Академический Документы
Профессиональный Документы
Культура Документы
Molecule Shapes
Загружено:
Sandunil JayasingheАвторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
Molecule Shapes
Загружено:
Sandunil JayasingheАвторское право:
Доступные форматы
Bonding in Molecules
Lecture 3
Molecular shapes
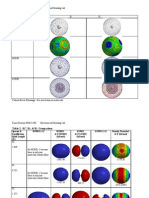
Known shape of some AH2 molecules Molecular species LiH2+ LiH2, BeH2+ BeH2, BH2+ BH2, AlH2, CH2+ CH2, SiH2, BH2-, NH2+ NH2, PH2, CH2-, OH2+ OH2, SH2, NH2-, FH2+ No. of valence electrons 2 3 4 5 6 7 8 Shape Bent Linear Linear Bent Bent Bent Bent
Molecular species BH2 CH2 SiH2 NH2 OH2 SH2 FH2+
angle 131 110 93 103 104.5 92.1 118
Dh
C2v
Walsh diagram
poorer overlap of H1s with 2px remains non-bonding
better overlap of H 1s orbitals First order effects
Walsh diagram
mixing can occur between 2 orbitals because they both transform as a1 in the lower symmetry
A second order effect (often called a second order Jahn Teller distortion)
1 E
Walsh diagram
Known shape of some AH2 molecules Molecular species No. of valence electrons 2 3 4 5 6 7 8 Shape
LiH2+ LiH2, BeH2+ BeH2, BH2+ BH2, AlH2, CH2+ CH2, SiH2, BH2-, NH2+ NH2, PH2, CH2-, OH2+ OH2, SH2, NH2-, FH2+
Bent Linear Linear Bent Bent Bent Bent
Recall: a molecule adopts the structure that best stabilises the HOMO. If the HOMO is unperturbed by the structural change under consideration, then the occupied MO lying closest to it governs the geometric preference.
Walsh diagram
OH2 vs SH2 vs SeH2? VSEPR? Decreased s/p hybridisation? (note bonding with pure p orbitals implies 90angles)
1a12 1b22 + BH2
2a11 131 CH2 NH2+ BH2AlH2 119 SiH2
2a12 110 115-120 102 93
2a12 1b11 NH2 OH2+ CH2PH2 AsH2 103 110.5 99 92 91
2a12 1b12 OH2 FH2+ NH2SH2 SeH2 TeH2 104.5 118 104 92.1 90.6 90
Dh
C2v
2 x sp hybrids + 2 x p lone pairs
3 x sp2 hybrids + 1 x p lone pair more p character in bonding orbitals
2nd order JT effect mixes s character (originally in 2g) into the lone pair (1u) so more p character accumulates in the bonding orbitals. Strength of 2nd order JT effect proportional to 1/E 2 factors to consider: Decrease in electronegativity destabilises u 3/4s orbitals of S/Se are lower than 2s of O (increased penetration) and also overlap more weakly with H 1s because they are diffuse. Both factors stabilise g both make 2nd order JT effect stronger in the heavier elements
PES revisited: origin of fine structure
PES revisited: origin of fine structure
PES revisited: HF
(0,0) band most intense
higher band most intense
narrow band
PES revisited: H2O (0,0) band most intense
The shapes of H3+ and H3-: 1st order JT effects, 3-centre-4-electron and 3-centre-2-electron bonds Consider MOs for the 2 limiting shapes for a 3-atom system: linear (Dh) and triangular (D3h)
D3h
(using the SALCS given in the handout)
The shapes of H3+ and H3-: 1st order JT effects, 3-centre-4-electron and 3-centre-2-electron bonds Consider MOs for the 2 limiting shapes for a 3-atom system: linear (Dh) and triangular (D3h)
Dh
Walsh diagram
increasing destructive overlap of terminal H 1s
increasing constructive overlap of terminal H 1s
Walsh diagram
(1st order) Jahn-Teller theorem If a non-linear molecule is in an orbitally degenerate state, it will distort to remove the degeneracy
orbitally degenerate therefore a first-order JT distortion
H3(a 3-centre-4-electron bond) c.f. hypervalent species such as HF2-, XeF2
H3+ (a 3-centre-2-electron bond) c.f. electron deficient species such as boranes
Bonding in Hypervalent Molecules XeF2 (hypervalent = 10 electrons on Xe) Possible explanations?
sp3d hybridisation?
Ionic resonance forms?
Bond order 0.5
Symmetry analysis (Dh)
Вам также может понравиться
- Physical Chemistry II: Quantum Chemistry Lecture 19:many-Electron Atoms & Atomic Term SymbolsДокумент42 страницыPhysical Chemistry II: Quantum Chemistry Lecture 19:many-Electron Atoms & Atomic Term SymbolsBo-Ji PengОценок пока нет
- Handout For Russell-Saunders Coupling PDFДокумент12 страницHandout For Russell-Saunders Coupling PDFAditiОценок пока нет
- Problem Set 6 KeyДокумент4 страницыProblem Set 6 KeyryezhuОценок пока нет
- Mark Schemes For The Units January 2008: ChemistryДокумент59 страницMark Schemes For The Units January 2008: ChemistryPhilip_830Оценок пока нет
- ACJC JC2 H2 Maths 2012 Year End Exam Solutions Paper 1Документ10 страницACJC JC2 H2 Maths 2012 Year End Exam Solutions Paper 1ofji4oОценок пока нет
- Picos Solvente RMNДокумент1 страницаPicos Solvente RMNYasminGomesОценок пока нет
- Amperes Law of ForceДокумент4 страницыAmperes Law of ForceAli khan7Оценок пока нет
- LaserДокумент15 страницLaserkaran5singh-12100% (1)
- Virial Equation of StateДокумент9 страницVirial Equation of StateSaba ArifОценок пока нет
- Code 0 p2 SolutionДокумент38 страницCode 0 p2 Solutionanon020202Оценок пока нет
- Inorganic Chap#3 And#4 Hom Take ExamДокумент22 страницыInorganic Chap#3 And#4 Hom Take Examwold100% (1)
- Final Exam KeyДокумент12 страницFinal Exam KeykitthiОценок пока нет
- Problem Set 5 KeyДокумент5 страницProblem Set 5 KeyYash KumarОценок пока нет
- Application of Partition FunctionДокумент2 страницыApplication of Partition FunctionNITISH KUMARОценок пока нет
- Ans 1Документ12 страницAns 1euphysics2025Оценок пока нет
- 1 IntroductoryДокумент45 страниц1 IntroductoryTuhin Sahu100% (1)
- Chapter 10Документ24 страницыChapter 10Lucy BrownОценок пока нет
- 12 Chemistry Impq CH09 Coordination Compounds 01Документ7 страниц12 Chemistry Impq CH09 Coordination Compounds 01Sudarshan PandeyОценок пока нет
- Topic 10 SL CHEM QuestionsДокумент32 страницыTopic 10 SL CHEM QuestionsWalter Jose Velasquez100% (1)
- Chemistry G11: G11 Constricted Response (CR) Questions Instructions and Examples Term One 2021-2022Документ6 страницChemistry G11: G11 Constricted Response (CR) Questions Instructions and Examples Term One 2021-2022ahmed aluОценок пока нет
- Problems SetДокумент10 страницProblems SetSajith KurianОценок пока нет
- Atomic Term SymbolsДокумент4 страницыAtomic Term SymbolsM IkhsanОценок пока нет
- Transition Metals and Coordination ChemistryДокумент80 страницTransition Metals and Coordination ChemistryVincent Choo100% (1)
- PhotochemistryДокумент24 страницыPhotochemistryVijay PradhanОценок пока нет
- ChelotropicДокумент11 страницChelotropicChemistry MESОценок пока нет
- 18 Quantitative Aspects of Chemical ChangeДокумент30 страниц18 Quantitative Aspects of Chemical Changeapi-235269401Оценок пока нет
- Chapter 7Документ20 страницChapter 7Ajeet SinghОценок пока нет
- Organic Reactions and Their MechanismsДокумент24 страницыOrganic Reactions and Their Mechanismsankita_friends100Оценок пока нет
- Chemistry 12: Solutions Manual Part AДокумент34 страницыChemistry 12: Solutions Manual Part ADerrick JamesОценок пока нет
- Torsion Pendulum PDFДокумент4 страницыTorsion Pendulum PDFVijayalakshmi PrabakaranОценок пока нет
- Magnetic Dipole MomentsДокумент44 страницыMagnetic Dipole MomentsOm SinghОценок пока нет
- PhotoChemistry PDFДокумент22 страницыPhotoChemistry PDFAnkitranjan GogoiОценок пока нет
- SN1 Vs SN2 ReactionsДокумент23 страницыSN1 Vs SN2 Reactionssamnas100Оценок пока нет
- Molecular Orbital Therory-Diatomic MoleculesДокумент25 страницMolecular Orbital Therory-Diatomic MoleculesDnyaneshwar ShindeОценок пока нет
- Topic 4: Bonding 4.2: Covalent BondingДокумент30 страницTopic 4: Bonding 4.2: Covalent Bondingapi-546066323Оценок пока нет
- Anti Baldwin CyclizationsДокумент14 страницAnti Baldwin CyclizationsLeandro SasiambarrenaОценок пока нет
- Ligand Field TheoryДокумент4 страницыLigand Field TheoryEca SCoutОценок пока нет
- AlkenesДокумент12 страницAlkenesDoc_CrocОценок пока нет
- F Block ElementsДокумент4 страницыF Block ElementsAfaf HucynОценок пока нет
- Boltzmann's and Saha's EquationsДокумент22 страницыBoltzmann's and Saha's Equationssujayan2005Оценок пока нет
- Einstein Relations: Prof - Siva PrasadДокумент11 страницEinstein Relations: Prof - Siva PrasadRenuОценок пока нет
- Energy Bands For Electrons in Crystals (Kittel Ch. 7)Документ39 страницEnergy Bands For Electrons in Crystals (Kittel Ch. 7)sabhanОценок пока нет
- Multiplet Splitting Phenomena in XPSДокумент5 страницMultiplet Splitting Phenomena in XPSmadihahbaik0% (1)
- Reaction Mechanism of Coordination ComplexesДокумент7 страницReaction Mechanism of Coordination ComplexesMartinMagu0% (1)
- Fluxionality 2Документ15 страницFluxionality 2GA GAОценок пока нет
- Chem Unit 5electrchemistry AnswersДокумент18 страницChem Unit 5electrchemistry Answersareyouthere92Оценок пока нет
- Experiment 1 Docx - Comp EngДокумент26 страницExperiment 1 Docx - Comp Engapi-344058913Оценок пока нет
- Determination of o of Chromium Using Tanabe-Sugano DiagramДокумент2 страницыDetermination of o of Chromium Using Tanabe-Sugano DiagramDozdiОценок пока нет
- LCAO MO Theory Illustrated by Its Application To H2Документ8 страницLCAO MO Theory Illustrated by Its Application To H2maugonzalezsuarezОценок пока нет
- MCQs Vibrations and Waves MITДокумент11 страницMCQs Vibrations and Waves MITcmegmhiОценок пока нет
- Lab Report 7 Borda PendulumДокумент7 страницLab Report 7 Borda PendulumazarmechОценок пока нет
- Signal Flow DiagramsДокумент20 страницSignal Flow DiagramsSingappuliОценок пока нет
- Lab Manual Math 209Документ75 страницLab Manual Math 209ReetОценок пока нет
- MatSci PrelimsДокумент17 страницMatSci Prelimssantovincent425100% (1)
- Chapter 13.2 - RothДокумент37 страницChapter 13.2 - Rothkishan patelОценок пока нет
- Electrons and Bonding LabДокумент6 страницElectrons and Bonding Lablovelylost100% (1)
- Primary Kinetic Isotope EffectДокумент7 страницPrimary Kinetic Isotope EffectAbhay KapkotiОценок пока нет
- Nuclear Magnetic Resonance SpectrosДокумент11 страницNuclear Magnetic Resonance Spectrosilias1973Оценок пока нет
- CHM 1321 Assignment 4 AnswersДокумент12 страницCHM 1321 Assignment 4 AnswersSara YuenОценок пока нет
- Chem310 MO TheoryДокумент18 страницChem310 MO TheoryNitinKumarОценок пока нет
- VocabularyДокумент42 страницыVocabularySandunil JayasingheОценок пока нет
- Lewis Structures Are A Way To Write Chemical Compounds Where All The Atoms and Electrons Are ShownДокумент3 страницыLewis Structures Are A Way To Write Chemical Compounds Where All The Atoms and Electrons Are ShownSandunil JayasingheОценок пока нет
- Mathematics For Information Technology 103Документ10 страницMathematics For Information Technology 103Sandunil JayasingheОценок пока нет
- Apch 03 SCДокумент3 страницыApch 03 SCScott AllredОценок пока нет
- Apch 02 SCДокумент6 страницApch 02 SCScott AllredОценок пока нет
- SQA Higher Physics Summary NotesДокумент115 страницSQA Higher Physics Summary Noteshhhhhhhhhhhhhhhhhhhhhhhhhhhhhf100% (2)
- 01) 100 Causes of Bad ShiftingДокумент2 страницы01) 100 Causes of Bad Shiftingbaracuda44Оценок пока нет
- Economics 101: Interactive BrokersДокумент11 страницEconomics 101: Interactive BrokersDennys FreireОценок пока нет
- Mid Year Test: Objective QuestionsДокумент3 страницыMid Year Test: Objective QuestionsNur SuHaОценок пока нет
- Final PPT of Carbon NanotubesДокумент29 страницFinal PPT of Carbon Nanotubesmkumar_5481450% (2)
- Design Aspects of Cathodic ProtectionДокумент24 страницыDesign Aspects of Cathodic ProtectionRahul AdityaОценок пока нет
- Autoclave CatalogueДокумент17 страницAutoclave CatalogueUMARALEKSANA, CVОценок пока нет
- Q4 - WEEK 2 - MEASURES OF POSITION For GROUPED DATAДокумент16 страницQ4 - WEEK 2 - MEASURES OF POSITION For GROUPED DATAEllaineeeeОценок пока нет
- Advantage of Oil CoolingДокумент2 страницыAdvantage of Oil CoolingYin ThoОценок пока нет
- Synchronous Generators - 2 Marks Questions and AnswersДокумент3 страницыSynchronous Generators - 2 Marks Questions and AnswersJoseph Harindranath67% (3)
- 3Документ8 страниц3K@mR@N D@uD P@nHw@RОценок пока нет
- Digital Logic Design Chapter 5Документ28 страницDigital Logic Design Chapter 5Okezaki TemoyoОценок пока нет
- Data Communication: By:Eng - Alaa I.HaniyДокумент8 страницData Communication: By:Eng - Alaa I.Haniypömo cОценок пока нет
- 1.1.1.A.VEX SimpleMachineInvestigationДокумент14 страниц1.1.1.A.VEX SimpleMachineInvestigationDivya Sureshkannan100% (2)
- SIGGRAPH2022 Advances Lumen Wright Et AlДокумент199 страницSIGGRAPH2022 Advances Lumen Wright Et AlmhazaniОценок пока нет
- Gauss Contest: Grade 7Документ4 страницыGauss Contest: Grade 7irwan syahОценок пока нет
- Phy Interface Pci Express Sata Usb31 Architectures Ver43 PDFДокумент99 страницPhy Interface Pci Express Sata Usb31 Architectures Ver43 PDFRaj Shekhar ReddyОценок пока нет
- Holmium Laser - Fibers - Brochure - A4 - PB-006360 - Rev K - WebДокумент4 страницыHolmium Laser - Fibers - Brochure - A4 - PB-006360 - Rev K - WebTigherMahОценок пока нет
- Principles of Communication Reviewer MidtermДокумент5 страницPrinciples of Communication Reviewer MidtermVon Ryan AlcazarОценок пока нет
- Nitrosyl Complexes of RutheniumДокумент26 страницNitrosyl Complexes of RutheniumBenjamín Marc Ridgway de SassouОценок пока нет
- KinematicsДокумент5 страницKinematicsUnicornpop PopОценок пока нет
- A330 FCOM Vol III PDFДокумент1 137 страницA330 FCOM Vol III PDFGraham Waterfield100% (1)
- Offshore Pipeline Decommissioning Scale and ContextДокумент4 страницыOffshore Pipeline Decommissioning Scale and ContextHieu Le TrungОценок пока нет
- B8 em WavesДокумент1 страницаB8 em Wavesbalikisyakubu64Оценок пока нет
- Performance of A Mid-Sized Harvester-Forwarder System in Integrated Harvesting of Sawmill, Pulpwood and FirewoodДокумент16 страницPerformance of A Mid-Sized Harvester-Forwarder System in Integrated Harvesting of Sawmill, Pulpwood and FirewoodAlexandru Gabriel CotituОценок пока нет
- Lin Guo - The First Line of Code - Android Programming With Kotlin-Springer (2022)Документ714 страницLin Guo - The First Line of Code - Android Programming With Kotlin-Springer (2022)İsmail SaygınОценок пока нет
- FG Wilson AtsДокумент5 страницFG Wilson AtsErwan Shidiq FathoniОценок пока нет
- Driver PDFДокумент211 страницDriver PDFAjay DhamijaОценок пока нет
- Maths - 2B Imp QuestionsДокумент93 страницыMaths - 2B Imp QuestionsBandaru Chiranjeevi100% (1)
- API TutorialДокумент22 страницыAPI TutorialKomarudinОценок пока нет
- Athene S Theory of Everything PDFДокумент19 страницAthene S Theory of Everything PDFAdriana Ealangi0% (1)
- Programming Exercise 2Документ2 страницыProgramming Exercise 2hamidahrazakОценок пока нет