasing urine volume.38,42 For a man in the seventh or eighth decade of life who voids 150 ml or more, a peak urinary-ow rate of 15 ml per second is normal, whereas the same rate would be considered abnormal in younger men or men voiding with very high intravesical pressure. When this information is considered together with the symptom score, the physician can determine whether the patients prostatism is mild, moderate, or severe and to what extent it improves during or after any treatment. For men in whom the symptom score and peak urinary-ow rate are not in agreement, additional urodynamic evaluation consisting of the measurement of residual urine volume after voiding and pressureow studies may be helpful. For each treatment discussed here, the efcacy is presented in terms of the change in symptom score, peak urinary-ow rate, and other urodynamic factors as appropriate. MEDICAL TREATMENTS
Androgen-Deprivation Therapy
BENIGN PROSTATIC HYPERPLASIA Medical and Minimally Invasive Treatment Options JOSEPH E. OESTERLING, M.D. ENIGN prostatic hyperplasia is a nonmalignant enlargement of the prostate that is due to excessive cellular growth of both the glandular and the stromal elements of the gland. The condition is very common in men over 40 years of age of all races and cultures.1-8 For the past 50 years, transurethral resection of the prostate has been the mainstay of treatment. Approximately 400,000 such resections are performed annually in the United States, making this operation the second most common after cataract extraction in men older than 65.9 The associated expense is considerable; the total cost is approximately $5 billion per year.10 Although transurethral resection of the prostate is an effective treatment for most men with symptomatic benign prostatic hyperplasia, it is by no means perfect. Approximately 20 to 25 percent of patients who undergo the operation do not have satisfactory long-term outcomes.11 The complications include retrograde ejaculation in 70 to 75 percent of men, impotence in 5 to 10 percent, postoperative urinary tract infection in 5 to 10 percent, and some degree of urinary incontinence in 2 to 4 percent. Approximately 5 to 10 percent receive a blood transfusion, with its risk of infection.12-14 Another concern is that the rate of reoperation is approximately 15 to 20 percent in men followed for 10 years or longer (2.2 percent per year).15 Also, in several preliminary retrospective studies, the life expectancy of men undergoing transurethral resection of the prostate was shorter than that of men undergoing open prostatectomy as treatment for benign prostatic hyperplasia16,17; additional studies will be necessary to verify these initial observations. Because of these problems, as well as the desire of many men to avoid surgery whenever possible, there has been much interest in alternative treatments (Table 1). Currently, the management of benign prostatic hyperplasia is in transition. Although surgical treatment will continue to be widely used, medical therapy will assume increasing importance. As a result, internists and primary care physicians will have more involvement in the care of men with this condition than previously. In this discussion, the most important medical and minimally invasive treatments for
From the Michigan Prostate Center, University of Michigan, 1500 E. Medical Center Dr., Ann Arbor, MI 48109, where reprint requests should be addressed to Dr. Oesterling.
Aging and androgens are required for the development of benign prostatic hyperplasia. Because the pros-
Downloaded from www.nejm.org by David Lyons on March 4, 2010 . The New England Journal of Medicine Copyright 1995 Massachusetts Medical Society. All rights reserved. Downloaded from nejm.org on March 26, 2013. For personal use only. No other uses without permission.
Copyright 1995 Massachusetts Medical Society. All rights reserved.
100
THE NEW ENGLAND JOURNAL OF MEDICINE
Jan. 12, 1995
tate gland is an androgen-sensitive organ, androgen deprivation decreases the size of the prostate and the resistance to outow through the prostatic urethra, and the ability of many patients to urinate improves.43,44 Producing a state of androgen deprivation means interrupting the hypothalamicpituitarygonadal axis (Fig. 1).45-48 Of the agents that diminish androgen secretion or action gonadotropin-releasing hormone (GnRH) analogues,49-53 antiandrogens,54-58 and 5a-reductase inhibitors59-65 the 5a-reductase inhibitors have received the most attention.
Drugs That Inhibit 5a-Reductase Activity
Table 2. Questionnaire Used by the American Urological Association to Determine Symptom Scores for Patients with Benign Prostatic Hyperplasia.
Question 1. Over the past month or so, how often have you had a sensation of not emptying your bladder completely after you nished urinating? 2. Over the past month or so, how often have you had to urinate again less than two hours after you nished urinating? 3. Over the past month or so, how often have you found you stopped and started again several times when you urinated? 4. Over the past month or so, how often have you found it difcult to postpone urination? 5. Over the past month or so, how often have you had a weak urinary stream? 6. Over the past month or so, how often have you had to push or strain to begin urination? 7. Over the last month, how many times did you most typically get up to urinate from the time you went to bed at night until the time you got up in the morning? Symptom score (sum of the answers) 07 mild prostatism 818 moderate prostatism 1935 severe prostatism
Type 2 5a-reductase catalyzes the conversion of testosterone to dihydrotestosterone in most androgensensitive tissues, and blocking the conversion causes androgen deciency in those tissues. Finasteride [N(2-methyl-2-propyl)3-oxo-4-aza-5a-androst-1-ene-17b carboxamide] is a potent inhibitor of 5a-reductase and can be given orally, once a day. It is the only such compound to be approved by the Food and Drug Administration (FDA) for the treatment of benign prostatic hyperplasia, although other 5a-reductase inhibitors are being developed. The initial studies of nasteride, previously summarized in the Journal by Rittmaster,64 indicated that prostate volume decreased by 18 percent, the symptom score decreased by 26 percent, and the peak urinaryow rate increased by approximately 23 percent when 5 mg of the drug was administered orally on a daily basis. A recent report described the long-term (three year) safety and efcacy of nasteride.65 Of 543 men with benign prostatic hyperplasia who were originally assigned to receive nasteride (5 mg orally daily) in the North American and international trials, 297 (55 percent) could be evaluated, 178 (33 percent) withdrew from the study for various reasons, and 68 (13 percent) could not be evaluated because of insufcient data. After three years, prostatic volume was reduced from base line by approximately 27 percent, the peak urinary-ow rate had improved by 2.3 ml per second, and
Table 1. Medical and Minimally Invasive Treatments for Benign Prostatic Hyperplasia.
Medical Androgen-deprivation therapy Gonadotropin-releasing hormone agonists18 Antiandrogens19 5a-Reductase inhibitors20 a-Adrenergic antagonists21 Minimally invasive Transurethral incision of the prostate22 Balloon dilation of the prostate23 Prostatic stents24 Microwave therapy Transrectal hyperthermia25 Transurethral hyperthermia26 Transurethral thermotherapy27 Laser prostatectomy Transurethral ultrasound-guided, laser-induced prostatectomy28 Visual laser ablation of the prostate29 Contact laser ablation of the prostate30 Transrectal high-intensity focused ultrasound therapy31 Transurethral needle ablation of the prostate32
Possible answers: 0 Not at all 1 Less than 1 time in 5 2 Less than half the time 3 About half the time 4 More than half the time 5 Almost always
0, 1, 2, 3, 4, or 5 times
the symptom score had improved by 3.6 points. Fortytwo percent of the men had decreases of 30 percent or more in prostatic volume, 40 percent had increases of 3 ml per second or more in the peak urinary-ow rate, and 48 percent had decreases of 50 percent or more in the symptom score. Thus, the improvement achieved after 12 months with 5 mg of nasteride as compared with placebo was maintained with extended treatment. Finasteride has few side effects. Approximately 5 percent of men treated with it in the two large trials subsequently had decreased libido, ejaculatory dysfunction, or impotence, as compared with 1.5 percent of the men receiving placebo (P0.05).20,63 Finasteride also caused a 50 percent decrease in serum concentrations of prostate-specic antigen, so the highest value that should be considered normal in men treated with nasteride is lower by half than that in other men.65-68 Although nasteride is a potent 5a-reductase inhibitor and causes a marked decrease in serum and tissue concentrations of dihydrotestosterone, it is only moderately effective in treating symptomatic benign prostatic hyperplasia. For many men, the clinical improvement is minimal. Perhaps the most attractive feature of nasteride is its excellent toxicity prole. As more men are treated with the drug, it should be possible to determine its effect on the subsequent development of prostate cancer and whether it alters the value of serum measurements of prostate-specic antigen as an indicator of prostate cancer.
a-AdrenergicAntagonist Drugs
The clinical manifestations of bladder-outlet obstruction in men with benign prostatic hyperplasia are caused by increased resistance to the ow of urine through the bladder neck and the prostatic urethra. Historically, this obstruction has been relieved by re-
Downloaded from www.nejm.org by David Lyons on March 4, 2010 . The New England Journal of Medicine Copyright 1995 Massachusetts Medical Society. All rights reserved. Downloaded from nejm.org on March 26, 2013. For personal use only. No other uses without permission.
Copyright 1995 Massachusetts Medical Society. All rights reserved.
Vol. 332
No. 2
Table 3. General Guidelines for the Interpretation of Peak Urinary-Flow Rates.*
RATE OF FLOW ml/sec DEGREE OF PROSTATISM
DRUG THERAPY
101
15 to 20 10 to 15 10
Mild Moderate Severe
*The correct interpretation can depend on the volume voided and on the patients age.38
moving the prostatic tissue that obstructs the prostatic urethra. Medical therapies with an endocrine basis have also been devised to decrease the size of the prostate. In an attempt to relieve infravesical obstruction, a-adrenergicantagonist drugs are given to block the adrenergic receptors in hyperplastic prostatic tissue, the prostatic capsule, and the bladder neck, so that the smooth-muscle tone of these structures is decreased. As a result, resistance to urinary ow through the bladder neck and the prostatic urethra decreases, and urinary ow increases. Benign prostatic hyperplasia has two components: a static component that is related to the enlargement of the prostate and a dynamic component that reects the tone or degree of contraction of smooth muscle within the prostate.69 This static as compared with dynamic model followed from early studies showing a contraction of prostatic tissue in response to the administration of norepinephrine. Caine and associates70 demonstrated the presence of a-adrenergic receptors in both the prostatic capsule and hyperplastic prostatic tissue; in the same tissues, the concentration of b-adrenergic receptors was low and cholinergic receptors were barely detectable. These tissues have two types of a-adrenergic receptors, denoted a1 and a2.71 Three subtypes of the a1 receptor a1a, a1b, and a1c have been identied and are present in prostatic tissue72-74; the contraction of prostatic smooth muscle appears to be mediated by the a1c subtype.75 On the basis of this physiologic makeup and the fact that tissue affected by benign prostatic hyperplasia is rich in smooth muscle, a1-adrenergicantagonist drugs should decrease bladder resistance to urinary outow and therefore be effective in treating the condition. Because of the relative sparsity of a-adrenergic receptors in the bladder proper, this effect should be achieved without interfering with bladder contraction. A variety of a-adrenergic antagonists with distinct properties have been investigated as possible treatments for benign prostatic hyperplasia (Table 4). The one most studied, particularly in North America, is terazosin. In a multicenter study of 285 men with symptomatic benign prostatic hyperplasia who were assigned to receive either placebo or 2, 5, or 10 mg of terazosin once daily, 237 (83 percent) completed the 4-week, single-blind, lead-in placebo period and the 12-week, double-blind treatment period.82 The men who received terazosin had a greater decrease in the symptom score than those who received placebo; the differences were signicant only among the men who
received 5 or 10 mg daily (P 0.04 and P0.001, respectively). With regard to the peak urinary-ow rate, all the men treated with terazosin had greater increases than the men receiving placebo, but the mean difference (1.5 ml per second) was signicant only among the men treated with 10 mg daily (P 0.009). For a given dosage, the maximal therapeutic effect, as measured by the symptom score or the urinary-ow rate, was reached four to six weeks after the start of therapy. The improvement in these measures did not, however, reach a plateau within the dose ranges evaluated in the study, suggesting that further benet may be achieved with higher doses of terazosin. In an open-label study of the long-term efcacy and safety of terazosin in 494 men with benign prostatic hyperplasia, 351, 206, and 64 men could be evaluated at 12, 24, and 30 months, respectively.83 Improvement in the symptom score and the peak urinary-ow rate after 12 weeks of therapy was maintained throughout the 30month follow-up period; treatment was considered to
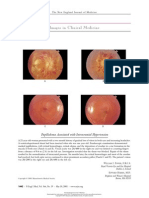
Hypothalamus GnRH Pituitary gland T GnRH LH agonists T Testicular Leydig cells
R T-R Antiandrogens 5a-Reductase DHT
Prostate gland
Nucleus
DHT-R
5a-Reductase inhibitors R
Figure 1. Regulation of Testicular Androgen Secretion and Mechanism of Androgen Action. Panel A shows the hypothalamicpituitarygonadal axis, with the site of action (X) of gonadotropin-releasing hormone (GnRH) agonists and the sites of the inhibitory () and stimulatory () actions of testosterone (T). LH denotes luteinizing hormone. (Adapted from Oesterling45 with the permission of the publisher.) Panel B shows the mechanism by which testosterone stimulates prostatic-cell activity, with the sites of action (X) of antiandrogens and 5a-reductase inhibitors. DHT denotes dihydrotestosterone, DHT-R dihydrotestosteronereceptor complex, R cytoplasmic receptor for androgens, and T-R testosteronereceptor complex. (Reprinted from Monda and Oesterling46 with the permission of the publisher.) Downloaded from www.nejm.org by David Lyons on March 4, 2010 . The New England Journal of Medicine Copyright 1995 Massachusetts Medical Society. All rights reserved. Downloaded from nejm.org on March 26, 2013. For personal use only. No other uses without permission.
Copyright 1995 Massachusetts Medical Society. All rights reserved.
102
THE NEW ENGLAND JOURNAL OF MEDICINE
Table 4. a-AdrenergicAntagonist Drugs for the Treatment of Benign Prostatic Hyperplasia.
Nonselective drugs Phenoxybenzamine76 Thymoxamine46 Selective drugs Short-acting Alfuzosin77 Indoramin78 Prazosin79 Long-acting Doxazosin80 Tamsulosin81 Terazosin82
Jan. 12, 1995
have failed in only 10 percent of the men. An important nding concerned the selective antihypertensive effect of terazosin. The mean decreases in systolic blood pressure in normotensive and hypertensive men during treatment were 4 and 18 mm Hg, respectively, an indication that terazosin can be an effective concomitant treatment for hypertension. The adverse effects of terazosin in men receiving 5 or 10 mg daily include asthenia in 6 to 10 percent, postural hypotension in 6 to 8 percent, and dizziness in 5 to 10 percent. Beginning therapy with a titration schedule (for example, 1 mg for 3 days, 2 mg for 11 days, 5 mg for 7 days, and 10 mg thereafter, given at bedtime) may minimize these adverse effects as well as any decrease in systolic or diastolic blood pressure. Terazosin has no effect on sexual function, and neither it nor any of the other selective a1-adrenergic antagonists alters the serum concentration of prostate-specic antigen. Doxazosin is another long-acting, selective a1-adrenergic antagonist that is being investigated in the treatment of benign prostatic hyperplasia. In a 16-week double-blind, placebo-controlled, dose-titration study involving 100 normotensive men, the mean peak urinary-ow rate increased by 2.9 ml per second in the doxazosin group and by 0.7 ml per second in the placebo group (P0.05).80 The mean symptom score improved by 5.7 points in the men treated with doxazosin and by 2.5 points in those given placebo (P0.05). Adverse effects consisting of dizziness, fatigue, and headache were more pronounced, though minimal, in the doxazosin-treated men; the mean decrease in systolic and diastolic blood pressures was approximately 5 mm Hg in these men. Tamsulosin is another long-acting, selective a1-adrenergic antagonist that has been used to treat men with benign prostatic hyperplasia. In a clinical trial evaluating its safety and efcacy,81 270 men with obstructive voiding symptoms were randomly assigned to receive placebo or 0.1, 0.2, or 0.4 mg of tamsulosin once daily for four weeks after a two-week lead-in placebo period; 12, 15, 40 and 36 percent, respectively, had improvement in the mean peak urinary-ow rate (P not significant); 10, 28, 38, and 39 percent had moderate-tomarked improvement in symptom scores; and 0, 1, 3, and 3 percent had adverse effects. Alfuzosin is a short-acting, selective a1-adrenergic antagonist that has been investigated extensively in
Europe.77 Among 518 men given either placebo or 7.5 or 10 mg of alfuzosin once daily for six months, the mean symptom score (calculated by the Boyarsky method) decreased from 9.5 to 5.5 (a 42 percent decrease) in the alfuzosin group and from 9.4 to 6.4 (a 32 percent decrease) in the placebo group (P0.001). The peak urinary-ow rate increased by 11 percent in the alfuzosin-treated men and by 12 percent in the men given placebo (P 0.21). Fifty percent of the men treated with alfuzosin rated their degree of improvement as good to very good, as compared with 40 percent of those given placebo. The frequency of adverse effects, including dizziness, headache, postural hypotension, asthenia, and impotence, was similar in the two groups. Although the various a1-adrenergicantagonist drugs have not been directly compared, their pharmacologic properties, clinical efcacy, and frequency of adverse effects are similar.46,76-85 Their therapeutic effect, as measured by improvement in the symptom score and the peak urinary-ow rate, is moderate. Their onset of action is rapid, and they may be effective in patients with mild-to-moderate hypertension. Because these drugs can cause postural hypotension, dizziness, headache, and lightheadedness, patients receiving them need to be monitored regularly.
Summary of Medical Therapies
In 1995, medical therapy for benign prostatic hyperplasia is a reality. Androgen-deprivation therapy (with GnRH agonists, antiandrogens, or 5a-reductase inhibitors) is effective because it reduces the static component of benign prostatic hyperplasia. Of these agents, the 5a-reductase inhibitors have the most promise because of their low toxicity. The others, though effective, cause impotence and loss of libido in most men. Selective a1-adrenergicantagonist drugs, which inhibit the dynamic component of benign prostatic hyperplasia, are also effective. As compared with androgen-deprivation therapy, treatment with these drugs offers several advantages. Their onset of action is more rapid, they have no effect on serum concentrations of prostate-specic antigen, and they can improve hypertension at the same time. As compared with a1-adrenergic antagonists, nasteride has minimal side effects, does not require titration at the start of therapy, and decreases prostatic size. Because it reduces androgenic stimulation to the prostate gland, nasteride may alter the natural history and progression of the disease process in addition to improving urinary symptoms. In the future, it may be possible to be selective in deciding which men should receive a 5a-reductase inhibitor and which an a1-adrenergic antagonist. The former may be more effective in men with a predominantly glandular component to their benign prostatic hyperplasia, whereas the latter may be more effective in those in whom stromal and smooth-muscle tissue is primarily affected. The only method currently available to determine the morphologic features of affected tissue is biopsy, but less invasive methods may be developed. Also, given the different mechanisms by
Downloaded from www.nejm.org by David Lyons on March 4, 2010 . The New England Journal of Medicine Copyright 1995 Massachusetts Medical Society. All rights reserved. Downloaded from nejm.org on March 26, 2013. For personal use only. No other uses without permission.
Copyright 1995 Massachusetts Medical Society. All rights reserved.
Vol. 332
No. 2
DRUG THERAPY
103
which these two types of medications act, the next principal advance may come from using them in combination. MINIMALLY INVASIVE TREATMENTS
Transurethral Incision of the Prostate
Transurethral incision of the prostate is an ideal procedure for any patient with bladder-outlet obstruction and an enlarged prostate gland weighing 30 g or less or in whom the primary obstruction is located at the bladder neck.86,87 Most commonly, the procedure is performed under regional or general anesthesia. Either a single incision is made at the six-oclock position (Fig. 2A) or two incisions are made at the ve- and sevenoclock positions (Fig. 2B).88 The incisions are begun distal to the interureteric ridge and extended across the bladder neck and prostatic urethra to the verumontanum. As the incisions are deepened, the bladder neck and prostatic urethra spring open and the bladder-outlet obstruction is relieved. Four prospective, randomized trials comparing transurethral incision of the prostate with transurethral resection have been conducted.89-92 The symptom score in the men who underwent transurethral incision decreased from 16.0 to 3.0 (an 81 percent decrease), whereas in the men who underwent transurethral resection the score decreased from 16.7 to 2.3 (an 86 percent decrease) (P0.001). The mean peak urinaryow rate increased from 8.5 to 15.0 ml per second (a 76 percent increase) in the men who underwent transurethral incision, and from 8.8 to 18.7 ml per second (a 112 percent increase) in the men who underwent transurethral resection (P0.001). The complication rates associated with incision were much lower than those associated with resection (impotence, 2 percent vs. 5 percent; retrograde ejaculation, 15 percent vs. 66 percent; incontinence, 1 percent vs. 6 percent; need for transfusion, 1 percent vs. 6 percent).89-92 In addi-
tion, surgery, hospitalization, and convalescence all required a shorter time. Transurethral incision of the prostate is ideal not only for men with a small prostate gland, but also for those in whom the preservation of potency and capacity for normal ejaculation are important considerations, as well as for debilitated men in whom the risks presented by surgery and anesthesia are substantial. The procedure is markedly underused to treat men with symptomatic benign prostatic hyperplasia.
Prostatic Stents
Several permanently indwelling endoprostheses are being tested for the treatment of benign prostatic hyperplasia.93-97 The two that have been evaluated most extensively in both Europe and the United States are the UroLume endoprosthesis (American Medical Systems, Minnetonka, Minn.) and the Intra-Prostatic Stent (Boston Scientic, Watertown, Mass.).98-100 The UroLume endoprosthesis is a biocompatible, inert prosthesis made from a nonmagnetic superalloy woven into a tubular mesh. It is exible and self-expanding, with no elastic recoil. When fully expanded, it has a wide (1.4 cm) internal diameter.101,102 The stent is made in six lengths: 1.5, 2.0, 2.5, 3.0, 3.5, and 4.0 cm. It can be placed in the prostatic urethra with a specially designed deployment tool under direct vision. The longest experience with the UroLume endoprosthesis has been in Europe, where the device has been used since 1989, primarily in patients who are considered to present poor surgical risks. In a multicenter study, it was used to treat 140 men with infravesical obstruction caused by enlarged prostate glands; 94 (67 percent) presented with symptoms of benign prostatic hyperplasia, and 46 (33 percent) had acute urinary retention.93 Before the placement of the stent, the mean peak urinary-ow rate in the nonretention group was 9.4 ml per second. After the insertion of the endopros-
Figure 2. Transurethral Incision of the Prostate. Panel A shows the incision at six oclock with the prostatic urethra and bladder neck springing open, and Panel B shows the incisions at ve oclock and seven oclock. The solid arrows indicate the trigone of the bladder, and the open arrows the bladder neck. (Reprinted from Monda and Oesterling46 with the permission of the publisher.) Downloaded from www.nejm.org by David Lyons on March 4, 2010 . The New England Journal of Medicine Copyright 1995 Massachusetts Medical Society. All rights reserved. Downloaded from nejm.org on March 26, 2013. For personal use only. No other uses without permission.
Copyright 1995 Massachusetts Medical Society. All rights reserved.
104
THE NEW ENGLAND JOURNAL OF MEDICINE
Jan. 12, 1995
thesis, the rate increased to 17.3 ml per second (an 84 percent improvement). The mean symptom score (on the MadsenIversen scale) declined from 15.7 to 7.6 (a 52 percent decrease). All the men who had acute urinary retention were able to void satisfactorily after placement of the stent; the mean peak urinary-ow rate was 13.5 ml per second and the mean symptom score was 3.0. A total of 14 stents were removed, 11 (79 percent) because of poor placement and 3 (21 percent) because of late untoward effects, such as persistent irritation on voiding (two men) and the formation of encrustations on the exposed ends at the bladder neck (one man). All 14 endoprostheses were removed intact transurethrally without sequelae. The North American UroLume Study Group used this endoprosthesis to treat 95 healthy men with obstructive benign prostatic hyperplasia.103 After 12 months, the mean symptom score had decreased from 15.0 before stent insertion to 6.3 (P0.001), and the mean (SD) peak urinary-ow rate had increased from 8.63.5 ml per second before insertion to 15.66.2 ml per second (P0.001). The mean residual urine volume after voiding decreased from 129 to 24 ml (P0.001). None of the men had any difculty postoperatively with infection, erosion, stent migration, incontinence, or impaired potency. However, 67 percent had some irritative symptoms (urgency, frequency, or dysuria) for at least one month. The stents were removed in eight men without injury to either the external urinary sphincter or the urethra.104 The preliminary results of these two studies suggest that this endoprosthesis may be a useful treatment option for men with obstructive benign prostatic hyperplasia. The Intra-Prostatic Stent is an inert device made of titanium, with excellent biocompatibility. When fully expanded, the stent has an internal diameter of 33 French (1.1 cm).105 The Intra-Prostatic Stent is available in lengths ranging from 1.9 to 5.8 cm, in 4-mm increments; thus, it can be matched precisely to the length of the patients prostate. Unlike the UroLume endoprosthesis, it is neither exible nor self-expanding, yet it provides enough outward radial force to maintain patency of the prostatic urethra. The Intra-Prostatic Stent has been studied primarily in older men and men in whom the risks of surgery were high. In a report from England of 50 such men who had urinary retention, the mean peak urinary-ow rate was 11.3 ml per second six months after the placement of the Intra-Prostatic Stent and 12.8 ml per second at one year among the 43 men available for evaluation.106 Most reported some urgency immediately after the placement of the device. In three men stent removal was necessary; this was accomplished without difculty with a specially designed retrieval tool. In another study of 30 men with urinary retention caused by benign prostatic hyperplasia who were treated with the Intra-Prostatic Stent, 25 (83 percent) could void satisfactorily after insertion of the stent; at one year the mean peak urinary-ow rate was 10.8 ml per second, and the mean residual urine volume after voiding was
56 ml.107 Ten men (33 percent) had subsequent urinary tract infections that resolved with appropriate antibiotic therapy. There were no other untoward effects. Experience with the Intra-Prostatic Stent in the United States has also been encouraging.94 Sixty-eight men, 38 of whom (56 percent) had acute urinary retention, were treated with the device and followed for up to 18 months in a multicenter study. The symptom score decreased from 16.8 before stent insertion to 3.2 at 18 months (P0.001), and the peak urinaryow rate increased from 3.9 to 14.4 ml per second (P0.001). Seventeen endoprostheses (25 percent) were removed because of technical failure (59 percent) or treatment failure (41 percent); all were removed transurethrally without sequelae. On the basis of these preliminary data, stenting the prostatic urethra to relieve bladder-outlet obstruction caused by an enlarged prostate gland seems reasonable. The prostatic endoprosthesis has several advantages. It can be placed quickly (in 15 minutes or less), with the patient under regional anesthesia or with only a prostatic block and intravenous sedation. The placement causes minimal intraoperative and postoperative hemorrhage. The patient does not need an indwelling urethral catheter after the operation and can be discharged from the hospital the same day or the next morning, with a minimal period of convalescence. The stent does not alter the serum concentration of prostate-specic antigen. There are also several potential disadvantages. The stent is a foreign body, and its long-term effects are unknown. Some men have irritative voiding symptoms after the procedure. Precise positioning of the stent in the prostatic urethra such that the proximal end does not protrude into the bladder and the distal end does not extend into the external urinary sphincter may be difcult. No catheter should be passed urethrally for six to eight weeks after placement to avoid dislodging the stent; postoperative urinary retention must be treated with a suprapubic catheter.
Microwave Therapy
Microwave energy can be delivered to the prostate gland transrectally108-112 and transurethrally.26,113,114 There are three forms of microwave treatment: hyperthermia, thermotherapy, and thermoablation. In hyperthermia, the prostatic tissue is heated to 42C to 44C in multiple sessions, with the microwave antenna placed in either the rectum or the urethra. In thermotherapy, the prostatic tissue is heated to between 45C and 60C in a single session, with a transurethral microwave antenna located inside a cooling catheter to protect the urethral lining.25 In thermoablation, the tissue is heated to a temperature between 60C and 75C.115 Combining heating with surface cooling is particularly important for producing high intraprostatic temperatures without injury to the urethra or rectum. In the absence of surface cooling, the heat generated by the microwave antenna is highest closest to the anten-
Downloaded from www.nejm.org by David Lyons on March 4, 2010 . The New England Journal of Medicine Copyright 1995 Massachusetts Medical Society. All rights reserved. Downloaded from nejm.org on March 26, 2013. For personal use only. No other uses without permission.
Copyright 1995 Massachusetts Medical Society. All rights reserved.
Vol. 332
No. 2
DRUG THERAPY
105
na, decreasing as a function of distance (Fig. 3A). As a result, the urethra or rectum is the tissue that is heated to the highest temperature. To protect these tissues, the microwave antenna is placed inside a catheter through which a coolant ows to keep the urethral or rectal epithelium at nearly normal temperatures. The urethra and rectum therefore remain uninjured while therapeutic temperatures are administered deep within the prostate (Fig. 3B). The following discussion focuses on the role of transurethral microwave thermotherapy, because it probably has the most potential to treat men with symptomatic benign prostatic hyperplasia. With transurethral thermotherapy using conductive cooling, prostatic temperatures ranging from 45C to 60C can be achieved while the urethral temperature remains below 44.5C and the rectal temperature below 42.5C.25 The treatment is generally administered in a single session without anesthesia. In the initial study of 37 men, both the symptom score and the residual urine volume after voiding decreased markedly, and the mean peak urinary-ow rate increased from 8.4 ml per second before treatment to 10.8 ml per second three months later (a 29 percent increase) (P0.03).25 Seven men (19 percent), however, had postoperative urinary retention and required indwelling catheters for one week. In a multicenter North American clinical trial of 150 men with benign prostatic hyperplasia treated with transurethral microwave thermotherapy, 94 (63 percent) were followed for 12 months.27 Their mean symptom score decreased from 13.7 to 5.4 (a 61 percent decrease) (P0.001), and the mean peak urinary-ow rate increased from 8.5 to 11.3 ml per second (a 33 percent increase) (P0.001). The residual urine volume after voiding, however, did not change signicantly after therapy. Forty-three men (46 percent) had urinary retention after the procedure for which urinary catheterization was required. Other adverse effects, including urethral bleeding, bladder spasm, and he-
matospermia, were rare. No men reported retrograde ejaculation or changes in sexual function. Seventy-four percent did not require anesthesia or analgesia during the procedure; 26 percent received some oral or intravenous analgesia. Three prospective clinical trials have been conducted in which transurethral microwave thermotherapy was compared with a sham procedure.116-118 The patients in both groups had decreases in symptom scores in all three studies, but the decrease in the thermotherapy group was signicantly greater (P0.05). With regard to the peak urinary-ow rate, the thermotherapy group had a mean increase of 3 ml per second (P0.05), whereas the group undergoing the sham procedure had no increase. Transurethral microwave thermotherapy has not been compared directly with transrectal or transurethral microwave hyperthermia in the treatment of men with symptomatic benign prostatic hyperplasia, but the former is probably superior. It is a single-session treatment that can be performed on an outpatient basis. Most men require no anesthesia or analgesia, and the complications are few. The improvement in symptom scores and peak urinary-ow rates is substantial and similar to the improvement with a1-adrenergicantagonist therapy. One disadvantage of transurethral microwave thermotherapy is postoperative urinary retention in approximately 35 percent of men, which may persist for up to 10 days.
Laser Prostatectomy
Another minimally invasive procedure that is receiving much attention is laser prostatectomy, several different methods of which are being evaluated. These laser-delivery systems include transurethral ultrasound-guided, laser-induced prostatectomy28,119; visual laser ablation of the prostate with 90-degree-ring freebeam bers30; and contact laser ablation of the prostate with contact bers. No meaningful data relating to
Temperature
Necrosis Tissue alteration Minimal change No reaction
Temperature
Distance from urethra
A B
Distance from urethra
Figure 3. Microwave Treatment of the Prostate. Panel A shows the temperature distribution around the microwave antenna (white central area) without surface conductive cooling, and Panel B shows the temperature with cooling. When the cooling catheter is used, the temperature where the antenna and the tissue touch is not increased, and the highest temperature is achieved deep within the prostatic tissue.
Downloaded from www.nejm.org by David Lyons on March 4, 2010 . The New England Journal of Medicine Copyright 1995 Massachusetts Medical Society. All rights reserved. Downloaded from nejm.org on March 26, 2013. For personal use only. No other uses without permission.
Copyright 1995 Massachusetts Medical Society. All rights reserved.
106
THE NEW ENGLAND JOURNAL OF MEDICINE
Jan. 12, 1995
the last of these are available. The results obtained with the ultrasound-guided procedure are similar to those with visual laser ablation, which is discussed in detail here. Visual laser ablation of the prostate uses a neodymium:yttriumaluminumgarnet laser as the energy source. The laser ber is positioned in the prostatic urethra through the working channel of a standard cystoscope under direct vision. In 17 men treated with this type of laser, the mean symptom score decreased from 15 before treatment to 4 in six weeks (a 73 percent decrease), and the mean peak urinary-ow rate increased from 5 to 9 ml per second (an 80 percent increase).29 Two men (12 percent) subsequently required transurethral resection of the prostate, and one (6 percent) underwent an incision of the bladder neck to relieve persistent bladder-outlet obstructive symptoms. In a randomized, double-blind clinical trial comparing visual laser ablation with transurethral resection in 43 men, 42 of whom completed three months of follow-up, the symptom score decreased by 10.3 points in the men who underwent ablation and by 11.5 points in the men who underwent resection (P 0.26).120 There was an increase in the mean peak urinary-ow rate from 9.4 to 12.2 ml per second (a 30 percent increase) in the ablation group, as compared with an increase from 7.5 to 17.4 ml per second (a 132 percent increase) in the resection group (P 0.04). Irritative voiding symptoms were both more severe and more frequent in the men treated with ablation. A study that had similar results with regard to efcacy six months after treatment was reported recently.121 These preliminary data suggest that visual laser ablation of the prostate is an effective and safe treatment for benign prostatic hyperplasia. Its advantages are a short operating time (20 minutes or less), ease of learning, the opportunity to observe the laser tip directly, and the absence of perioperative hemorrhage. The procedure can be performed on outpatients, but most men require catheter drainage afterward. Because the treated tissue is gradually sloughed, the maximal therapeutic effect may not be appreciated by the patient for six to eight weeks. Laser prostatectomy may be a viable alternative to transurethral resection of the prostate for men with symptomatic benign prostatic hyperplasia. However, the follow-up of the men treated so far has been short. Laser prostatectomy, like prostatic stents and microwave therapy, does not yet have the approval of the FDA for this use.
Summary of Minimally Invasive Treatments
chased, and no additional training is required. Indeed, transurethral incision has been proved with time to be applicable to 80 percent of the men now undergoing transurethral resection. Prostatic stents, microwave therapy, and laser prostatectomy are all being studied at present, both in this country and abroad. RIGHT TREATMENT FOR EACH PATIENT In the foregoing review, the results obtained with a wide variety of medical and minimally invasive treatments for benign prostatic hyperplasia have been presented as they were reported by the primary investigators. Unfortunately, there have been no studies comparing these various treatments with each other, with surgery (either transurethral resection or open prostatectomy), or with watchful waiting. In fact, the report by Wasson et al. in this issue of the Journal122 is the rst study in which watchful waiting has been compared with transurethral resection in men with moderate prostatism. As a result, there are no denitive guidelines indicating that a particular treatment or the lack thereof (that is, watchful waiting) is preferred in the care of a specic patient. Until more comparative trials are conducted, practicing physicians must rely on clinical judgment and intuition, as well as on the recommendations of the Agency for Health Care Policy and Research.123 Although intervention may be appropriate for many men with prostatism, doing nothing may be the better management. On the basis of ve studies of the natural history of prostatism among men with moderate prostatism who were followed for ve years, approximately 40 percent will improve, 45 percent will have no change in symptoms, and only 15 percent will have deterioration.4-8 The effect of placebo on symptoms of prostatism is also well recognized. Among 1260 men with benign prostatic hyperplasia in several studies who received placebo for 2 to 24 weeks, 42 percent improved, 46 percent had no change, and 12 percent had worsening of symptoms.124 Therefore, all clinical trials evaluating forms of medical therapy must have a placebo group, and all trials assessing minimally invasive procedures must have a no-treatment group. Watchful waiting must be considered a management option for men with prostatism. Transurethral resection of the prostate and open prostatectomy must also continue to be considered valid treatment options in 1995. Of all the treatments available, they result in the most improvement in symptoms and in the urinary-ow rate.11 On long-term follow-up, more than 75 percent of men undergoing these procedures are satised and have good ability to urinate.125 How then does a practicing clinician decide which treatment is best for each patient? There is no simple answer. Because the indications for treating men with symptomatic benign prostatic hyperplasia are relative rather than absolute, it is very important to consult the patient. Symptoms considered
ON THE
DECIDING
As we have seen regarding the medical therapy of benign prostatic hyperplasia, much progress has been made in recent years to develop a minimally invasive procedure that is effective, easy to perform, safe, and associated with a minimal hospitalization and a short convalescence. Transurethral incision of the prostate is such a treatment. No new equipment need be pur-
Downloaded from www.nejm.org by David Lyons on March 4, 2010 . The New England Journal of Medicine Copyright 1995 Massachusetts Medical Society. All rights reserved. Downloaded from nejm.org on March 26, 2013. For personal use only. No other uses without permission.
Copyright 1995 Massachusetts Medical Society. All rights reserved.
Vol. 332
No. 2
DRUG THERAPY
107
Table 5. Recommended Treatment Options for Patients with Benign Prostatic Hyperplasia, According to Severity of Prostatism.
TREATMENT SEVERITY
MILD (07) OF
PROSTATISM (SYMPTOM SCORE*)
SEVERE (1935) WITH URINARY RETENTION
MODERATE (818)
Watchful waiting 5a-Reductase inhibitors a1-Adrenergic antagonists Microwave therapy Laser prostatectomy Prostatic stents Transurethral incision of the prostate Transurethral resection of the prostate Open prostatectomy
Yes No No No No No No No No
Yes Yes Yes Yes Yes Yes Yes Yes No
No Yes Yes Yes Yes Yes Yes Yes Yes
No No No No No Yes Yes Yes Yes
*Symptom scores are as determined with the American Urological Association questionnaire shown in Table 2. Involving placement of permanent endoprostheses.
bothersome or even disabling by one man may be neither to another. Thus, a man who considers his symptoms annoying may want some treatment, whereas another will elect watchful waiting. Men seeking treatment must be informed of the potential benets and harms associated with each treatment. One man may prefer medical therapy to avoid an operation; another may choose a minimally invasive procedure to avoid having to take medication for the rest of his life. As for the range of therapies available medical, minimally invasive, and surgical most are suitable for any man with moderate prostatism. Watchful waiting, however, is the only one recommended for men with mild prostatism. Surgery and permanent prostatic stenting are suggested for men with acute urinary retention. Recommendations for the use of the various treatments are shown in Table 5. However, the nal decision about the best treatment for a particular man must take into account the patients preference after he has been appropriately informed. REFERENCES
1. Walsh PC. Human benign prostatic hyperplasia: etiological considerations. Prog Clin Biol Res 1984;145:1-25. 2. Oesterling JE. Benign prostatic hyperplasia: its natural history, epidemiologic characteristics, and surgical treatment. Arch Fam Med 1992;1:25766. 3. Barry MJ. Epidemiology and natural history of benign prostatic hyperplasia. Urol Clin North Am 1990;17:495-507. 4. Clarke R. The prostate and the endocrines: a control series. Br J Urol 1937; 9:254-71. 5. Craigen AA, Hickling JB, Saunders CR, Carpenter RG. Natural history of prostatic obstruction: a prospective survey. J R Coll Gen Pract 1969;18: 226-32. 6. Birkhoff JD, Wiederhorn AR, Hamilton ML, Zinsser HH. Natural history of benign prostatic hypertrophy and acute urinary retention. Urology 1976; 7:48-52. 7. Ball AJ, Feneley RCL, Abrams PH. The natural history of untreated prostatism. Br J Urol 1981;53:613-6. 8. Barnes RW, Marsh C. Progression of obstruction and symptoms. In: Hinman F Jr, ed. Benign prostatic hypertrophy. New York: Springer-Verlag, 1983:711-3. 9. National Center for Health Statistics, Graves EJ. Detailed diagnoses and procedures, National Hospital Discharge Survey, 1987. Vital and Health Statistics. Series 13. No. 100. Washington, D.C.: Government Printing Ofce, 1989:295. (DHHS publication no. (PHS) 89-1761.)
10. Graversen PH, Gasser TC, Wasson JH, Hinman F Jr, Bruskewitz RC. Controversies about indications for transurethral resection of the prostate. J Urol 1989;141:475-81. 11. Lepor H, Rigaud G. The efcacy of transurethral resection of the prostate in men with moderate symptoms of prostatism. J Urol 1990;143:533-7. 12. Mebust WK, Holtgrewe HL, Cockett ATK, Peters PC. Transurethral prostatectomy: immediate and postoperative complications: a cooperative study of 13 participating institutions evaluating 3,885 patients. J Urol 1989;141: 243-7. 13. Holtgrewe HL, Mebust WK, Dowd JB, Cockett ATK, Peters PC, Proctor C. Transurethral prostatectomy: practice aspects of the dominant operation in American urology. J Urol 1989;141:248-53. 14. Fowler FJ Jr, Wennberg JE, Timothy RP, Barry MJ, Mulley AG Jr, Hanley D. Symptom status and quality of life following prostatectomy. JAMA 1988;259:3018-22. 15. Wennberg JE, Roos N, Sola L, Schori A, Jaffe R. Use of claims data systems to evaluate health care outcomes: mortality and reoperation following prostatectomy. JAMA 1987;257:933-6. 16. Roos NP, Wennberg JE, Malenka DJ, et al. Mortality and reoperation after open and transurethral resection of the prostate for benign prostatic hyperplasia. N Engl J Med 1989;320:1120-4. 17. Malenka DJ, Roos N, Fisher ES, et al. Further study of the increased mortality following transurethral prostatectomy: a chart-based analysis. J Urol 1990;144:224-7. 18. Peters CA, Walsh PC. The effect of nafarelin acetate, a luteinizing-hormonereleasing hormone agonist, on benign prostatic hyperplasia. N Engl J Med 1987;317:599-604. [Erratum, N Engl J Med 1988;318:580.] 19. Caine M, Perlberg S, Gordon R. The treatment of benign prostatic hypertrophy with utamide (SCH: 13521): a placebo-controlled study. J Urol 1975;114:564-8. 20. Gormley GJ, Stoner E, Bruskewitz RC, et al. The effect of nasteride in men with benign prostatic hyperplasia. N Engl J Med 1992;327:118591. 21. Caine M, Pfau A, Perlberg S. The use of alpha-adrenergic blockers in benign prostatic obstruction. Br J Urol 1976;48:255-63. 22. Orandi A. Transurethral incision of the prostate. J Urol 1973;110:229-31. 23. Lepor H, Sypherd D, Machi G, Derus J. Randomized double-blind study comparing the effectiveness of balloon dilation of the prostate and cystoscopy for the treatment of symptomatic benign prostatic hyperplasia. J Urol 1992;147:639-42. 24. Oesterling JE. A permanent, epithelializing stent for the treatment of benign prostatic hyperplasia: preliminary results. J Androl 1991;12:4238. 25. Devonec M, Berger N, Perrin P. Transurethral microwave heating of the prostate or from hyperthermia to thermotherapy. J Endourol 1991;5: 129-35. 26. Baert L, Willemen P, Ameye F, Petrovich Z. Treatment response with transurethral microwave hyperthermia in different forms of benign prostatic hyperplasia: a preliminary report. Prostate 1991;18:315-20. 27. Blute ML, Tomera KM, Hellerstein DK, et al. Transurethral microwave thermotherapy for management of benign prostatic hyperplasia: results of the United States Prostatron Cooperative Study. J Urol 1993;150: 1591-6. 28. McCullough DL, Roth RA, Babayan RK, et al. Transurethral ultrasoundguided laser-induced prostatectomy: National Human Cooperative Study results. J Urol 1993;150:1607-11. 29. Costello AJ, Bowsher WG, Bolton DM, Braslis KG, Burt J. Laser ablation of the prostate in patients with benign prostatic hypertrophy. Br J Urol 1992;69:603-8. 30. Dixon CM, Lepor H. Laser ablation of the prostate. Semin Urol 1992;10: 273-7. 31. Madersbacher S, Kratzik C, Szabo N, Susani M, Vingers L, Marberger M. Tissue ablation in benign prostatic hyperplasia with high-intensity focused ultrasound. Eur Urol 1993;23:Suppl 1:39-43. 32. Schulman CC, Zlotta AR, Rasor JS, Hourriez L, Noel JC, Edwards SD. Transurethral needle ablation (TUNA): safety, feasibility, and tolerance of a new ofce procedure for treatment of benign prostatic hyperplasia. Eur Urol 1993;24:415-23. 33. Madsen PO, Iversen P. A point system for selecting operative candidates. In: Hinman F Jr, ed. Benign prostatic hypertrophy. New York: SpringerVerlag, 1983:763-5. 34. Boyarsky S, Jones G, Paulson DF, Prout GR Jr. A new look at bladder neck obstruction by the Food and Drug Administration regulators: guide lines for investigation of benign prostatic hypertrophy. Trans Am Assoc Genitourin Surg 1976;68:29-32. 35. Fowler FJ Jr, Wennberg JE, Timothy RP, Barry MJ, Mulley AG Jr, Hanley D. Symptom status and quality of life following prostatectomy. JAMA 1988;259:3018-22. 36. Barry MJ, Fowler FJ Jr, OLeary MP, et al. The American Urological Association Symptom Index for benign prostatic hyperplasia. J Urol 1992; 148:1549-57.
Downloaded from www.nejm.org by David Lyons on March 4, 2010 . The New England Journal of Medicine Copyright 1995 Massachusetts Medical Society. All rights reserved. Downloaded from nejm.org on March 26, 2013. For personal use only. No other uses without permission.
Copyright 1995 Massachusetts Medical Society. All rights reserved.
108
THE NEW ENGLAND JOURNAL OF MEDICINE
Jan. 12, 1995
37. Barry MJ, Fowler FJ Jr, OLeary MP, et al. Correlation of the American Urological Association Symptom Index with self-administered versions of the Madsen-Iversen, Boyarsky and Maine Medical Assessment Program Symptom Indexes. J Urol 1992;148:1558-63. 38. Drach GW, Layton TN, Binard WJ. Male peak urinary ow rate: relationships to volume voided and age. J Urol 1979;122:210-4. 39. Siroky MB, Olsson CA, Krane RJ. The ow rate nomogram. II. Clinical correlation. J Urol 1980;123:208-10. 40. Hald T. Urodynamics in benign prostatic hyperplasia: a survey. Prostate Suppl 1989;2:69-77. 41. McGuire EJ. The role of urodynamic investigation in the assessment of benign prostatic hypertrophy. J Urol 1992;148:1133-6. 42. Drach GW, Layton T, Bottaccini MR. A method of adjustment of male peak urinary ow rate for varying age and volume voided. J Urol 1982;128:9602. 43. White JW. The results of double castration in hypertrophy of the prostate. Ann Surg 1895;22:1-80. 44. Huggins C, Clark PJ. Quantitative studies of prostatic secretion. II. The effect of castration and of estrogen injection on the normal and on the hyperplastic prostate glands of dogs. J Exp Med 1940;72:747-61. 45. Oesterling JE. LHRH agonists: a nonsurgical treatment for benign prostatic hyperplasia. J Androl 1991;12:381-8. 46. Monda JM, Oesterling JE. Medical and minimally invasive treatments for benign prostatic hyperplasia. Curr Probl Urol 1992;2:100-42. 47. Huggins C, Stevens RA. The effect of castration on benign hypertrophy of the prostate in man. J Urol 1940;43:705-14. 48. Grifn JE, Wilson JD. Disorders of the testes and male reproductive tract. In: Wilson JD, Foster DW, eds. Textbook of endocrinology. 7th ed. Philadelphia: W.B. Saunders, 1985:259-311. 49. Conn PM, Crowley WF Jr. Gonadotropin-releasing hormone and its analogues. N Engl J Med 1991;324:93-103. 50. Blum JJ, Conn PM. Gonadotropin-releasing hormone stimulation of luteinizing hormone release: a ligand-receptor-effector model. Proc Natl Acad Sci U S A 1982;79:7307-11. 51. Schroeder FH, Westerhof M, Bosch RJLH, Kurth KH. Benign prostatic hyperplasia treated by castration or the LH-RH analogue buserelin: a report on 6 cases. Eur Urol 1986;12:318-21. 52. Matzkin H, Chen J, Lewysohn O, Braf Z. Treatment of benign prostatic hypertrophy by a long-acting gonadotropin-releasing hormone analogue: 1-year experience. J Urol 1991;145:309-12. 53. Weber JP, Oesterling JE, Peters CA, Partin AW, Chan DW, Walsh PC. The inuence of reversible androgen deprivation on serum prostate-specic antigen levels in men with benign prostatic hyperplasia. J Urol 1989;141:98792. 54. McConnell JD. Androgen ablation and blockade in the treatment of benign prostatic hyperplasia. Urol Clin North Am 1990;17:661-70. 55. Sufrin G, Coffey DS. Flutamide: mechanism of action of a new nonsteroidal antiandrogen. Invest Urol 1976;13:429-34. 56. Neri R. Pharmacology and pharmacokinetics of utamide. Urology 1989; 34:Suppl:19-21. 57. Stone NN. Flutamide in treatment of benign prostatic hypertrophy. Urology 1989;34:Suppl:64-8. 58. Stone NN, Clejan SJ. Response of prostate volume, prostate-specic antigen, and testosterone to utamide in men with benign prostatic hyperplasia. J Androl 1991;12:376-80. 59. Walsh PC, Madden JD, Harrod MJ, Goldstein JL, MacDonald PC, Wilson JD. Familial incomplete male pseudohermaphroditism, type 2: decreased dihydrotestosterone formation in pseudovaginal perineoscrotal hypospadias. N Engl J Med 1974;291:944-9. 60. Imperato-McGinley J, Guerrero L, Gautier T, Peterson RE. Steroid 5areductase deciency in man: an inherited form of male pseudohermaphroditism. Science 1974;186:1213-5. 61. Stoner E. The clinical development of a 5a-reductase inhibitor, nasteride. J Steroid Biochem Mol Biol 1990;37:375-8. 62. Lamb JC, English H, Levandoski PL, Rhodes GR, Johnson RK, Isaacs JT. Prostatic involution in rats induced by a novel 5a-reductase inhibitor, SK&F 105657: role for testosterone in the androgenic response. Endocrinology 1992;130:685-94. 63. The Finasteride Study Group. Finasteride (MK-906) in the treatment of benign prostatic hyperplasia. Prostate 1993;22:291-9. 64. Rittmaster RS. Finasteride. N Engl J Med 1994;330:120-5. 65. Stoner E. Three-year safety and efcacy data on the use of nasteride in the treatment of benign prostatic hyperplasia. Urology 1994;43:284-94. 66. Guess HA, Heyse JF, Gormley GJ. The effect of nasteride on prostatespecic antigen in men with benign prostatic hyperplasia. Prostate 1993; 22:31-7. 67. Guess HA, Heyse JF, Gormley GJ, Stoner E, Oesterling JE. Effect of nasteride on serum PSA concentration in men with benign prostatic hyperplasia: results from the North American Phase III Clinical Trial. Urol Clin North Am 1993;20:627-36.
68. Oesterling JE, Jacobsen SJ, Chute CG, et al. Serum prostate-specic antigen in a community-based population of healthy men: establishment of age-specic reference ranges. JAMA 1993;270:860-4. 69. Caine M. The present role of alpha-adrenergic blockers in the treatment of benign prostatic hypertrophy. J Urol 1986;136:1-4. 70. Caine M, Raz S, Zeigler M. Adrenergic and cholinergic receptors in the human prostate, prostate capsule and bladder neck. Br J Urol 1975;47:193202. 71. Lepor H, Gup DI, Baumann M, Shapiro E. Laboratory assessment of terazosin and alpha-1 blockade in prostatic hyperplasia. Urology 1988;32: Suppl:21-6. 72. Minneman KP, Han C, Abel PW. Comparison of alpha1-adrenergic receptor subtypes distinguished by chlorethylclonidine and WB 4101. Mol Pharmacol 1988;33:509-14. 73. Schwinn DA, Lomasney JW, Lorenz W, et al. Molecular cloning and expression of the cDNA for a novel a1-adrenergic receptor subtype. J Biol Chem 1990;265:8183-9. 74. Lepor H, Tang R, Meretyk S, Shapiro E. Alpha1 adrenoceptor subtypes in the human prostate. J Urol 1993;149:640-2. 75. Lepor H, Tang R, Shapiro E. The alpha-adrenoceptor subtype mediating the tension of human prostatic smooth muscle. Prostate 1993;22:301-7. 76. Caine M, Perlberg S, Meretyk S. A placebo-controlled double-blind study of the effect of phenoxybenzamine in benign prostatic obstruction. Br J Urol 1978;50:551-4. 77. Jardin A, Bensadoun H, Delauche-Cavallier MC, Attali P. Alfuzosin for treatment of benign prostatic hypertrophy. Lancet 1991;337:1457-61. 78. Lepor H. Medical therapy for benign prostatic hyperplasia. Urology 1993; 42:483-501. 79. Kirby RS, Coppinger SWC, Corcoran MO, Chapple CR, Flannigan M, Milroy EJG. Prazosin in the treatment of prostatic obstruction: a placebocontrolled study. Br J Urol 1987;60:136-42. 80. Gillenwater JY, Mobley DL. A sixteen week, double-blind, placebo-controlled, dose-titration study using doxazosin tablets for the treatment of benign prostatic hyperplasia (BPH) in normotensive males. J Urol 1993;149: Suppl:324A. abstract. 81. Kawabe K, Ueno A, Takimoto Y, Aso Y, Kato H. Use of an a1-blocker, YM617, in the treatment of benign prostatic hypertrophy. J Urol 1990;144: 908-12. 82. Lepor H, Auerbach S, Puras-Baez A, et al. A randomized, placebo-controlled multicenter study of the efcacy and safety of terazosin in the treatment of benign prostatic hyperplasia. J Urol 1992;148:1467-74. 83. Lepor H, Abbott Terazosin BPH Group. Long-term safety and efcacy of terazosin for the treatment of clinical BPH: an interim analysis. J Urol 1993;149:Suppl:324A. abstract. 84. Christensen MM, Bendix Holme J, Rasmussen PC, et al. Doxazosin treatment in patients with prostatic obstruction: a double-blind placebo-controlled study. Scand J Urol Nephrol 1993;27:39-44. 85. Janknegt RA, Chapple CR. Efcacy and safety of the alpha-1 blocker doxazosin in the treatment of benign prostatic hyperplasia: analysis of 5 studies. Eur Urol 1993;24:319-26. 86. Keitzer WA, Cervantes L, Demaculangan A, Cruz B. Transurethral incision of bladder neck for contracture. J Urol 1961;86:242-6. 87. Orandi A. Transurethral incision of the prostate. J Urol 1973;110:229-31. 88. Kletcher BA, Oesterling JE. Transurethral incision of the prostate: a viable alternative to transurethral resection. Semin Urol 1992;10:265-72. 89. Christensen MM, Aagaard J, Madsen PO. Transurethral resection versus transurethral incision of the prostate: a prospective randomized study. Urol Clin North Am 1990;17:621-30. 90. Dringer T, ster M, Larsen JF, Walter S, Krarup T. Transurethral prostatectomy or incision of the prostate in the treatment of prostatism caused by small benign prostates. Scand J Urol Nephrol Suppl 1987;104:7781. 91. Larsen EH, Dringer T, Gasser TC, Graversen PH, Bruskewitz RC. Transurethral incision versus transurethral resection of the prostate for the treatment of benign prostatic hypertrophy: a preliminary report. Scand J Urol Nephrol Suppl 1987;104:83-6. 92. Hellstrom P, Lukkarinen O, Kontturi M. Bladder neck incision or transurethral electroresection for the treatment of urinary obstruction caused by a small benign prostate? A randomized urodynamic study. Scand J Urol Nephrol 1986;20:187-92. 93. Milroy E, Chapple CR. The UroLume stent in the management of benign prostatic hyperplasia. J Urol 1993;150:1630-5. 94. Kaplan SA, Merrill DC, Mosely WG, et al. The titanium intraprostatic stent: the United States experience. J Urol 1993;150:1624-9. 95. Kemppainen E, Talja M, Riihela M, Pohjonen T, Trml P, Alfthan O. A bioresorbable urethral stent: an experimental study. Urol Res 1993;21: 235-8. 96. Kiyota H, Machida T, Ohishi Y, Yamazaki H, Nakada J, Madarame J. Intraurethral catheter made of niti-shape-memory alloys for benign prostatic hyperplasia. J Urol 1994;151:397A. abstract.
Downloaded from www.nejm.org by David Lyons on March 4, 2010 . The New England Journal of Medicine Copyright 1995 Massachusetts Medical Society. All rights reserved. Downloaded from nejm.org on March 26, 2013. For personal use only. No other uses without permission.
Copyright 1995 Massachusetts Medical Society. All rights reserved.
Vol. 332
No. 2
DRUG THERAPY
109
97. Mori K, Okamoto S, Akimoto M. Placement of the urethral stent made of shape memory alloy (SMA) in the management of benign prostatic hypertrophy for patients contra-indicating other less invasive procedures. J Urol 1994;151:398A. abstract. 98. Dotter CT. Transluminally-placed coilspring endarterial tube grafts: longterm patency in canine popliteal artery. Invest Radiol 1969;4:329-32. 99. Sigwart U, Puel J, Mirkovitch V, Joffre F, Kappenberger L. Intravascular stents to prevent occlusion and restenosis after transluminal angioplasty. N Engl J Med 1987;316:701-6. 100. Palmaz JC, Kopp DT, Hayashi H, et al. Normal and stenotic renal arteries: experimental balloon-expandable intraluminal stenting. Radiology 1987; 164:705-8. 101. Milroy EJG, Chapple CR, Cooper JE, et al. A new treatment for urethral strictures. Lancet 1988;1:1424-7. 102. Oesterling JE. Urologic applications of a permanent, epithelializing urethral endoprosthesis. Urology 1993;41:Suppl:10-8. 103. Oesterling JE, Epstein H, North American UroLume Study Group. The North American experience with the UroLume endoprosthesis as a treatment for symptomatic BPH: long-term results. J Urol 1993;149:Suppl: 216A. abstract. 104. Chapple CR, Milroy EGJ. Use of stents for treating obstruction of urinary outow. BMJ 1989;299:795. 105. Kaplan SA. Can a stent succeed in keeping the prostatic urethra open? Contemp Urol 1993;5:19-33. 106. Abrams P, Gillatt D, Chadwick D. Intraprostatic stent experience with the ASI stent to treat bladder outow obstruction. J Urol 1991;145:Suppl: 373A. abstract. 107. Kirby RS, Heard SR, Miller P, et al. Use of the ASI titanium stent in the management of bladder outow obstruction due to benign prostatic hyperplasia. J Urol 1992;148:1195-7. 108. Yerushalmi A, Servadio C, Leib Z, Fishelovitz Y, Rokowsky E, Stein JA. Local hyperthermia for treatment of carcinoma of the prostate: a preliminary report. Prostate 1982;3:623-30. 109. Servadio C, Leib Z. Hyperthermia in the treatment of prostate cancer. Prostate 1984;5:205-11. 110. Yerushalmi A, Fishelovitz Y, Singer D, et al. Localized deep microwave hyperthermia in the treatment of poor operative risk patients with benign prostatic hyperplasia. J Urol 1985;133:873-6. 111. Servadio C, Braf Z, Siegel Y, Leib Z, Saranga R, Lindner A. Local thermotherapy of the benign prostate: a 1-year follow-up. Eur Urol 1990;18:16973.
112. Lindner A, Siegel YI, Saranga R, Korzcak D, Matzkin H, Braf Z. Complications in hyperthermia treatment of benign prostatic hyperplasia. J Urol 1990;144:1390-1. 113. Harada T, Etori K, Kumazaki T, Nishizawa O, Noto H, Tsuchida S. Microwave surgical treatment of diseases of prostate. Urology 1985;26:572-6. 114. Sapozink MD, Boyd SD, Astrahan MA, Jozsef G, Petrovich Z. Transurethral hyperthermia for benign prostatic hyperplasia: preliminary clinical results. J Urol 1990;143:944-50. 115. Devonec M, Fendler J-P, Nasser M, Joubert P, Perrin P. The clinical response to transurethral microwave thermotherapy is dose-dependent: from thermo-coagulation to thermo-ablation. J Urol 1993;149:Suppl:249A. abstract. 116. Ogden CW, Reddy P, Johnson H, Ramsay JW, Carter SS. Sham versus transurethral microwave thermotherapy in patients with symptoms of benign prostatic bladder outow obstruction. Lancet 1993;341:14-7. 117. Bdesha AS, Bunce CJ, Kelleher JP, Snell ME, Vukusic J, Witherow RO. Transurethral microwave treatment for benign prostatic hypertrophy: a randomised controlled clinical trial. BMJ 1993;306:1293-6. 118. Debruyne FMJ, Bloem FAG, de la Rosette JJMCH, Laduc R. Transurethral thermotherapy (TUMT) in benign prostatic hyperplasia: placebo versus TUMT. J Urol 1993;149:Suppl:250A. abstract. 119. Fuselier HA, Roth RA, Babayan RK, et al. TULIP national human cooperative study results. J Urol 1993;149:Suppl:215A. abstract. 120. Dixon C, Machi G, Theune C, Lepor H. A prospective, double-blind, randomized study comparing laser ablation of the prostate and transurethral prostatectomy for the treatment of BPH. J Urol 1993;149:Suppl:215A. abstract. 121. Kabalin JN. Laser prostatectomy performed with a right angle ring neodymium: YAG laser ber at 40 watts power setting. J Urol 1993;150:95-9. 122. Wasson JH, Reda DJ, Bruskewitz RC, et al. A comparison of transurethral surgery with watchful waiting for moderate symptoms of benign prostatic hyperplasia. N Engl J Med 1995;332:75-9. 123. McConnell JD, Barry MJ, Bruskewitz RC, et al. Benign prostatic hyperplasia: diagnosis and treatment. Quick reference guide for clinicians. No. 8. Rockville, Md.: Department of Health and Human Services, 1994. (AHCPR publication no. 94-0583.) 124. Isaacs JT. Importance of the natural history of benign prostatic hyperplasia in the evaluation of pharmacologic intervention. Prostate Suppl 1990;3:1-7. 125. Bruskewitz RC, Larsen EH, Madsen PO, Dringer T. 3-Year followup of urinary symptoms after transurethral resection of the prostate. J Urol 1986; 136:613-5.
Downloaded from www.nejm.org by David Lyons on March 4, 2010 . The New England Journal of Medicine Copyright 1995 Massachusetts Medical Society. All rights reserved. Downloaded from nejm.org on March 26, 2013. For personal use only. No other uses without permission.
Copyright 1995 Massachusetts Medical Society. All rights reserved.
Вам также может понравиться
- How To Make Nasi UdukДокумент2 страницыHow To Make Nasi UdukChris WariakaОценок пока нет
- ArrocasДокумент9 страницArrocasChris WariakaОценок пока нет
- Iqbaal Dhiafakhri RamadanДокумент2 страницыIqbaal Dhiafakhri RamadanChris WariakaОценок пока нет
- Helen 1Документ30 страницHelen 1Chris WariakaОценок пока нет
- AA-4-Total Prostate Rulan DN PakasiДокумент12 страницAA-4-Total Prostate Rulan DN PakasiChris WariakaОценок пока нет
- 04ref Append PDFДокумент0 страниц04ref Append PDFChris WariakaОценок пока нет
- MR 30 FilsaДокумент18 страницMR 30 FilsaChris WariakaОценок пока нет
- Lion Air Eticket Itinerary / ReceiptДокумент0 страницLion Air Eticket Itinerary / ReceiptChris WariakaОценок пока нет
- Nej M 20010510344hfg1905Документ1 страницаNej M 20010510344hfg1905Chris WariakaОценок пока нет
- 1316 2 FullДокумент4 страницы1316 2 FullChris WariakaОценок пока нет
- 345 2010 Article 612Документ6 страниц345 2010 Article 612Chris WariakaОценок пока нет
- Dosis Obat2an PediatricsДокумент1 страницаDosis Obat2an PediatricsChris WariakaОценок пока нет
- 4 Poisoning Our ChildrenДокумент182 страницы4 Poisoning Our ChildrenChris WariakaОценок пока нет
- Dosing Forms MalariaДокумент3 страницыDosing Forms MalariaChris WariakaОценок пока нет
- Dosing Forms MalariaДокумент3 страницыDosing Forms MalariaChris WariakaОценок пока нет
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceОт EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceРейтинг: 4 из 5 звезд4/5 (894)
- The Yellow House: A Memoir (2019 National Book Award Winner)От EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Рейтинг: 4 из 5 звезд4/5 (98)
- The Little Book of Hygge: Danish Secrets to Happy LivingОт EverandThe Little Book of Hygge: Danish Secrets to Happy LivingРейтинг: 3.5 из 5 звезд3.5/5 (399)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeОт EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeРейтинг: 4 из 5 звезд4/5 (5794)
- Never Split the Difference: Negotiating As If Your Life Depended On ItОт EverandNever Split the Difference: Negotiating As If Your Life Depended On ItРейтинг: 4.5 из 5 звезд4.5/5 (838)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureОт EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureРейтинг: 4.5 из 5 звезд4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryОт EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryРейтинг: 3.5 из 5 звезд3.5/5 (231)
- The Emperor of All Maladies: A Biography of CancerОт EverandThe Emperor of All Maladies: A Biography of CancerРейтинг: 4.5 из 5 звезд4.5/5 (271)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreОт EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreРейтинг: 4 из 5 звезд4/5 (1090)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyОт EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyРейтинг: 3.5 из 5 звезд3.5/5 (2219)
- Team of Rivals: The Political Genius of Abraham LincolnОт EverandTeam of Rivals: The Political Genius of Abraham LincolnРейтинг: 4.5 из 5 звезд4.5/5 (234)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersОт EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersРейтинг: 4.5 из 5 звезд4.5/5 (344)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaОт EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaРейтинг: 4.5 из 5 звезд4.5/5 (265)
- The Unwinding: An Inner History of the New AmericaОт EverandThe Unwinding: An Inner History of the New AmericaРейтинг: 4 из 5 звезд4/5 (45)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)От EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Рейтинг: 4.5 из 5 звезд4.5/5 (119)
- A Guide To Testosterone: Boost Levels Through Diet And SupplementsДокумент5 страницA Guide To Testosterone: Boost Levels Through Diet And SupplementsNick Graham TrinhОценок пока нет
- Endocrine-Disrupting Chemicals: An Endocrine Society Scientific StatementДокумент50 страницEndocrine-Disrupting Chemicals: An Endocrine Society Scientific StatementRobin KristófiОценок пока нет
- Androgen Receptor Gene CAG Repeat Length andДокумент10 страницAndrogen Receptor Gene CAG Repeat Length anddouglas 87Оценок пока нет
- AAEs and PsychyatricsДокумент10 страницAAEs and PsychyatricsJorge FernándezОценок пока нет
- Discuss Formation and Development of Gender RolesДокумент3 страницыDiscuss Formation and Development of Gender Rolesmickykitson0% (1)
- International Journal Electro EjaculatorДокумент11 страницInternational Journal Electro EjaculatorAllya AzahraОценок пока нет
- Bony To BrawnyДокумент50 страницBony To Brawnyartus14100% (5)
- How to Naturally Increase Testosterone in 3 Simple StepsДокумент149 страницHow to Naturally Increase Testosterone in 3 Simple Stepsverve1977100% (6)
- Natural Penis Enlargement Volume 2Документ143 страницыNatural Penis Enlargement Volume 2MJ0% (1)
- Sex Continuum Adolescence Psychological TraitsДокумент11 страницSex Continuum Adolescence Psychological TraitsDa CuОценок пока нет
- Telephone ScatologiaДокумент5 страницTelephone ScatologiaEdaОценок пока нет
- Perceptions of Sexual HarassmentДокумент10 страницPerceptions of Sexual Harassmenttatapanminda100% (1)
- Hormones Receptors REPRO - COMPAÑERO - 2005Документ308 страницHormones Receptors REPRO - COMPAÑERO - 2005Juancho MontalicoОценок пока нет
- Aquaculture Fish Fisheries - 2022 - Aziz - The Efficacy of Using Pine Pinus Massoniana Pollen As An Alternative ToДокумент9 страницAquaculture Fish Fisheries - 2022 - Aziz - The Efficacy of Using Pine Pinus Massoniana Pollen As An Alternative ToAs DvgОценок пока нет
- PSY4122 - Original - Male Steroid Hormones and Women's AttractionДокумент11 страницPSY4122 - Original - Male Steroid Hormones and Women's AttractionJason WongОценок пока нет
- Ginseng and Male Reproductive FunctionДокумент6 страницGinseng and Male Reproductive FunctionHavard MorkhagenОценок пока нет
- The Chemistry of DesireДокумент9 страницThe Chemistry of DesireSyncOrSwimОценок пока нет
- Biological Theories of Gender DevelopmentДокумент2 страницыBiological Theories of Gender DevelopmentAshley Mae FajardoОценок пока нет
- 80 20 Fat Loss PDFДокумент127 страниц80 20 Fat Loss PDFSanelicasam100% (1)
- Energy Balance and Body Composition in Sports and ExerciseДокумент15 страницEnergy Balance and Body Composition in Sports and ExerciseHeny KurniasariОценок пока нет
- EroxelДокумент3 страницыEroxelPills Villa100% (1)
- Anabolic Peptide Beta EbookДокумент226 страницAnabolic Peptide Beta EbookMatei Cipri80% (5)
- Hirsutism and HomoeopathyДокумент9 страницHirsutism and HomoeopathyDr. Rajneesh Kumar Sharma MD HomОценок пока нет
- Anatomy-of-Adrenal Fatigue S PDFДокумент202 страницыAnatomy-of-Adrenal Fatigue S PDFVivy Faria67% (3)
- IB Psychology HL Biological LOAДокумент11 страницIB Psychology HL Biological LOAgfore3Оценок пока нет
- Drugs Used in Disorders of Endocrine System Ppt. Book (Lectures 1-6)Документ467 страницDrugs Used in Disorders of Endocrine System Ppt. Book (Lectures 1-6)Marc Imhotep Cray, M.D.Оценок пока нет
- Septiembre 2021 Telegram - @anabolictienda: Sustenon (Testosterona Blend) ApresentaçãoДокумент27 страницSeptiembre 2021 Telegram - @anabolictienda: Sustenon (Testosterona Blend) ApresentaçãoMiguel Fernandez SangradorОценок пока нет
- Case 12-2016: An 8-Year-Old Boy With An Enlarging Mass in The Right BreastДокумент10 страницCase 12-2016: An 8-Year-Old Boy With An Enlarging Mass in The Right BreastbayutrihatmajaОценок пока нет
- Testosterone Therapy Fact SheetДокумент2 страницыTestosterone Therapy Fact SheetJonathan CastroОценок пока нет
- Male Reproductive Physiology - UpToDate - 2020Документ18 страницMale Reproductive Physiology - UpToDate - 2020Karaca AzizОценок пока нет