Академический Документы
Профессиональный Документы
Культура Документы
Clinical Manifestations and Evaluation of The Patient With Suspected Heart Failure PDF
Загружено:
cristianamihailaОригинальное название
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
Clinical Manifestations and Evaluation of The Patient With Suspected Heart Failure PDF
Загружено:
cristianamihailaАвторское право:
Доступные форматы
Clinical manifestations and evaluation of the patient with suspected heart ...
http://www.uptodate.com/online/content/topic.do?topicKey=hrt_fail/73...
Official reprint from UpToDate www.uptodate.com
Back
Clinical manifestations and evaluation of the patient with suspected heart failure
Author Wilson S Colucci, MD Section Editor Stephen S Gottlieb, MD Deputy Editor Susan B Yeon, MD, JD, FACC
Last literature review for version 16.1: January 31, 2008 | This topic last updated: February 13, 2008 INTRODUCTION Heart failure (HF) is a common clinical syndrome representing the end-stage of a number of different cardiac diseases [1] . The initial evaluation of the patient with suspected HF will be reviewed here. The management and prognosis of this disorder are discussed separately. ( See "Overview of the therapy of heart failure due to systolic dysfunction" and see "Prognosis of heart failure"). DEFINITION Heart failure (HF) is a complex clinical syndrome that can result from any structural or functional cardiac disorder that impairs the ability of the ventricle to fill with or eject blood. It is characterized by specific symptoms, such as dyspnea and fatigue, and signs, such as fluid retention. There are many ways to assess cardiac function. However, there is no diagnostic test for HF, since it is largely a clinical diagnosis that is based upon a careful history and physical examination ( show table 1). Classification of severity The classification system that is most commonly used to quantify the degree of functional limitation imposed by HF is one first developed by the New York Heart Association (NYHA). This system assigns patients to one of four functional classes, depending on the degree of effort needed to elicit symptoms (show table 2): Class I - symptoms of HF only at activity levels that would limit normal individuals Class II - symptoms of HF with ordinary exertion Class III - symptoms of HF with less than ordinary exertion Class IV - symptoms of HF at rest Stages of HF There are several stages in the evolution of HF, as outlined by an ACC/AHA task force [2] : Stage A High risk for HF, without structural heart disease or symptoms Stage B Heart disease with asymptomatic left ventricular dysfunction Stage C Prior or current symptoms of HF Stage D Advanced heart disease and severely symptomatic or refractory HF This staged system, in contrast to the NYHA classification, emphasizes the progressive nature of HF and defines the appropriate therapeutic approach for each stage (show figure 1). ETIOLOGY There are two basic pathophysiologic mechanisms by which reduced cardiac output and HF occur: systolic dysfunction and diastolic dysfunction. Systolic and diastolic dysfunction each may be due to a variety of etiologies. Effective management is often dependent upon establishing the correct etiologic
1 of 23
7/23/2008 7:14 PM
Clinical manifestations and evaluation of the patient with suspected heart ...
http://www.uptodate.com/online/content/topic.do?topicKey=hrt_fail/73...
diagnosis. As an example, coronary revascularization may be beneficial in patients with ischemic cardiomyopathy who have evidence of hibernating myocardium ( see "Detection of coronary heart disease" below). Systolic dysfunction The most common causes of systolic dysfunction are coronary (ischemic) heart disease, idiopathic dilated cardiomyopathy (DCM), hypertension, and valvular disease ( show table 3). Effective therapy of hypertension has led to a changing pattern in which coronary disease has become more prevalent as a cause of HF [3,4] . In one review, coronary disease and hypertension accounted for 62 and 10 percent of cases, respectively [3] . (See "Epidemiology and causes of heart failure"). Not surprisingly, the distribution of etiologies is different in patients who present with initially unexplained DCM. After a complete evaluation of 1230 such patients, the relative frequency of the different causes was as follows [5] : Idiopathic 50 percent Myocarditis 9 percent Ischemic heart disease 7 percent Infiltrative disease 5 percent Peripartum cardiomyopathy 4 percent Hypertension 4 percent HIV infection 4 percent Connective tissue disease 3 percent Substance abuse 3 percent Doxorubicin 1 percent Other 10 percent Familial disease Among patients with idiopathic DCM, it is estimated that at least 25 percent have familial disease. No clinical or histologic criteria, other than family history and careful examination of relatives (including those who are asymptomatic), have been derived to distinguish familial from nonfamilial disease. The mode of inheritance is usually autosomal dominant, although autosomal recessive, X-linked, and mitochondrial inheritance have also been described. These disorders, as well as recommendations for screening and counseling family members, are discussed elsewhere. ( See "Genetics of dilated cardiomyopathy", section on Familial dilated cardiomyopathy). Diastolic dysfunction Diastolic dysfunction can be induced by many of the same conditions that lead to systolic dysfunction. The most common causes are hypertension, ischemic heart disease, hypertrophic obstructive cardiomyopathy, and restrictive cardiomyopathy. However, many patients with symptoms suggestive of HF (shortness of breath, ankle edema, or paroxysmal nocturnal dyspnea) who have intact left ventricular systolic function may not have diastolic dysfunction, but have other etiologies that can account for their symptoms, including obesity, lung disease, or occult coronary ischemia [ 6] . (See "Clinical manifestations and diagnosis of diastolic heart failure" ). CLINICAL PRESENTATION The approach to the patient with HF uses the history and physical examination, chest x-ray, and a series of diagnostic tests to establish the diagnosis, determine the etiology, and assess acuity and severity. Recommendations for the evaluation of patients with HF were published in the 2005 ACC/AHA guidelines on chronic heart failure ( show table 4 and show algorithm 1) [2] .
2 of 23
7/23/2008 7:14 PM
Clinical manifestations and evaluation of the patient with suspected heart ...
http://www.uptodate.com/online/content/topic.do?topicKey=hrt_fail/73...
History There are two major classes of symptoms in HF: those due to excess fluid accumulation (dyspnea, edema, hepatic congestion, and ascites) and those due to a reduction in cardiac output (fatigue, weakness) that is most pronounced with exertion. Fluid retention in HF is initiated by the fall in cardiac output, leading to alterations in renal function, due in part to activation of the sodium-retaining renin-angiotensin-aldosterone and sympathetic nervous systems. ( See "Pathophysiology of heart failure: Neurohumoral adaptations"). Important information concerning the acuity of HF is suggested by the presenting symptoms: Acute and subacute presentations (days to weeks) are characterized primarily by shortness of breath, at rest and/or with exertion. Also common are orthopnea, paroxysmal nocturnal dyspnea, and, with right HF, right upper quadrant discomfort due to acute hepatic congestion, which can be confused with acute cholecystitis. Patients with atrial and/or ventricular tachyarrhythmias may complain of palpitations with or without lightheadedness. ( See "Pathophysiology and evaluation of acute decompensated heart failure" ). Chronic presentations (months) differ in that fatigue, anorexia, bowel distension, and peripheral edema may be more pronounced than dyspnea. The anorexia is secondary to several factors including poor perfusion of the splanchnic circulation, bowel edema, and nausea induced by hepatic congestion. Over time, pulmonary venous capacitance accommodates to the chronic state of volume overload, leading to less or no fluid accumulation in the alveoli, despite the increase in total lung water. These patients present with excessive fatigue and low-output symptoms. The value of the history and physical examination in predicting the presence of HF was assessed in a study of 259 primary care patients referred to an echocardiography service for suspected HF, 41 of whom had significant left ventricular (LV) systolic dysfunction on echocardiography [ 7] . Absence of dyspnea on exertion essentially ruled out the presence of HF due to LV dysfunction, although this may reflect the fact that only nine asymptomatic patients were referred for assessment. The sensitivity and specificity of the following symptoms were reported: dyspnea on exertion 100 and 17 percent, respectively; orthopnea 22 and 74 percent; paroxysmal nocturnal dyspnea 39 and 80 percent; peripheral edema 49 and 47 percent. Thus, while exertional dyspnea is non-specific for HF, a history of paroxysmal nocturnal dyspnea, although insensitive, is much more specific. A past history of myocardial infarction had the best combination of sensitivity, specificity, and positive and negative predictive value of any item in the history. A second study assessed the clinical characteristics of elderly patients (ages 70 to 84 years) in a primary care setting who had LV systolic dysfunction diagnosed by echocardiography [ 8] . Factors in the history that independently predicted the presence of LV dysfunction on echocardiography were breathlessness when walking and a history of myocardial infarction or angina. However, no single clinical symptom was both sensitive and specific, and a substantial number of patients had asymptomatic LV dysfunction. These findings confirm that the history alone is insufficient to make the diagnosis of HF with certainty. Nevertheless, a detailed history remains the single best discriminator to determine the acuity, etiology, and rate of progression of HF. The history often provides important clues to the cause of HF. As examples: Classic exertional angina usually indicates ischemic heart disease. Acute HF after an antecedent flu-like illness suggests viral myocarditis. Long-standing hypertension or alcohol use suggests hypertensive or alcoholic cardiomyopathy. Amyloidosis should be excluded in patients who also have a history of heavy proteinuria. It
3 of 23
7/23/2008 7:14 PM
Clinical manifestations and evaluation of the patient with suspected heart ...
http://www.uptodate.com/online/content/topic.do?topicKey=hrt_fail/73...
should be appreciated, however, that mild proteinuria can be seen with heart failure alone. Primary valvular dysfunction should be considered in a patient with a history of murmurs. ( See "Auscultation of cardiac murmurs"). HF may be provoked or worsened by drugs, including antiarrhythmic agents such as disopyramide and flecainide; calcium channel blockers, particularly verapamil; beta blockers; and nonsteroidal antiinflammatory drugs (NSAIDs) [ 9] . (See "NSAIDs: Cardiovascular effects", section on heart failure). Acute pulmonary edema occurring during, or shortly after, infusion of blood products suggests transfusional volume overload. (See "Transfusion reactions caused by chemical and physical agents", section on Volume overload). Physical examination The physical examination can provide important information concerning the degree to which the cardiac output is reduced as well as the degree of volume overload, ventricular enlargement, and pulmonary hypertension. Heart sounds An S3 gallop have been associated with left atrial pressures exceeding 20 mmHg, increased left ventricular end-diastolic pressures (>15 mmHg) and elevated serum brain natriuretic peptide concentrations. However, there is appreciable interobserver variability in the ability to detect an S3 that cannot be solely explained by the experience of the observer [10,11] . In addition, in a phonocardiographic study of patients who were undergoing cardiac catheterization, an S3 was not very sensitive (40 to 50 percent) for the detection of an elevated left ventricular end-diastolic pressure or a reduced left ventricular ejection fraction; however, the S3 was highly specific (90 percent) for these parameters and for an elevated serum brain natriuretic peptide concentration [ 12] . (See "Auscultation of heart sounds", section on Left ventricular gallops). Decreased cardiac output Patients with advanced HF may show evidence of a major decline in cardiac output and therefore a decrease in tissue perfusion. Both the cardiac disease itself and the secondary neurohumoral adaptation contribute to the low output state. As mentioned above, patients compensate for a fall in cardiac output by increasing sympathetic outflow. This results in shunting of the cardiac output to vital organs, leading to three major findings that vary directly with the severity of the cardiac dysfunction: sinus tachycardia, diaphoresis, and peripheral vasoconstriction. The last abnormality is manifested as cool, pale, and sometimes cyanotic extremities (due to the combination of decreased perfusion and increased oxygen extraction). A decrease in cardiac output should be suspected when the pulse pressure is reduced below 25 mmHg. Two other signs that may be present in advanced HF are a palpable dicrotic notch pulsation and pulsus alternans. The dicrotic notch represents closure of the aortic valve. With more severe left ventricular dysfunction, the compensatory increase in total peripheral resistance decreases aortic compliance, thereby accentuating the closure pulsation and increasing the contribution of the dicrotic notch to the carotid upstroke. (See "Examination of the arterial pulse", section on Dicrotic pulse). Pulsus alternans, if present, is virtually pathognomonic of severe left ventricular failure. This phenomenon is characterized by evenly spaced alternating strong and weak peripheral pulses. It is best appreciated by applying light pressure on the peripheral arterial pulse, and can be confirmed by measuring the blood pressure. When the cuff pressure is slowly released, phase I Korotkoff sounds are initially heard only during the alternate strong beats; with further release of cuff pressure, the softer sounds of the weak beat also appear. The degree of pulsus alternans can be quantitated by measuring the difference in systolic pressure between the strong and the weak beat. (See "Examination of the arterial pulse", section on Pulsus alternans).
4 of 23
7/23/2008 7:14 PM
Clinical manifestations and evaluation of the patient with suspected heart ...
http://www.uptodate.com/online/content/topic.do?topicKey=hrt_fail/73...
The pathophysiology of pulsus alternans is not well understood. The severe ventricular dysfunction may be associated with variations in contractility secondary to shifts in afterload, preload, and electrical excitability [13] . Volume overload There are three major manifestations of volume overload in patients with HF: pulmonary congestion, peripheral edema, and elevated jugular venous pressure. Pulmonary congestion is more prominent in acute or subacute disease. As noted above, chronic HF is associated with increases in venous capacitance and lymphatic drainage of the lungs; as a result, rales are often absent even though the pulmonary capillary pressure is still elevated. Continued sodium retention in this setting preferentially accumulates in the periphery although a chronic elevation in pulmonary venous pressure can lead to pleural effusions. Peripheral edema is manifested by swelling of the legs (which is more prominent when the patient is upright), ascites, hepatomegaly, and splenomegaly [ 14] . (See "Approach to the adult patient with splenomegaly and other splenic disorders"). Manual compression of the right upper quadrant in this setting may elevate the central venous pressure via increased venous return due to compression of the inferior vena cava. This sign is known as hepatojugular reflux. Elevated jugular venous pressure is usually present if peripheral edema is due to HF, since it is the high intracapillary pressure that is responsible for fluid movement into the interstitium. Jugular venous pressure can be estimated with the patient sitting at 45 either from the height above the left atrium of venous pulsations in the internal jugular vein or, if visible, of the fluid column in the external jugular vein. Ventricular enlargement Ventricular chamber size can be estimated by precordial palpation. An apical impulse that is laterally displaced past the midclavicular line is usually indicative of left ventricular enlargement. Left ventricular dysfunction can also lead to sustained apical impulse which may be accompanied by a parasternal heave. The S3 may be palpable in severe ventricular failure. (See "Examination of the precordial pulsation" ). Pulmonary hypertension Patients with chronic HF often develop secondary pulmonary hypertension, which can contribute to dyspnea as pulmonary pressures rise with exertion. These patients may also complain of substernal chest pressure, typical of angina. In this setting, elevated right ventricular end-diastolic pressure leads to secondary right ventricular subendocardial ischemia. Physical signs of pulmonary hypertension can include increased intensity of P2, a murmur of pulmonary insufficiency, and a palpable pulmonic tap (felt in the left second intercostal space). (See "Auscultation of cardiac murmurs" and see "Auscultation of heart sounds"). In the study of primary care patients cited above, the physical finding of a displaced apical impulse had the best combination of sensitivity, specificity, and positive and negative predictive value of any physical sign of HF [7] . Other strong predictors of HF included a gallop rhythm and elevated jugular venous pressure. BLOOD TESTS Overview Recommended initial blood tests for patients with signs or symptoms of HF include: A complete blood count since anemia can exacerbate preexisting HF. ( See "Overview of the therapy of heart failure due to systolic dysfunction" section on Anemia). Serum electrolytes and creatinine as a baseline to follow when initiating therapy with diuretics and/or angiotensin converting enzyme inhibitors.
5 of 23
7/23/2008 7:14 PM
Clinical manifestations and evaluation of the patient with suspected heart ...
http://www.uptodate.com/online/content/topic.do?topicKey=hrt_fail/73...
Liver function tests, which may be affected by hepatic congestion. Fasting blood glucose to detect underlying diabetes mellitus. ( See "Heart failure in diabetes mellitus"). In addition, if it is determined that dilated cardiomyopathy is responsible for HF and the cause is not apparent, several other blood tests may be warranted. (See "Causes of dilated cardiomyopathy"): Thyroid function tests, particularly in patients over the age of 65 or in patients with atrial fibrillation. Thyrotoxicosis is associated with atrial fibrillation and hypothyroidism may present as HF. (See "Cardiovascular effects of hyperthyroidism" and see "Cardiovascular effects of hypothyroidism"). Iron studies (ferritin and TIBC) to screen for hereditary hemochromatosis (HH). In the past, prior to increased screening, cardiac disease was the presenting manifestation in up to 15 percent of patients with HH. Thus, the absence of other characteristic findings of HH does not preclude the diagnosis. (See "Clinical manifestations of hereditary hemochromatosis" , section on Heart disease). Other studies that may be undertaken depending upon the potential findings identified in the history and physical examination include: ANA and other serologic tests for lupus Viral serologies and antimyosin antibody if myocarditis is suspected Evaluation for pheochromocytoma Thiamine, carnitine, and selenium levels Genetic testing and counseling (eg, in patients suspected of familial cardiomyopathy after obtaining a detailed family history) Plasma BNP With chronic HF, atrial myocytes secrete increased amounts of atrial natriuretic peptide (ANP) and ventricular myocytes secrete both ANP and brain natriuretic peptide (BNP) in response to the high atrial and ventricular filling pressures. The plasma concentrations of both hormones are increased in patients with asymptomatic and symptomatic left ventricular dysfunction, permitting their use in diagnosis. (See "Brain natriuretic peptide measurement in left ventricular dysfunction and other cardiac diseases"). Rapid bedside measurement of plasma BNP is useful for distinguishing between HF due to systolic and/or diastolic dysfunction and a pulmonary cause of dyspnea. Based upon the following findings, plasma BNP measurement was approved by the FDA as an aid in HF diagnosis and it is recommended that measurement of BNP levels should be part of the diagnostic approach to patients with suspected HF. The cost for a BNP test is about $20. The value of this approach has been best evaluated in the Breathing Not Properly study of 1586 patients presenting to the emergency department or urgent care setting with a major complaint of acute dyspnea [15] . The final diagnosis was HF in 47 percent (primarily confirmed by chest x-ray and/or echocardiography), no HF in 49 percent, and noncardiac dyspnea in patients with a past history of left ventricular dysfunction in 5 percent. The following findings were noted: Plasma concentrations of BNP were markedly higher in patients with clinically diagnosed HF (including patients with right HF due to cor pulmonale) compared to those without HF (mean 675 versus 110 pg/mL). Intermediate values were found in the patients with baseline left ventricular dysfunction without an acute exacerbation (346 pg/mL) ( show figure 2).
6 of 23
7/23/2008 7:14 PM
Clinical manifestations and evaluation of the patient with suspected heart ...
http://www.uptodate.com/online/content/topic.do?topicKey=hrt_fail/73...
A value >100 pg/mL diagnosed HF with a sensitivity, specificity, and predictive accuracy of 90, 76, and 83 percent, respectively. Choosing values >125 or >150 pg/mL decreased sensitivity, increased specificity, and did not change overall predictive accuracy. The predictive accuracy of plasma BNP for HF was equivalent to or better than other parameters such as cardiomegaly on chest x-ray, a history of HF, or rales on physical examination. Another study compared plasma BNP to initial clinical judgment [ 16] . Among patients judged on clinical grounds to have a 80 percent probability of HF, a plasma BNP >100 pg/mL was more sensitive (90 versus 49 percent) but less specific (73 versus 96 percent) than clinical judgment [ 16] . Adding BNP to clinical judgment increased diagnostic accuracy (HF versus no HF) from 74 to 81 percent. Among patients with a 21 to 79 percent clinical probability of HF, a plasma BNP >100 pg/mL had a diagnostic accuracy of 74 percent and only misclassified 7 percent of patients who had HF. Among patients originally thought to have less than a 21 percent clinical probability of HF, 17 percent had a final diagnosis of HF and plasma BNP correctly diagnosed 90 percent of these patients. The cutoff points for a normal and abnormal BNP have varied in other reports, due in part to different assays, as have the sensitivity and specificity. With the rapid bedside assay, most dyspneic patients with HF have values above 400 pg/mL, while left ventricular dysfunction without exacerbation, pulmonary embolism, and cor pulmonale should be excluded in dyspneic patients with plasma BNP concentrations between 100 and 400 pg/mL [17] . A consistent finding in most studies is a high negative predictive value of low BNP concentrations, suggesting that BNP may have particular value in ruling out HF and saving the need to perform additional costly tests. In the BNP study, for example, a plasma BNP cutoff of 50 pg/mL compared to 100 pg/mL increased the sensitivity for HF (97 versus 90 percent), at a cost of reduced specificity [ 15] . Thus, only 3 and 10 percent of patients with HF had plasma BNP values below 50 and 100 pg/mL, respectively. An additional factor that can affect the cutoff used is the presence of atrial fibrillation (AF). This was illustrated in an analysis in which permanent or paroxysmal AF (present in 20 percent of patients) was associated with higher BNP levels in the patients who did not have a final diagnosis of HF (119 versus 25 pg/mL in patients without AF) [ 18] . As a result, a BNP cutoff of 100 pg/mL was associated with a specificity of only 40 percent compared to 79 percent in patients without AF. Using a cutoff of 200 pg/mL in patients with AF increased specificity from 40 to 73 percent with a smaller reduction in sensitivity from 95 to 85 percent. Plasma N-terminal pro-BNP The active BNP hormone is cleaved from the C-terminal end of its prohormone, pro-BNP. The N-terminal fragment, NT-proBNP, is also released into the circulation. In normal subjects, the plasma concentrations of BNP and NT-proBNP are similar (approximately 10 pmol/L). However, in patients with LV dysfunction, plasma NT-proBNP concentrations are approximately four-fold higher than BNP concentrations [19] . (See "Brain natriuretic peptide measurement in left ventricular dysfunction and other cardiac diseases" , section on Plasma N-terminal-pro-BNP). Measurement of NT-proBNP can improve the accuracy of diagnosis of HF above that attained with routine clinical evaluation in both acute care and primary care settings [ 20,21] . The utility of NT-proBNP in the acute setting is best illustrated by a retrospective cohort study that combined and analyzed data from three previous prospective studies [20] . Differences in plasma NT-proBNP among 1256 patients with and without acute heart failure were compared, and the relationship between plasma NT-proBNP and symptoms was examined. The following findings were noted: The optimal value for distinguishing HF from other causes of dyspnea varied with patient age. For patients <50, 50 to 75, and >75 years of age, the optimal plasma NT-proBNP cutoffs for diagnosing HF were 450 pg/mL, 900 pg/mL, and 1800 pg/mL respectively. Overall, these cutoffs yielded a sensitivity and specificity of 90 and 84 percent, respectively.
7 of 23
7/23/2008 7:14 PM
Clinical manifestations and evaluation of the patient with suspected heart ...
http://www.uptodate.com/online/content/topic.do?topicKey=hrt_fail/73...
Across the entire population, NT-proBNP levels below 300 pg/mL were optimal for excluding a diagnosis of HF, with a negative predictive value of 98 percent. In addition, use of NT-proBNP to guide diagnostic assessment of dyspnea in the emergency department may result in cost savings as well as improvement in selected clinical outcomes [ 22] . Limitations of plasma BNP and NT-proBNP Confounding factors may complicate interpretation of these measurements. Plasma BNP and NT-proBNP levels tend to be lower in obese patients and are elevated in patients with renal failure, right heart failure (due to pulmonary embolism or pulmonary disease), and some acute noncardiac illnesses such as sepsis. Measurement and interpretation of BNP and NT-proBNP levels is discussed in detail separately. ( See "Brain natriuretic peptide measurement in left ventricular dysfunction and other cardiac diseases" ) CHEST X-RAY The chest x-ray is a useful first diagnostic test, particularly in the evaluation of patients who present with dyspnea, to differentiate HF from primary pulmonary disease [ 23-25] . Findings suggestive of HF include cardiomegaly (cardiac-to-thoracic width ratio above 50 percent), cephalization of the pulmonary vessels, Kerley B-lines, and pleural effusions ( show radiograph 1A-1E). The cardiac size and silhouette may also reveal signs of congenital anomalies (ventricular or atrial septal defect) or valvular disease (mitral stenosis or aortic stenosis). A systematic review of the utility of the chest x-ray to diagnose LV dysfunction concluded that redistribution and cardiomegaly were the best predictors of increased preload and reduced ejection fraction, respectively [24] . Neither finding, however, was sufficient to make a definitive diagnosis of HF. In a multicenter study of 880 patients, alveolar edema, interstitial edema, and cephalization all had a specificity of >90 percent for HF, but only cardiomegaly had a sensitivity >50 percent [ 25] . ELECTROCARDIOGRAM The electrocardiogram may show findings that favor the presence of a specific cause of HF and can also detect arrhythmias such as asymptomatic ventricular premature beats, runs of nonsustained ventricular tachycardia, or atrial fibrillation, which may be the cause of or exacerbate HF. (See "Tachycardia-mediated cardiomyopathy"). Patients with dilated cardiomyopathy frequently have first degree AV block, left bundle branch block, left anterior fascicular block, or a nonspecific intraventricular conduction abnormality. Among the potentially diagnostic findings on ECG are: Evidence of ischemic heart disease. Left ventricular hypertrophy due to hypertension; a pseudoinfarct pattern may also be present representing significant posterior forces of the increased left ventricular mass. Low limb lead voltage on the surface ECG with a pseudo-infarction pattern (loss of precordial R wave progression in leads V1-V6) can suggest an infiltrative process such as amyloidosis. Low limb lead voltage with precordial criteria for left ventricular hypertrophy is most suggestive of idiopathic dilated cardiomyopathy. A widened QRS complex and/or a left bundle branch block pattern is also consistent with this diagnosis. Heart block, that may be complete, and various types of intraventricular conduction defects are observed in patients with cardiac sarcoidosis. The presence of a persistent tachycardia such as atrial fibrillation with a rapid ventricular response may lead to some confusion, since this arrhythmia itself can lead to a cardiomyopathy (tachycardia-mediated cardiomyopathy). Most patients with HF due to systolic dysfunction have a significant abnormality on ECG. A normal ECG
8 of 23
7/23/2008 7:14 PM
Clinical manifestations and evaluation of the patient with suspected heart ...
http://www.uptodate.com/online/content/topic.do?topicKey=hrt_fail/73...
makes systolic dysfunction extremely unlikely (98 percent negative predictive value) [ 26] . ECHOCARDIOGRAPHY Echocardiography should be performed in all patients with new onset HF and can provide important information about ventricular size and function ( show echocardiogram 1 and show echocardiogram 2 show echocardiogram 3 show echocardiogram 4 show echocardiogram 5. (See "Echocardiographic recognition of cardiomyopathies" ). The sensitivity and specificity of two dimensional echocardiography for the diagnosis of myocardial failure are as high as 80 and 100 percent, respectively [27] . A number of other important findings also can be detected: Although regional wall motion abnormalities are compatible with coronary heart disease, they are not specific for ischemia since they also occur in 50 to 60 percent of patients with idiopathic dilated cardiomyopathy [ 28] . However, regional wall motion assessment using dobutamine stress echocardiography may increase the ability to distinguish among ischemic and nonischemic cardiomyopathies. The presence of six or more akinetic segments, for example, was 80 percent sensitive and 96 percent specific for ischemic dilated cardiomyopathy in one report [29] . (See "Stress echocardiography in the diagnosis and prognosis of coronary heart disease"). Pericardial thickening in constrictive pericarditis. Mitral or aortic valve disease, as well as interatrial and interventricular shunts. Abnormal myocardial texture in infiltrative cardiomyopathies, with a "sparkling" pattern being specific for cardiac amyloidosis show echocardiogram 6 show echocardiogram 7 show echocardiogram 8. (See "Echocardiographic recognition of cardiomyopathies" ). Right ventricular size and function in right HF. Estimation of pulmonary capillary wedge pressure (PCWP) via the ratio (E/Ea or E/E') of tissue Doppler of early mitral inflow velocity (E) to early diastolic velocity of the mitral annulus (Ea or e'). An E/e' ratio >15 suggests a PCWP >15 mm Hg when e' is the mean of medial and lateral mitral annulus early diastolic velocities [ 30] . However, the E/e' ratio does not predict PCWP in patients with significant mitral valve disease (either mitral stenosis or mitral regurgitation). In patients with mitral valve disease, a preliminary report indicates that the ratio of isovolumetric relaxation time (IVRT) to the time interval between the onset of E and Ea (TE-Ea) correlates with PCWP [31] . A short deceleration time (125 ms) is an independent predictor of poor prognosis in patients with left ventricular dysfunction, regardless of the presence or absence of symptoms [ 32] . Right atrial and pulmonary artery pressures, determined by the peak velocity of tricuspid regurgitation on Doppler echocardiography. These findings correlate with the pulmonary artery wedge pressure, regardless of the etiology of HF or severity of tricuspid regurgitation; they can be used to assess changes in left ventricular filling pressures resulting from therapy [ 33] . The cardiac output can be measured accurately by pulsed-wave Doppler from the left ventricular outflow tract, even in the presence of a low output state or tricuspid regurgitation (show figure 3) [34] . Patients with idiopathic dilated cardiomyopathy typically have both left and right ventricular enlargement (four chamber dilatation) with decreased left systolic ventricular function. Ventricular thrombi can occasionally be found and are frequently observed at the left ventricular apex.
9 of 23
7/23/2008 7:14 PM
Clinical manifestations and evaluation of the patient with suspected heart ...
http://www.uptodate.com/online/content/topic.do?topicKey=hrt_fail/73...
Echocardiography, especially with the use of dobutamine, is also useful in predicting recovery of cardiac function [35,36] . (See "Prognosis of heart failure"). DETECTION OF CORONARY HEART DISEASE Most patients with HF due to ischemic cardiomyopathy have known coronary heart disease. As noted above, however, occult disease is a not uncommon cause of dilated cardiomyopathy, accounting for as many as 7 percent of initially unexplained cases [5] . On the other hand, chest pain alone is not sufficient to make the diagnosis since up to one-third of patients with nonischemic cardiomyopathy have chest pain that may resemble angina or be atypical. Heart failure resulting from coronary disease is usually irreversible due to myocardial infarction and subsequent ventricular remodeling. However, revascularization may be of benefit in the appreciable number of patients in whom hibernating myocardium is in part responsible for the decline in myocardial function. This was illustrated in a meta-analysis of 24 studies involving 3088 patients (mean ejection fraction 32 percent) [37] . The 42 percent of patients with documented viability by thallium perfusion imaging, PET scanning, or dobutamine echocardiography had a significant 80 percent reduction in annual mortality with revascularization (3.2 versus 16 percent for medical therapy). There was a direct relationship between the severity of left ventricular dysfunction and the magnitude of benefit. In contrast, there was no difference in outcome with revascularization or medical therapy in patients without viability (annual mortality 7.7 versus 6.2 percent). (See "Diagnosis and management of ischemic cardiomyopathy" ). Exercise testing Exercise testing should be part of the initial evaluation of any patient with HF. In addition to detection of ischemic heart disease, assessment of exercise capacity can be used for risk stratification and determining prognosis; serial measurements also can assess the efficacy of therapy and clinical stability of patients over time. With severe heart failure, measurement of the maximal oxygen uptake (VO2max) provides an objective estimate of the functional severity of the myocardial dysfunction. VO2max is one of the best indices of prognosis in patients with symptomatic HF and can aid in the determination of the necessity and timing of listing for cardiac transplantation. A simple alternative that provides an estimate of exercise function is the six minute walk test. (See "Exercise capacity and VO2 in heart failure" and see "Predictors of survival in heart failure due to systolic dysfunction" , section on Six minute walk test). Coronary arteriography The 2005 ACC/AHA guidelines on heart failure made the following recommendations for coronary arteriography, unless the patient is not eligible for revascularization of any kind (show table 4) [2] : Coronary arteriography should be performed in patients presenting with HF who have angina or significant ischemia. Coronary arteriography is reasonable in patients who have chest pain that may or may not be cardiac in origin in whom cardiac anatomy is not known and in patients with known or suspected coronary artery disease who do not have angina (class IIa). The diagnosis of an ischemic cardiomyopathy is an independent predictor of mortality; the extent of coronary artery disease as determined by angiography contributes more prognostic information than the clinical diagnosis of ischemic cardiomyopathy alone [ 38] . However, the angiographic findings must be considered in the context of the patient's history and other data. The presence of asymptomatic angiographic coronary artery disease in patients with dilated cardiomyopathy does not prove causality unless there is evidence of prior infarction or hibernating myocardium [39,40] .
10 of 23
7/23/2008 7:14 PM
Clinical manifestations and evaluation of the patient with suspected heart ...
http://www.uptodate.com/online/content/topic.do?topicKey=hrt_fail/73...
This was illustrated in a study of 55 patients who underwent heart transplantation; the patients carried a diagnosis of idiopathic DCM and all had had a normal coronary arteriogram within the preceding ten years and no ischemic events [39] . Examination of the explanted heart revealed critical lesions in at least one coronary artery segment in 15 patients (27 percent) with no evidence of scars. In addition to defining coronary artery anatomy, other useful information can be obtained from cardiac catheterization: Measurements of cardiac output, the degree of left ventricular dysfunction, and left ventricular end-diastolic pressure are helpful in assigning a severity score. Right (or left) ventricular endomyocardial biopsy can be performed if an unusual cause of myocarditis is suspected [40] . (See "Endomyocardial biopsy" below). Intracardiac shunts/anomalies and anomalous coronary arteries can be detected and the magnitude of secondary pulmonary hypertension can be assessed. If significant coronary disease is documented, an improvement in outcome with revascularization compared to medical therapy appears to be limited to those with hibernating myocardium as noted above [37] . Myocardial viability can be assessed by thallium perfusion imaging, PET scanning, or dobutamine echocardiography. (See "Evaluation of hibernating myocardium" ). Are there alternatives to angiography? Cardiac catheterization with coronary angiography is an invasive procedure with potentially serious complications such as atheroemboli and rarely myocardial infarction, ventricular tachyarrhythmias, stroke, and death. ( See "Complications of diagnostic cardiac catheterization"). Because of these concerns, noninvasive methods have been evaluated for the diagnosis of ischemic cardiomyopathy, although none is as yet recommended as a replacement for cardiac catheterization in patients with HF. Cardiac magnetic resonance imaging (CMR) can be used to look for late myocardial enhancement after the injection of gadolinium, which is much more common in ischemic than nonischemic cardiomyopathy and is associated with a different enhancement pattern. ( See "Clinical utility of cardiovascular magnetic resonance imaging" , section on Cardiomyopathy). The presence of coronary artery calcification (CAC) on electron beam computed tomography (EBCT) was, in several reports, highly suggestive of ischemic cardiomyopathy. It seems safe to assume that the presence of CAC on multidetector CT (MDCT) has the same diagnostic implications. On the other hand, the absence of CAC strongly favors a nonischemic cardiomyopathy. (See "Diagnostic and prognostic implications of coronary artery calcification detected by computed tomography", section on CAC and diagnosis of cardiomyopathy). The coronary artery lumen can be directly imaged by cardiac CT or CMR angiography. Early studies of 16 slice MDCT have shown this to be a promising approach to the noninvasive distinction between ischemic and nonischemic cardiomyopathy ( See "Noninvasive coronary arteriography with cardiac computed tomography and cardiovascular magnetic resonance" , section on Other uses). Noninvasive imaging The 2005 ACC/AHA guidelines on HF concluded that noninvasive imaging to detect myocardial ischemia and viability is reasonable in patients with known coronary artery disease and no angina unless the patient is not a candidate for revascularization of any kind ( show table 4) [2] . Although the evidence of efficacy is less well established, noninvasive imaging may be considered to define the likelihood of coronary artery disease in all patients with HF and left ventricular dysfunction. (See "Evaluation of hibernating myocardium" and see "Diagnosis and management of ischemic
11 of 23
7/23/2008 7:14 PM
Clinical manifestations and evaluation of the patient with suspected heart ...
http://www.uptodate.com/online/content/topic.do?topicKey=hrt_fail/73...
cardiomyopathy"). ENDOMYOCARDIAL BIOPSY The potential benefits and risks of endocardial biopsy in identifying the etiology of dilated cardiomyopathy are discussed in detail separately. ( See "Indications for endomyocardial biopsy"). ASSESSMENT OF SEVERITY The history, including assessment of NYHA functional class, and physical examination in conjunction with the diagnostic tests reviewed above should both establish the primary cause of the heart failure and provide a reasonable estimate of its severity. ( See "Classification of severity" above). The long-term prognosis can also be estimated. The peak VO2 is the most accurate predictor of prognosis, but functional class and exercise capacity, the magnitude of the reduction in ejection fraction with systolic dysfunction, serum BNP concentrations, and a variety of other factors are also important. (See "Predictors of survival in heart failure due to systolic dysfunction" and see "Prognosis of heart failure", section on Predictive models). HEART FAILURE AND COPD Chronic obstructive pulmonary disease (COPD) and HF are among the most common diagnoses encountered in clinical practice. Because of the high prevalence of these disorders, their similar presentations, and their frequent coexistence, it is reasonable to consider both diagnoses, not only in patients presenting with dyspnea for the first time, but also in any patient with one of these diagnoses who presents with a deterioration in pulmonary status [ 41] . This issue is discussed in detail separately. (See "Chronic obstructive pulmonary disease: definition, clinical manifestations, diagnosis, and staging" section on COPD and heart failure). INFORMATION FOR PATIENTS Educational materials on this topic are available for patients. ( See "Patient information: Heart failure causes, symptoms, and diagnosis" and see "Patient information: Heart failure treatments"). We encourage you to print or e-mail these topic reviews, or to refer patients to our public web site, www.uptodate.com/patients, which includes these and other topics. SUMMARY A thorough history and physical examination are the initial steps in the evaluation of patients with suspected HF. The absence of dyspnea on exertion makes the diagnosis of HF unlikely, while a history of myocardial infarction and a displaced apical impulse, or S3 on physical examination, strongly suggest the diagnosis. Initial blood tests should include a complete blood count, plasma electrolytes, BUN and creatinine, liver function tests, and urinalysis; these tests do not make the diagnosis of HF, but are performed for other reasons. An elevated plasma BNP suggests the diagnosis of HF, while a low plasma BNP may be particularly valuable in ruling out HF in patients in whom it is difficult to distinguish between a pulmonary and cardiac cause of dyspnea (see "Plasma BNP" above). A number of other tests may be warranted to establish the etiology of dilated cardiomyopathy if this is diagnosed on echocardiography. ( See "Causes of dilated cardiomyopathy"). A chest x-ray and electrocardiogram should be performed in all patients. The presence of pulmonary vascular congestion and cardiomegaly on chest x-ray support the diagnosis of HF; chest x-ray is also useful for ruling out pulmonary disease in patients who present with dyspnea. A normal ECG is unusual in patients with symptomatic systolic dysfunction (98 percent negative predictive value). Echocardiography should be performed in all patients with new onset HF. Echocardiography has a high sensitivity and specificity for the diagnosis of myocardial dysfunction, and may also establish the etiology of HF. Virtually all patients with unexplained HF should be evaluated for the presence of coronary heart disease. Noninvasive exercise testing is a reasonable first step as it not only provides information about the
12 of 23
7/23/2008 7:14 PM
Clinical manifestations and evaluation of the patient with suspected heart ...
http://www.uptodate.com/online/content/topic.do?topicKey=hrt_fail/73...
presence of ischemic heart disease, but also can be used for risk stratification and prognostic purposes. Coronary catheterization with angiography should be considered in patients with angina or a positive exercise test. Even in patients with a normal exercise test, however, cardiac catheterization should be strongly considered if HF is otherwise unexplained because of the relatively high prevalence of occult coronary disease in patients with HF. Cardiac catheterization may also establish the presence of severity of underlying valvular heart disease, which may contribute to HF. Endomyocardial biopsy should be reserved for patients with suspected systemic disease known to affect the myocardium, including hemochromatosis, amyloidosis, and sarcoidosis.
Use of UpToDate is subject to the Subscription and License Agreement.
REFERENCES
1. 2. Ho, KK, Pinsky, JL, Kannel, WB, Levy, D. The epidemiology of heart failure: the Framingham Study. J Am Coll Cardiol 1993; 22:6A. Hunt, SA, Abraham, WT, Chin, MH, et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation 2005; 112:e154. He, J, Ogden, LG, Bazzano, LA, et al. Risk factors for congestive heart failure in US men and women: NHANES I epidemiologic follow-up study. Arch Intern Med 2001; 161:996. Baldasseroni, S, Opasich, C, Gorini, M, et al. Left bundle-branch block is associated with increased 1-year sudden and total mortality rate in 5517 outpatients with congestive heart failure: a report from the Italian network on congestive heart failure. Am Heart J 2002; 143:398. Felker, CM, Thompson, RE, Hare, JM, et al. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N Engl J Med 2000; 342:1077. Caruana, L, Petrie, MC, Davie, AP, McMurray, JJ. Do patients with suspected heart failure and preserved left ventricular systolic function suffer from "diastolic heart failure" or from misdiagnosis? A prospective descriptive study. BMJ 2000; 321:215. Davie, AP, Francis, CM, Caruana, L, et al. Assessing diagnosis in heart failure: which features are any use?. QJM 1997; 90:335. Morgan, S, Smith, H, Simpson, I, et al. Prevalence and clinical characteristics of left ventricular dysfunction among elderly patients in general practice setting: cross sectional survey. BMJ 1999; 318:368. Page, J, Henry, D. Consumption of NSAIDs and the development of congestive heart failure in elderly patients. Arch Intern Med 2000; 160:777. Ishmail, AA, Wing, S, Ferguson, J, et al. Interobserver agreement by auscultation in the presence of a third heart sound in patients with congestive heart failure. Chest 1987; 91:870. Lok, CE, Morgan, CD, Ranganathan, N. The accuracy and interobserver agreement in detecting the'gallop sounds'by cardiac auscultation. Chest 1998; 114:1283. Marcus, GM, Gerber, IL, McKeown, BH, et al. Association between phonocardiographic third and fourth heart sounds and objective measures of left ventricular function. JAMA 2005; 293:2238. Gleason, WL, Braunwald, E. Studies on Starling's law of the heart. VI. Relationships between left ventricular end-diastolic volume and stroke volume in man with observations on the mechanism of pulsus alternans. Circulation 1962; 25:841. O'Reilly, RA. Splenomegaly in 2,505 patients at a large university medical center from 1913 to 1995. 1963 to 1995: 449 patients. West J Med 1998; 169:88.
3. 4.
5. 6.
7. 8.
9. 10. 11. 12.
13.
14.
13 of 23
7/23/2008 7:14 PM
Clinical manifestations and evaluation of the patient with suspected heart ...
http://www.uptodate.com/online/content/topic.do?topicKey=hrt_fail/73...
15. 16.
Maisel, AS, Krishnaswamy, P, Nowak, RM, et al. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med 2002; 347:161. McCullough, PA, Nowak, RM, McCord, J, et al. B-type natriuretic peptide and clinical judgment in emergency diagnosis of heart failure: analysis from Breathing Not Properly (BNP) multinational study. Circulation 2002; 106:416. Maisel, A. B-type natriuretic peptide levels: diagnostic and prognostic in congestive heart failure: what's next?. Circulation 2002; 105:2328. Knudsen, CW, Omland, T, Clopton, P, et al. Impact of atrial fibrillation on the diagnostic performance of B-type natriuretic peptide in dyspneic patients: an analysis from the Breathing Not Properly multinational study. J Am Coll Cardiol 2005; 46:838. Hunt, PJ, Richards, AM, Nicholls, MG, et al. Immunoreactive amino-terminal pro-brain natriuretic peptide (NT-PROBNP): a new marker of cardiac impairment. Clin Endocrinol (Oxf) 1997; 47:287. Januzzi, JL, van Kimmenade, R, Lainchbury, J, et al. NT-proBNP testing for diagnosis and short-term prognosis in acute destabilized heart failure: an international pooled analysis of 1256 patients: the International Collaborative of NT-proBNP Study. Eur Heart J 2006; 27:330. Wright, SP, Doughty, RN, Pearl, A, et al. Plasma amino-terminal pro-brain natriuretic peptide and accuracy of heart-failure diagnosis in primary care: a randomized, controlled trial. J Am Coll Cardiol 2003; 42:1793. Moe, GW, Howlett, J, Januzzi, JL, Zowall, H. N-terminal pro-B-type natriuretic peptide testing improves the management of patients with suspected acute heart failure: primary results of the Canadian prospective randomized multicenter IMPROVE-CHF study. Circulation 2007; 115:3103. Gillespie, ND, McNeil, G, Pringle, T, et al. Cross sectional study of contribution of clinical assessment and simple cardiac investigations in patients admitted with acute dyspnea. BMJ 1997; 314:936. Badgett, RG, Mulrow, CD, Otto, PM, Ramirez, G. How well can the chest radiograph diagnose leftventricular dysfunction?. J Gen Intern Med 1996; 11:625. Knudsen, CW, Omland, T, Clopton, P, et al. Diagnostic value of B-Type natriuretic peptide and chest radiographic findings in patients with acute dyspnea. Am J Med 2004; 116:363. Davie, AP, Francis, CM, Love, MP, et al. Value of the ECG in identifying heart failure due to left ventricular systolic dysfunction. BMJ 1996; 312:222. Erbel, R, Schweizer, P, Krebs, W, et al. Sensitivity and specificity of two-dimensional echocardiography in detection of impaired left ventricular function. Eur Heart J 1984; 5:477. Yamaguchi, S, Tsuiki, K, Hayasaka, M, Yasui, S. Segmental wall motion abnormalities in dilated cardiomyopathy: Hemodynamic characteristics and comparison with thallium-201 myocardial scintigraphy. Am Heart J 1987; 113:1123. Vigna, C, Russo, A, DeRito, V, et al. Regional wall motion analysis by dobutamine stress echocardiography to distinguish between ischemic and nonischemic dilated cardiomyopathy. Am Heart J 1996; 131:537. Dokainish, H, Zoghbi, WA, Lakkis, NM, et al. Optimal noninvasive assessment of left ventricular filling pressures: a comparison of tissue Doppler echocardiography and B-type natriuretic peptide in patients with pulmonary artery catheters. Circulation 2004; 109:2432. Diwan, A, McCulloch, M, Lawrie, GM, et al. Doppler estimation of left ventricular filling pressures in patients with mitral valve disease. Circulation 2005; 111:3281. Giannuzzi, P, Temporelli, PL, Bosimini, E, et al. Independent and incremental prognostic value of Doppler-derived mitral deceleration time in early filling in both symptomatic and asymptomatic patients with left ventricular dysfunction. J Am Coll Cardiol 1996; 28:383. Drazner, MH, Hamilton, MA, Fonarow, G, et al. Relationship between right and left-sided filling pressures in 1000 patients with advanced heart failure. J Heart Lung Transplant 1999; 18:1126. Gola, A, Pozzoli, M, Capomolla, S, et al. Comparison of Doppler echocardiography with thermodilution for assessing cardiac output in advanced congestive heart failure. Am J Cardiol 1996; 78:708. Naqvi, TZ, Goel, RK, Forrester, JS, Siegel, RJ. Myocardial contractile reserve on dobutamine
17. 18.
19.
20.
21.
22.
23.
24. 25. 26. 27. 28.
29.
30.
31. 32.
33.
34.
35.
14 of 23
7/23/2008 7:14 PM
Clinical manifestations and evaluation of the patient with suspected heart ...
http://www.uptodate.com/online/content/topic.do?topicKey=hrt_fail/73...
echocardiography predicts late spontaneous improvement in cardiac function in patients with recent onset idiopathic dilated cardiomyopathy. J Am Coll Cardiol 1999; 34:1537. 36. Naqvi, TZ, Goel, RK, Forrester, JS, et al. Usefulness of left ventricular mass in predicting recovery of left ventricular systolic function in patients with symptomatic idiopathic dilated cardiomyopathy. Am J Cardiol 2000; 85:624. Allman, KC, Shaw, LJ, Hachamovitch, R, Udelson, JE. Myocardial viability testing and impact of revascularization on prognosis in patients with coronary artery disease and left ventricular dysfunction: a meta-analysis. J Am Coll Cardiol 2002; 39:1151. Bart, BA, Shaw, LK, McCants, CB Jr, et al. Clinical determinants of mortality in patients with angiographically diagnosed ischemic or nonischemic cardiomyopathy. J Am Coll Cardiol 1997; 30:1002. Repetto, A, Bello, BD, Pasotti, M, et al. Coronary atherosclerosis in end-stage idiopathic dilated cardiomyopathy: an innocent bystander?. Eur Heart J 2005; 26:1519. Frustaci, A, Chimenti. C. Maseri, A. Global biventricular dysfunction in patients with asymptomatic coronary artery disease may be caused by myocarditis. Circulation 1999; 99:1295. Le Jemtel, TH, Padeletti, M, Jelic, S. Diagnostic and therapeutic challenges in patients with coexistent chronic obstructive pulmonary disease and chronic heart failure. J Am Coll Cardiol 2007; 49:171.
37.
38.
39. 40.
41.
GRAPHICS
Modified Framingham clinical criteria for the diagnosis of heart failure
Major
Paroxysmal nocturnal dyspnea Orthopnea Elevated jugular venous pressure Pulmonary rales Third heart sound Cardiomegaly on chest x-ray Pulmonary edema on chest x-ray Weight loss 4.5 kg in five days in response to treatment of presumed heart failure
Minor
Bilateral leg edema Nocturnal cough Dyspnea on ordinary exertion Hepatomegaly Pleural effusion Tachycardia (heart rate 120 beats/min) Weight loss 4.5 kg in five days
Diagnosis
The diagnosis of heart failure requires that 2 major or 1 major and 2 minor criteria cannot be attributed to another medical condition.
From Senni, M, Tribouilloy, CM, Rodeheffer, RJ, et al, Circulation 1998; 98:2282; ada pted from McKee, PA, Castelli, WP, McNamara, PM, Kannel, WB. N Engl J Med 1971; 85:1441.
Comparison of three methods of assessing cardiovascular disability
New York Heart Association functional classification Canadian Cardiovascular Society functional classification
Class
Specific activity scale
15 of 23
7/23/2008 7:14 PM
Clinical manifestations and evaluation of the patient with suspected heart ...
http://www.uptodate.com/online/content/topic.do?topicKey=hrt_fail/73...
Patients with cardiac disease but without resulting limitations of physical activity. Ordinary physical activity does not cause undue fatigue, palpitation, dyspnea, or anginal pain.
II
III
IV
Patients can perform to completion any activity requiring 7 metabolic equivalents, eg, can carry 24 lb up eight steps; do outdoor work (shovel snow, spade soil); do recreational activities (skiing, basketball, squash, handball, jog/walk 5 mph). Patients with cardiac disease Slight limitation of ordinary activity. Patients can perform to resulting in slight limitation Walking or climbing stairs rapidly, completion any activity of physical activity.They are walking uphill, walking or stair 5 metabolic requiring comfortable at rest. Ordinary climbing after meals, in cold, in wind, equivalents, e.g., have sexual physical activity results in intercourse without stopping, or when under emotional stress, or fatigue, palpitation, dyspnea, only during the few hours after garden, rake, weed, roller or anginal pain. skate, dance fox trot, walk at awakening. Walking more than two 4 mph on level ground, but blocks on the level and climbing cannot and do not perform to more than one flight of ordinary stairs at a normal pace and in normal completion activities requiring conditions. 7 metabolic equivalents. Patients with cardiac disease Marked limitation of ordinary Patients can perform to resulting in marked physical activity. Walking one to two completion any activity 2 metabolic limitation of physical blocks on the level and climbing requiring equivalents, eg, shower activity. They are more than one flight in normal without stopping, strip and comfortable at rest. Less conditions. make bed, clean windows, than ordinary physical walk 2.5 mph, bowl, play golf, activity causes fatigue, dress without stopping, but palpitation, dyspnea, or cannot and do not perform to anginal pain. completion any activities requiring > 5 metabolic equivalents. Patient with cardiac disease Inability to carry on any physical Patients cannot or do not resulting in inability to carry activity without discomfort - anginal perform to completion syndrome may be present at rest. on any physical activity activities requiring > 2 without discomfort. metabolic equivalents. Cannot Symptoms of cardiac carry out activities listed insufficiency or of the above (Specific activity scale anginal syndrome may be III). present even at rest. If any physical activity is undertaken, discomfort is increased.
Ordinary physical activity, such as walking and climbing stairs, does not cause angina. Angina with strenuous or rapid prolonged exertion at work or recreation.
ACC/AHA guidelines on management of chronic heart failure: stages in the evolution of heart failure and recommended therapy by stage
16 of 23
7/23/2008 7:14 PM
Clinical manifestations and evaluation of the patient with suspected heart ...
http://www.uptodate.com/online/content/topic.do?topicKey=hrt_fail/73...
Data from Hunt, SA, Abraham, WT, Chin, MH, et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult. Circulation 2005; 112:e154.
Major causes of congestive heart failure
Coronary artery disease Hypertension Cardiomyopathy
Idiopathic dilated cardiomyopathy Hypertrophic cardiomyopathy Alcohol Diabetes mellitus Viral - Coxsachie B, echovirus Infiltrative - amyloidosis, sarcoidosis Toxins - adriamycin Metabolic disorders - hypothyroidism Aging
Valvular heart disease Pericardial disease Incessant tachyarrhythmias High output states - hyperthyroidism, arteriovenous fistula
ACC/AHA guideline summary: Initial evaluation of patients with heart failure (HF)
Class I - There is evidence and/or general agreement that the initial evaluation of patients presenting with HF should include the following:
A complete history and physical examination to identify cardiac and noncardiac disorders or behaviors that might cause or accelerate the development or progression of HF. A careful history of current and past use of alcohol, illicit drugs, standard or "alternative" therapies, and chemotherapy drugs.
17 of 23
7/23/2008 7:14 PM
Clinical manifestations and evaluation of the patient with suspected heart ...
http://www.uptodate.com/online/content/topic.do?topicKey=hrt_fail/73...
An assessment of the ability to perform routine and desired activities of daily living. An assessment of the volume status, orthostatic blood pressure changes, height and weight, and calculation of body mass index. Laboratory studies including complete blood count, urinalysis, serum electrolytes (including calcium and magnesium), blood urea nitrogen, serum creatinine, fasting blood glucose (glycohemoglobin), lipid profile, liver function tests, and serum thyroid-stimulating hormone. A twelve-lead electrocardiogram and chest radiograph (posteroanterior and lateral). Two-dimensional echocardiography with Doppler to assess left ventricular ejection fraction (LVEF), left ventricular size, wall thickness, and valve function. Radionuclide ventriculography can be performed to assess LVEF and volumes. Coronary arteriography if there is a history or angina or significant ischemia unless the patient is not eligible for revascularization of any kind.
Class IIa - The weight of evidence or opinion is in favor of benefit from performing the following studies as part of the initial evaluation of patients presenting with HF:
Coronary arteriography in patients who have chest pain that may or may not be of cardiac origin who have not had a prior evaluation of their coronary anatomy and are eligible for coronary revascularization. Coronary arteriography in patients with known or suspected coronary artery disease who do not have angina and are eligible for revascularization. Noninvasive imaging to detect myocardial ischemia and viability in patients with known or suspected coronary artery who do not have angina and are eligible for revascularization. When the contribution of HF to exercise limitation is uncertain, maximal exercise testing with or without measurement of respiratory gas exchange and/or blood oxygen saturation. To identify candidates for cardiac transplantation or other advanced treatments, maximal exercise testing with measurement of respiratory gas exchange. In selected patients, screening for hemochromatosis, sleep disturbed breathing, or human immunodeficiency virus (HIV) infection. When suspected clinically, diagnostic tests for rheumatologic disease, amyloidosis, or pheochromocytoma. Endomyocardial biopsy when a specific diagnosis is suspected that would influence therapy. Measurement of serum B-type natriuretic peptide (BNP) in the urgent care setting if the clinical diagnosis of HF is uncertain.
Class IIb - The weight of evidence or opinion is less well established for the following testing in the initial evaluation of patients with HF
Noninvasive imaging to define the likelihood of coronary artery disease in patients with left ventricular dysfunction. Holter monitoring in patients who have a history of myocardial infarction and are being considered for electrophysiologic study to document the inducibility of ventricular tachycardia.
Class III - There is evidence and/or general agreement that the following tests are not useful or may be harmful in the initial evaluation of patients with HF
Routine endomyocardial biopsy in the absence of suspicion of a specific diagnosis that would influence therapy suspected. Routine signal-averaged electrocardiography. Routine measurement of serum neurohormones other than BNP (eg, norepinephrine or endothelin).
Data from Hunt, SA, Abraham, WT, Chin, MH, et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation 2005; 112:e154.
Flow diagram for the work-up of patients with dilated cardiomyopathy
18 of 23
7/23/2008 7:14 PM
Clinical manifestations and evaluation of the patient with suspected heart ...
http://www.uptodate.com/online/content/topic.do?topicKey=hrt_fail/73...
ECG: electrocardiography; echo: echocardiography. * The timing of endomyocardial biopsy in patients who fail to improve on medical therapy is controversial. If the patient's left ventricular function and symptoms are not stable after one week of treatment, additional time before endomyocardial biopsy could be considered, if appropriate in the treating physician's clinical judgment, to allow for delayed recovery to occur.
Reproduced with permission from: Wu, LA, Lapeyre, AC 3rd, Cooper, LT. Mayo Clin Proc 2001; 76:1030. Copyright 2001 Mayo Clinic Proceedings.
Plasma BNP in the diagnosis of dyspnea
19 of 23
7/23/2008 7:14 PM
Clinical manifestations and evaluation of the patient with suspected heart ...
http://www.uptodate.com/online/content/topic.do?topicKey=hrt_fail/73...
Shown are the plasma B-type natriuretic peptide (BNP) concentrations in patients presenting with acute dyspnea who are diagnosed noncardiac causes without or with a history of left ventricular (LV) dysfunction, or heart failure (HF). Patients with a diagnosis of HF have significantly higher median values of plasma BNP compared to those without heart failure (675 versus 110 pg/mL). The boxes show the interquarile ranges, and the I bars represent the highest and lowest values. Data from Maisel,
AS, Krishnaswamy, P, Nowak, RM, et al, N Engl J Med 2002; 347:161.
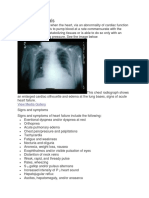
Cardiomyopathy Lateral
Cardiomyopathy
This plain frontal radiograph of the chest in a 51-year-old male demonstrates marked enlargement of the cardiac silhouette compatible with a cardiomyopathy. Cardiomegaly is nonspecific and can be seen with any etiology of cardiomyopathy. Courtesy of Jonathan Kruskal, MD, PhD.
Heart failure PA I
Congestive heart failure
20 of 23
7/23/2008 7:14 PM
Clinical manifestations and evaluation of the patient with suspected heart ...
http://www.uptodate.com/online/content/topic.do?topicKey=hrt_fail/73...
This chest radiograph of a 65-year-old male with dyspnea and orthopnea demonstrates mild pulmonary vascular congestion, septal lymphatic distention (white arrow), interstitial veiling, and enlarged hilar shadows (black arrow), indicative of left ventricular decompensation. Courtesy of
Jonathan Kruskal, MD.
Interstitial pulmonary edema PA
Pulmonary edema
This plain frontal chest radiograph of a 55-year-old male with known coronary artery disease demonstrates characteristic radiographic features of heart failure with interstitial pulmonary edema, bilateral perihilar alveolar edema producing a characteristic butterfly pattern and bilateral pleural effusions. Photo courtesy of Jonathan Kruskal, MD.
Heart failure PA II
Severe heart failure
This chest radiography shows severe heart failure with cardiomegaly,
21 of 23
7/23/2008 7:14 PM
Clinical manifestations and evaluation of the patient with suspected heart ...
http://www.uptodate.com/online/content/topic.do?topicKey=hrt_fail/73...
pulmonary vascular congestion with infiltrates in the mid lung fields (white arrow), and a small pleural effusion (black arrow). Courtesy of
Jonathan Kruskal, MD.
Cardiogenic pulmonary edema
Pulmonary edema in a "butterfly distribution" due to left ventricular failure. Chest radiograph shows large perihilar opacities in patient with enlarged cardiac silhouette. Courtesy of Paul Stark, MD.
Dilated cardiomyopathy
M-mode scan of the heart is obtained by moving the transducer from a cephalad to caudal direction, recording the aortic root, mital valve and mitral valve annulus, and the left ventricular chamber. In this patient with a severe dilated cardiomyopathy, there is significant enlargement of the left atrium and left ventricle. In addition, the septum and posterior left ventricular wall are thinned and hypokinetic.
Mitral valve motion in dilated cardiomyopathy
22 of 23
7/23/2008 7:14 PM
Clinical manifestations and evaluation of the patient with suspected heart ...
http://www.uptodate.com/online/content/topic.do?topicKey=hrt_fail/73...
M-mode echocardiogram, recorded at the level of the mitral valve leaflets, shows a dilated left ventricle. The interventricular septum and posterior left ventricular wall are thinned and hypokinetic. The movement of both the anterior and posterior mitral valve leaflets is well seen and there are prominent echoes from the chordae tendinae due to the enlarged left ventricular chamber.
Pulsed wave Doppler flow signals from the left ventricular outflow tract
The pulsed wave Doppler signal is obtained from the anteriorly angulated apical four chamber view. The arrow indicates the dashed line positioned by the sonographer to outline the signal. The area under the curve described by the tracing is integrated and expressed as the velocity time integral (VTI). In this example, the value of 0.16 M or 16 cm represents an abnormally low value (normal about 20 cm). 2008 UpToDate, Inc. All rights reserved. | Subscription and License Agreement | Support Tag: [ecapp1003p.utd.com-82.76.6.152-6EBAB4CF9B-2579] Licensed to: UpToDate Guest Pass | Your UpToDate subscription will expire in 9 day(s). Click here to
renew your subscription.
23 of 23
7/23/2008 7:14 PM
Вам также может понравиться
- Valvular Heart DiseaseДокумент85 страницValvular Heart DiseaseWilliam Lie100% (2)
- Shelf IM Video SlidesДокумент69 страницShelf IM Video SlidesRuth SanmooganОценок пока нет
- Aortic RegurgitationДокумент19 страницAortic RegurgitationsunilgenextОценок пока нет
- 6th Central Pay Commission Salary CalculatorДокумент15 страниц6th Central Pay Commission Salary Calculatorrakhonde100% (436)
- 3 Heart InternetNewДокумент55 страниц3 Heart InternetNewCoral Srinivasa RamaluОценок пока нет
- Paces Guide 2012 From Online NotesДокумент228 страницPaces Guide 2012 From Online NotesThistell ThistleОценок пока нет
- Cardiology Plabable PDFДокумент22 страницыCardiology Plabable PDFTirtha Taposh100% (1)
- Pathology Board QuestionsДокумент86 страницPathology Board QuestionsJulius Matthew LuzanaОценок пока нет
- Mcqs Faculty of General Medicine VI YearДокумент110 страницMcqs Faculty of General Medicine VI YearAbdimajiid100% (2)
- Adult Cardiac Surgery - Nursing Care and ManagementДокумент225 страницAdult Cardiac Surgery - Nursing Care and ManagementOsama Elsayed AhmedОценок пока нет
- Seminar On CV Thoracic SurgeriesДокумент81 страницаSeminar On CV Thoracic SurgeriesMegha lakraОценок пока нет
- Inotropes in Cardiothoracic SurgeryДокумент44 страницыInotropes in Cardiothoracic SurgeryMarce8118100% (1)
- Congestive Heart FailureДокумент19 страницCongestive Heart FailureIlavenil PanduranganОценок пока нет
- Heart Failure: Amanda Ryan, D.O. Cardiology Fellow February 14th, 2008Документ87 страницHeart Failure: Amanda Ryan, D.O. Cardiology Fellow February 14th, 2008IfaОценок пока нет
- Ventricular Assist DeviceДокумент12 страницVentricular Assist DevicesamadonyОценок пока нет
- Jansenkoh MRCP PacesДокумент209 страницJansenkoh MRCP PacesBob Yong83% (6)
- Cardiology - Valvular Disease PDFДокумент2 страницыCardiology - Valvular Disease PDFPshtiwan MahmoodОценок пока нет
- Pathophysiology of Cardiogenic Pulmonary EdemaДокумент8 страницPathophysiology of Cardiogenic Pulmonary EdemaLili Fiorela CRОценок пока нет
- Heart Failure: Clinical Manifestations and Diagnosis in Adults - UpToDateДокумент44 страницыHeart Failure: Clinical Manifestations and Diagnosis in Adults - UpToDateJulia Pelissaro CarboniОценок пока нет
- Heart Failure - Clinical Manifestations and Diagnosis in Adults - UpToDateДокумент39 страницHeart Failure - Clinical Manifestations and Diagnosis in Adults - UpToDateArthurОценок пока нет
- Congestive Heart Failure: Diagnosis, Pathophysiology, Therapy, and Implications For Respiratory CareДокумент10 страницCongestive Heart Failure: Diagnosis, Pathophysiology, Therapy, and Implications For Respiratory CareKevinDilianSugandaОценок пока нет
- Insuficiência Ventricular DireitaДокумент20 страницInsuficiência Ventricular Direitayvvs5f9m4mОценок пока нет
- CHFДокумент10 страницCHFPowpOw SangalangОценок пока нет
- Ghid Heart FailureДокумент117 страницGhid Heart FailureIlie RoxanaОценок пока нет
- Congestive Heart Failure: Diagnosis, Pathophysiology, Therapy, and Implications For Respiratory CareДокумент10 страницCongestive Heart Failure: Diagnosis, Pathophysiology, Therapy, and Implications For Respiratory CareIntan PurnamasariОценок пока нет
- Heart FailureДокумент8 страницHeart FailureSophia MarieОценок пока нет
- Approach To Cardiac PatientДокумент6 страницApproach To Cardiac PatientDemuel Dee L. BertoОценок пока нет
- Heart Failure OlderДокумент18 страницHeart Failure OlderRESIDENTES MEDICINA INTERNAОценок пока нет
- 403 Full PDFДокумент10 страниц403 Full PDFKuroto YoshikiОценок пока нет
- Jurnal Anes 1Документ7 страницJurnal Anes 1Chanvira Aria CandrayanaОценок пока нет
- Heart FailureДокумент11 страницHeart FailureWeqy Dwi PermataОценок пока нет
- I. Acute Heart Failure: What Every Physician Needs To KnowДокумент13 страницI. Acute Heart Failure: What Every Physician Needs To KnowRubie Ann TillorОценок пока нет
- Congestive Heart Failure: Diagnosis, Pathophysiology, Therapy, and Implications For Respiratory CareДокумент10 страницCongestive Heart Failure: Diagnosis, Pathophysiology, Therapy, and Implications For Respiratory CareMirachel AugustОценок пока нет
- Lec 4Документ9 страницLec 4fbbqbcht6yОценок пока нет
- Chronic Heart FailureДокумент11 страницChronic Heart FailurelaydyОценок пока нет
- Jurnal Print 3Документ17 страницJurnal Print 3Elok ZakiyyaОценок пока нет
- CH 011Документ16 страницCH 011Arash SamieiОценок пока нет
- Clinical PresentationsДокумент37 страницClinical PresentationsJim Christian EllaserОценок пока нет
- Clinical Manifestations and Diagnosis of Advanced Heart FailureДокумент11 страницClinical Manifestations and Diagnosis of Advanced Heart FailureJorge Silva MineiroОценок пока нет
- Jurnal NCBI Gagal JantungДокумент16 страницJurnal NCBI Gagal JantungMAS MANTRIОценок пока нет
- Chronicheartfailure Inolderadults: Ali AhmedДокумент23 страницыChronicheartfailure Inolderadults: Ali AhmedRaul LaraОценок пока нет
- Heart Failure in Older Adults PDFДокумент23 страницыHeart Failure in Older Adults PDFcuisilaisОценок пока нет
- Congestive Heart FailureДокумент16 страницCongestive Heart FailureRahmat Burhanudin RamdaniОценок пока нет
- Falla Cardiaca ReviewДокумент9 страницFalla Cardiaca ReviewYasmin CarhuamacaОценок пока нет
- Heart FailureДокумент70 страницHeart FailureNabin BhusalОценок пока нет
- Heart Failure Differential DiagnosesДокумент3 страницыHeart Failure Differential DiagnosesAizel ManiagoОценок пока нет
- Acute Lung Edema Management PracticeДокумент5 страницAcute Lung Edema Management PracticeYunia DuanaОценок пока нет
- Clinical Manifestations and Diagnosis of Edema in AdultsДокумент7 страницClinical Manifestations and Diagnosis of Edema in AdultsOscar Castro CuaquiraОценок пока нет
- CHF PathophysiologyДокумент4 страницыCHF PathophysiologyVirtudazo JessaОценок пока нет
- EPP SEMESTER 6 Baru BNGTДокумент8 страницEPP SEMESTER 6 Baru BNGTLisa FitrianiОценок пока нет
- ThiroidДокумент9 страницThiroidAhmed EmadОценок пока нет
- De Novo Acute Heart Failure and Acutely Decompensated Chronic Heart Failure PDFДокумент14 страницDe Novo Acute Heart Failure and Acutely Decompensated Chronic Heart Failure PDFEffika PutriОценок пока нет
- CHF PathДокумент7 страницCHF PathGeraldine Gallaron - CasipongОценок пока нет
- Congestive Heart Failure in The ElderlyДокумент13 страницCongestive Heart Failure in The ElderlyKezia MarsilinaОценок пока нет
- Clinical Manifestations and Diagnosis of Advanced Heart Failure - UpToDateДокумент17 страницClinical Manifestations and Diagnosis of Advanced Heart Failure - UpToDatearturitouОценок пока нет
- Congestive Heart Failure: Martin M. ZdanowiczДокумент6 страницCongestive Heart Failure: Martin M. ZdanowiczAnonymous wpUzJBОценок пока нет
- Stenoza AorticaДокумент31 страницаStenoza Aorticamicaella07Оценок пока нет
- Clinical Features and Diagnosis of Lower Extremity Peripheral Artery DiseaseДокумент16 страницClinical Features and Diagnosis of Lower Extremity Peripheral Artery DiseasePetru GlavanОценок пока нет
- Insufic Cardiaca 1Документ13 страницInsufic Cardiaca 1Ronaldo DuarteОценок пока нет
- Heart FailureДокумент36 страницHeart FailurenathanОценок пока нет
- Essential Heart FailureДокумент24 страницыEssential Heart FailuredeffyОценок пока нет
- Heart Failure Practice EssentialsДокумент12 страницHeart Failure Practice EssentialsdeffyОценок пока нет
- Chronic Heart Failure CHFДокумент11 страницChronic Heart Failure CHFChen BrionesОценок пока нет
- Etiology: Table 279-1Документ31 страницаEtiology: Table 279-1CatharinaNoviaОценок пока нет
- SINCOPE Journal of Internal MedicineДокумент9 страницSINCOPE Journal of Internal MedicineIlse MarquezОценок пока нет
- Heart Failure With Preserved Ejection Fraction - Concept, Pathophysiology, Diagnosis and Challenges For TreatmentДокумент7 страницHeart Failure With Preserved Ejection Fraction - Concept, Pathophysiology, Diagnosis and Challenges For TreatmentOngky AristianОценок пока нет
- Acute Decompensated Heart Failure: ReviewДокумент11 страницAcute Decompensated Heart Failure: ReviewFercee PrimulaОценок пока нет
- Etiology: Low-Output Versus High-Output FailureДокумент7 страницEtiology: Low-Output Versus High-Output FailureFitri Tri PutriОценок пока нет
- Clinical Features and Complications: ABC of Heart FailureДокумент4 страницыClinical Features and Complications: ABC of Heart FailureMohammed Ali Haydar JafferiОценок пока нет
- Heart Failure Clinical Presentation - History, Physical Examination, Predominant Right-Sided Heart Failure PDFДокумент11 страницHeart Failure Clinical Presentation - History, Physical Examination, Predominant Right-Sided Heart Failure PDFislamiah 89Оценок пока нет
- Acute Heart FailureДокумент9 страницAcute Heart FailureChen Briones100% (1)
- Clinical Cases in Heart FailureОт EverandClinical Cases in Heart FailureRavi V. ShahОценок пока нет
- Public Health and Legal Implications of OSA PDFДокумент9 страницPublic Health and Legal Implications of OSA PDFcristianamihailaОценок пока нет
- Pathophysiology of OSAДокумент20 страницPathophysiology of OSAMihaela-Alexandra PopОценок пока нет
- Ambulatory Diagnosis of OSA and New Technologies PDFДокумент14 страницAmbulatory Diagnosis of OSA and New Technologies PDFcristianamihailaОценок пока нет
- Uptodate Cor Pulmonale PDFДокумент13 страницUptodate Cor Pulmonale PDFcristianamihailaОценок пока нет
- Pathophysiology of Heart Failure - Neurohumoral Adaptations PDFДокумент10 страницPathophysiology of Heart Failure - Neurohumoral Adaptations PDFcristianamihailaОценок пока нет
- VHD Guidelines Slide Set GL VHDДокумент220 страницVHD Guidelines Slide Set GL VHDNASERZALLOUMОценок пока нет
- Cardiac Radiology.Документ13 страницCardiac Radiology.Haluk AlibazogluОценок пока нет
- Talley Sum UpДокумент51 страницаTalley Sum UpRozana Bawareth100% (1)
- 2012 MitraClip For The Treatment of Mitral RegurgitationДокумент11 страниц2012 MitraClip For The Treatment of Mitral RegurgitationThanh BinhОценок пока нет
- Requirements For European Class 3 Medical Certification of Air Traffic ControllersДокумент56 страницRequirements For European Class 3 Medical Certification of Air Traffic ControllersmalidalkiranОценок пока нет
- Ms 1 Cardiac DiseasesДокумент65 страницMs 1 Cardiac DiseasesWilliam ApostolОценок пока нет
- Valvular Heart Disease Def PDFДокумент3 страницыValvular Heart Disease Def PDFAfif Al BaalbakiОценок пока нет
- Person Vue 8 2016-1Документ49 страницPerson Vue 8 2016-1Adrian JamalОценок пока нет
- Vezzosi 2021Документ9 страницVezzosi 2021wilverОценок пока нет
- P Wave AbnormalitiesДокумент20 страницP Wave AbnormalitiesToufiqurRahmanОценок пока нет
- Valvular Esc Book ChapterДокумент96 страницValvular Esc Book ChapterkikiwulandariОценок пока нет
- Pathophysiology of Cardiogenic Pulmonary Edema - UpToDateДокумент14 страницPathophysiology of Cardiogenic Pulmonary Edema - UpToDateStefani AtlleОценок пока нет
- Vena Contracta Width Is A Semi-Quantitative Parameter To Assess The Severity of Mitral RegurgitationДокумент20 страницVena Contracta Width Is A Semi-Quantitative Parameter To Assess The Severity of Mitral Regurgitationalex leeОценок пока нет
- 38 Endocardial Cushion DefectsДокумент12 страниц38 Endocardial Cushion DefectsVictor PazОценок пока нет
- Comprehensive Nursing Management For Valvular DiseaseДокумент8 страницComprehensive Nursing Management For Valvular Diseasezaqqi ubaidillahОценок пока нет
- IVMS ICM-Heart MurmursДокумент22 страницыIVMS ICM-Heart MurmursMarc Imhotep Cray, M.D.Оценок пока нет