Академический Документы
Профессиональный Документы
Культура Документы
Chemical Kinetics Exam
Загружено:
Mišel VuittonАвторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
Chemical Kinetics Exam
Загружено:
Mišel VuittonАвторское право:
Доступные форматы
Chemical Kinetics Exam
Name:______________________________ Part A: Multiple Choice Value: 42 marks Suggested Time: 60 minutes INSTRUCTIONS: For each question, select the best answer and record your choice on the Response Form provided. Using an HB pencil, completely fill in the circle that has the letter corresponding to your answer. 1. Consider the reaction: Ca(s) + 2H2O(l) Ca(OH)2(aq) + H2(g) At a certain temperature 2.!" g Ca reacts completel# in $"." seconds. %he rate o& consumption o& Ca is A. ".""2"' mol ( min ). "."'$$ mol ( min C. ".12! mol ( min *. !."" mol ( min 2. %he minimum amount o& energ# required to o+ercome the energ# ,arrier in a chemical reaction is the A. heat o& reaction. ). acti+ation energ#. C. -. o& the reactants. *. enthalp# o& the products. $. An acti+ated comple/ is a chemical species that is A. sta,le and has lo0 1.. ). sta,le and has high 1.. C. unsta,le and has lo0 1.. *. unsta,le and has high 1.. 2. Consider the &ollo0ing reaction mechanism: 3tep 1: 2NO + H2 N2 + H2O2 3tep 2: H2O2 + H2 2H2O 4n this reaction H2 is a A. product. ). catal#st. C. reactant.
*. reaction intermediate. !. A certain reaction is a,le to proceed ,# +arious mechanisms. .ach mechanism has a di&&erent .a and results in a di&&erent o+erall rate. 5hich o& the &ollo0ing ,est descri,es the relationship ,et0een the .a +alues and the rates6
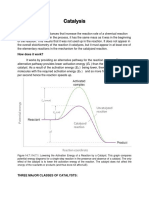
7. Consider the following P diagram!
"he forward reaction can #e descri#ed as H $k%& + 2" + 2" 8 2" 8 2" Activati n T!"e # Energ! $k%& 'eacti n '" endothermic 7" e/othermic '" e/othermic 1"" endothermic
A. ). C. *.
9. %he slo0est o& the &ollo0ing reactions is A. Ag(aq)+ + Cl8(aq) AgCl(s) ) ). H$O+(aq) + OH8(aq) 2H2O(l C C. $)a2+(aq) + 21O2$8(aq) )a$(1O2)2(s) *. Cu(s) + 2Ag+(aq) Cu2+(aq) + 2Ag(s) '. %he rate o& a chemical reaction is equal to the slope o& a graph 0ith the a/es la,elled x(axis y(axis A. time rate ). mass time C. +olume o& gas time *. time concentration :. Consider the &ollo0ing reaction: CH2(g) + 2O2(g) CO2(g) + 2H2O(g) + heat %he diagram 0hich represents the relationship ,et0een rate and temperature is:
1". 5hich o& the &ollo0ing descri,es the energ# o& colliding particles as reacting molecules approach each other6 KE )E A. decreases increases ). increases decreases C. decreases remains constant *. remains constant increases
11. %he a+erage ;inetic energ# o& colliding particles can ,e increased ,# A. adding a catal#st. ). increasing pressure. C. increasing temperature. *. increasing reactant concentration. 12. $ su#stance that increases the rate of a chemical reaction and may #e recovered unchanged at the end of the reaction is a%n& $. product. B. catalyst. C. activated comple'. (. reaction intermediate. )*. Consider the following P diagram for a reversi#le reaction!
+hich of the following descri#es this reaction, *irecti n A. ). C. *. re+erse &or0ard &or0ard re+erse Activati n Energ! $k%& $" 2" $" 2" H $k%& 8 1" 8 1" + 1" + 1"
12. +hich of the following can #e used to represent the rate of a reaction, $. g - . B. g - mol C. %g / min& - mol (. 0 - min
1!. Consider the &ollo0ing reaction: 2H2O2(l) 2H2O(l) + O2(g) 5hich graph sho0s the relationship ,et0een rate o& consumption o& H2O2 and time6
17. Consider the &ollo0ing reaction: <g(s) + 2HCl(aq) H2(g) + <gCl2(aq) %he rate o& this reaction increases 0hen more magnesium is added. %his change is caused ,# the A. addition o& a catal#st. ). increase in sur&ace area. C. change in nature o& the reactants. *. increase in concentration o& reactants. 19. A catal#st changes the rate o& a reaction ,# A. changing H. ). increasing the temperature. C. decreasing the energ# o& the products. *. pro+iding an alternate reaction mechanism.
1'. 5hich graph sho0s the relationship ,et0een acti+ation energ# (.a) and temperature6
1:. Consider the &ollo0ing reaction: CH2(g) + 2O2(g) CO2(g) + 2H2O(g) At a certain temperature 1." mol CH2 is consumed in 2." minutes. %he rate o& production o& H2O is A. ".2! mol ( min ). ".!" mol ( min C. 2." mol ( min *. '." mol ( min 2". %he changes in 1. and -. as reactant molecules approach each other can ,e represented ,#
21. Consider the &ollo0ing 1. diagram:
+hich of the following descri#es this reaction, H $k%& 8 2" 8 2" + 2" + 2" Activati n Energ! $k%& 2" 7" 2" 7" 'eacti n catal#=ed catal#=ed uncatal#=ed uncatal#=ed
A. ). C. *.
22. $ chemical reaction that gives off energy is $. e'othermic and H is positive. B. e'othermic and H is negative. C. endothermic and H is positive. (. endothermic and H is negative. 2$. Consider the &ollo0ing reaction in+ol+ing 1." g o& po0dered =inc: >n(s) + 2HCl(aq) >nCl2(aq) + H2(g)
Trial 1 2 3 Temperature ( C) 40 20 40 Concentration of HCl (M) 3.0 3.0 6.0
%he rates in order o& &astest to slo0est are A. 1 2 $ ). 2 1 $
C. $ 1 2 *. $ 2 1 12. Acti+ation energ# can ,e descri,ed as the A. energ# o& motion. ). energ# o& the acti+ated comple/. C. energ# di&&erence ,et0een the reactants and the products. *. energ# di&&erence ,et0een the reactants and the acti+ated comple/. 2!. 4ncreasing the temperature o& a reaction increases the reaction rate ,# 4. increasing &requenc# o& collisions 44. increasing the ;inetic energ# o& collision 444. decreasing the potential energ# o& collision A. ). C. *. 4 onl#. 4 and 44 onl#. 44 and 444 onl#. 4 44 and 444.
27. Consider the graph &or the &ollo0ing reaction: CaCO$(s) + 2HCl(aq) CaCl2(aq) + CO2(g) + H2O(l)
%he a+erage rate o& reaction is greatest in the time inter+al A. " 8 1 minute. ). " 8 2 minutes. C. " 8 $ minutes. *. " 8 2 minutes. 29. Consider the &ollo0ing mechanism &or a reaction: 3tep 1: H)r + O2 HOO)r 3tep 2: H)r + HOO)r 2HO)r 3tep $: 2HO)r + 2H)r 2)r2 + 2H2O 5hich o& the &ollo0ing statements is correct6
A. )r2 is a reactant. ). H)r is a product. C. HO)r is a catal#st. *. HOO)r is a reaction intermediate. 2'. Consider the &ollo0ing reaction: >n(s) + 2HCl(aq) >nCl2(aq) + H2(g) A graph o& concentration o& HCl +s time could ,e represented ,#
2:. Consider the &ollo0ing e/periments each in+ol+ing equal masses o& =inc and 1"." m? o& acid:
%he rate o& reaction in order &rom &astest to slo0est is A. 4 @ 44 @ 444 ). 44 @ 4 @ 444 C. 444 @ 4 @ 44 *. 444 @ 44 @ 4
$1. Consider the &ollo0ing reaction mechanism: 3tep 1: ClO8 + H2O HClO + OH8 3tep 2: 48 + HClO H4O + Cl8 3tep $: H4O + OH8 4O8 + H2O %he catal#st is A. 4O8 ). H2O C. ClO8 *. HClO $2. %he rate8determining step in a reaction mechanism is al0a#s the A. last step. ). &irst step. C. &astest step. *. slo0est step $$. %he addition o& a catal#st to an e/othermic reaction increases the rate o& the reaction ,ecause it decreases the A. H ). acti+ation energ# C. H and the acti+ation energ# *. acti+ation energ# and the amount o& energ# released. +se the # ll ,ing " tential energ! diagram t ans,er -uesti n .4/
$2. %he a,o+e energ# diagram represents a &our step reaction mechanism. 5hich is the rate determining step6 A. 1
). 2 C. $ *. 2 $!. Consider the &ollo0ing two3step reaction mechanism! 4tep )! 2 + 2 56 7 56* %slow& 4tep 1! 56* + C6 561 + C61 %fast& +hich one of the following changes would result in the greatest increase in reaction rate, $. increase 8C69 B. decrease 8569 C. increase 85619 (. decrease 856*9 $7. 5hich o& the &ollo0ing &actors a&&ects the rate o& heterogeneous reactions ON?A6 A. 1resence o& a catal#st. ). %emperature o& the reactants. C. 3ur&ace area o& reactants. *. Concentration o& reactants. $9. Consider the &ollo0ing reaction: CaCO$(s) + 2HCl(aq) CO2(g) + CaCl2(aq) + H2O(l) + heat 4n 0hich o& the &ollo0ing 0ill )O%H o& the descri,ed procedures cause an increase in the rate at 0hich products are &ormed6 A. 4ncrease BHClC and decrease pressure. ). 4ncrease BHClC and increase temperature. C. 4ncrease BHClC and decrease temperature. *. Drind up the CaCO$ and decrease temperature. $'. %he &ollo0ing equation represents the reaction ,et0een copper metal and aqueous sil+er nitrate solution: Cu(s) + 2AgNO$(aq) Cu(NO$)2(aq) + 2Ag(s) ,ro0n colorless ,lue gre# solid solution solution solid 5hich o& the &ollo0ing properties 0ould ).3% monitor the rate o& this reaction6 A. Concentration o& NO$8(aq) ). Das pressure. C. Color o& the solution. *. <ass o& the s#stem. $:. (ust particles suspended in the air inside unheated grain elevators can sometimes
react e'plosively #ecause the dust particles have a $. high :inetic energy. B. high activation energy. C. catalytic effect on the reaction. (. large surface area for the reaction. 2". Consider the &ollo0ing collisions each occurring at the same temperature:
+hich one of the following factors e'plains why collision one is successful while collision two is not successful, $. Catalyst. B. ;eometry. C. Concentration. (. <inetic energy. Use the following diagram to answer questions 41 and 42
2). 4elect the true statement concerning the a#ove potential energy diagram.
$. "he cataly=ed reaction has a larger H. B. "he uncataly=ed reaction has a larger H. C. "he cataly=ed reaction has a greater rate of reaction. (. "he uncataly=ed reaction has a greater rate of reaction. 21. +hich point on the diagram a#ove represents the potential energy of the activated comple' formed in the uncataly=ed reaction, $. > B. >> C. >>> (. >? 2$. %he class that #ou attend e+er# %uesda# and %hursda# &rom 7:2! to ::2! pm and 0ouldnEt miss &or the 0orld (e+en on Hallo0een and Christmas) is A. %ric; or %reating 12 ). 3anta 12 C. Chemistr# 12 *. )ite <e 12 22. %he instructor &or this course is A. %he Drinch ). Fac; O. ?antern C. <r. Cho# *. .aster )unn# Part B: Written Response Value: 26 marks Suggested Time: 40 minutes 01ST'+CT021S: Aou 0ill ,e e/pected to communicate #our ;no0ledge and understanding o& chemical principles in a clear and logical manner. Aour steps and assumptions leading to a solution must ,e 0ritten in the spaces ,elo0 the questions. Ans0ers must include units 0here appropriate and ,e gi+en to the correct num,er o& signi&icant &igures. 3 r -uesti ns inv lving calculati n4 #ull marks ,ill 12T 5e given # r "r viding nl! an ans,er/ 1. Consider the &ollo0ing reaction mechanism: 3tep 1 3tep 2 3tep $ 3tep 2 6 H2 + Cl HCl + H H + Cl2 HCl + Cl Cl + Cl Cl2
O+erall a) 5rite the equation &or 3tep 1.
H2 + Cl2 2HCl $2 marks&
,) *e&ine the term Greaction intermediateH and identi&# the reaction intermediate(s) in the a,o+e reaction mechansim. $2 marks&
1. $n e'periment is done to determine the rate of the following reaction! 1$l%s& 7 @HCl%aq& *H1%g& 7 1$lCl*%aq&
a& Calculate the rate of formation of H1 in mol-min. !2 mar"s#
#& Calculate the rate of consumption of $l in g-sec. !2 mar"s#
*. Consider the following reaction! CaC6*%s& 7 1H7%aq& Ca17%aq& 7 H16%l& 7 C61%g& a& .ist four factors that would increase the rate of the a#ove reaction. !4 mar"s# i. ii. iii. iv. #& Using collision theory, e'plain how each of these four factors increase the rate of the reaction. !4 mar"s# i.
ii.
iii.
iv.
2. Consider this reaction! 0gC6*%s& 7 1HCl%aq& 0gCl1%aq& 7 H16%l& 7 C61%g& 4uggest two properties which could #e used to determine the rate of this reaction, and state what would happen to each property as the reaction proceeds. !4 mar"s# i.
ii.
A. (escri#e the relationship #etween activation energy and the rate of a chemical reaction. !2 mar"s#
7. Consider the following potential energy diagram for a reversi#le reaction!
a& Calculate the activation energy for the forward reaction. !1 mar"#
#& Calculate H for the forward reaction. !1 mar"#
c& Calculate the activation energy for the reverse reaction. !1 mar"#
d& 6n the diagram a#ove, s:etch a curve that could result when a catalyst is added. !1 mar"#
Part C: Bonus Question ). Consider the following < distri#ution curve for colliding
particles! a) On the diagram a,o+e s;etch a line &or the distri,ution o& collisions at a higher temperature. $2 marks& * ,) 3hade in the area representing the collisions that could result in &orming an acti+ated comple/ at the lo0er temperature. $6 mark&
Вам также может понравиться
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeОт EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeРейтинг: 4 из 5 звезд4/5 (5794)
- The Little Book of Hygge: Danish Secrets to Happy LivingОт EverandThe Little Book of Hygge: Danish Secrets to Happy LivingРейтинг: 3.5 из 5 звезд3.5/5 (399)
- UV Damaged DNA Repair & Tolerance in PlantsДокумент25 страницUV Damaged DNA Repair & Tolerance in PlantsMišel VuittonОценок пока нет
- DNA Repair Mechanisms in Plants Bray - Et - Al-2005-New - PhytologistДокумент18 страницDNA Repair Mechanisms in Plants Bray - Et - Al-2005-New - PhytologistMišel VuittonОценок пока нет
- The Disease The Cure Imam Ibn Al Qayyim Compressed - CroppedДокумент553 страницыThe Disease The Cure Imam Ibn Al Qayyim Compressed - CroppedMišel Vuitton100% (1)
- Chapter 8 Measuring The Cost of Living (CPI)Документ31 страницаChapter 8 Measuring The Cost of Living (CPI)Mišel VuittonОценок пока нет
- 9 PhytochemalphazamДокумент7 страниц9 PhytochemalphazamMišel VuittonОценок пока нет
- Genetics and Bioengineering CoursesДокумент10 страницGenetics and Bioengineering CoursesMišel VuittonОценок пока нет
- 3.2.23 Bio418 VirologyДокумент1 страница3.2.23 Bio418 VirologyMišel VuittonОценок пока нет
- DNA Repair Pathways and MechanismsДокумент15 страницDNA Repair Pathways and MechanismsAxel Trinidad LopezОценок пока нет
- DNA Damage Repair in The Context of Plant ChromatinДокумент13 страницDNA Damage Repair in The Context of Plant ChromatinMišel VuittonОценок пока нет
- Articulo PDFДокумент10 страницArticulo PDFDaniela Guerrero.Оценок пока нет
- Protein Structure Prediction: Faruk Berat AkcesmeДокумент44 страницыProtein Structure Prediction: Faruk Berat AkcesmeMišel VuittonОценок пока нет
- Chapter 3Документ5 страницChapter 3Mišel VuittonОценок пока нет
- 6th Central Pay Commission Salary CalculatorДокумент15 страниц6th Central Pay Commission Salary Calculatorrakhonde100% (436)
- 9701 Chemistry Applications BookletДокумент161 страница9701 Chemistry Applications BookletZain RehanОценок пока нет
- 9700 s03 QP 2Документ12 страниц9700 s03 QP 2Mišel VuittonОценок пока нет
- 9700 s06 QP 2Документ16 страниц9700 s06 QP 2Mišel VuittonОценок пока нет
- Aqa Ad03 W PM Jun06Документ12 страницAqa Ad03 W PM Jun06Mišel VuittonОценок пока нет
- 9700 s03 Ms 1+2+3+4+5+6Документ37 страниц9700 s03 Ms 1+2+3+4+5+6Mišel VuittonОценок пока нет
- 6th Central Pay Commission Salary CalculatorДокумент15 страниц6th Central Pay Commission Salary Calculatorrakhonde100% (436)
- His Throne Was On Water PDFДокумент145 страницHis Throne Was On Water PDFArfaq HussainОценок пока нет
- 9701 s13 Ms 21Документ7 страниц9701 s13 Ms 21Mišel VuittonОценок пока нет
- GermanДокумент40 страницGermanMišel VuittonОценок пока нет
- Syllabus: Cambridge International AS and A Level BiologyДокумент72 страницыSyllabus: Cambridge International AS and A Level BiologysandsphilipОценок пока нет
- 1.4 Assessed Homework Mark Scheme: 10.4 Periodicity HW MSДокумент2 страницы1.4 Assessed Homework Mark Scheme: 10.4 Periodicity HW MSMišel VuittonОценок пока нет
- 9702 Y14 SyДокумент68 страниц9702 Y14 SyMišel VuittonОценок пока нет
- Bonding, Structure and Periodicity TestДокумент9 страницBonding, Structure and Periodicity TestMišel VuittonОценок пока нет
- 9701 s13 Ms 22Документ7 страниц9701 s13 Ms 22Mišel VuittonОценок пока нет
- PhysicsДокумент68 страницPhysicsMišel VuittonОценок пока нет
- Uses of ICT in Data ManagementДокумент6 страницUses of ICT in Data ManagementMišel VuittonОценок пока нет
- AS LEVEL CHEMISTRY BONDING AND PERIODICITY SELF-ASSESSMENTДокумент16 страницAS LEVEL CHEMISTRY BONDING AND PERIODICITY SELF-ASSESSMENTMišel VuittonОценок пока нет
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryОт EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryРейтинг: 3.5 из 5 звезд3.5/5 (231)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceОт EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceРейтинг: 4 из 5 звезд4/5 (894)
- The Yellow House: A Memoir (2019 National Book Award Winner)От EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Рейтинг: 4 из 5 звезд4/5 (98)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureОт EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureРейтинг: 4.5 из 5 звезд4.5/5 (474)
- Never Split the Difference: Negotiating As If Your Life Depended On ItОт EverandNever Split the Difference: Negotiating As If Your Life Depended On ItРейтинг: 4.5 из 5 звезд4.5/5 (838)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaОт EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaРейтинг: 4.5 из 5 звезд4.5/5 (265)
- The Emperor of All Maladies: A Biography of CancerОт EverandThe Emperor of All Maladies: A Biography of CancerРейтинг: 4.5 из 5 звезд4.5/5 (271)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersОт EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersРейтинг: 4.5 из 5 звезд4.5/5 (344)
- Team of Rivals: The Political Genius of Abraham LincolnОт EverandTeam of Rivals: The Political Genius of Abraham LincolnРейтинг: 4.5 из 5 звезд4.5/5 (234)
- The Unwinding: An Inner History of the New AmericaОт EverandThe Unwinding: An Inner History of the New AmericaРейтинг: 4 из 5 звезд4/5 (45)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyОт EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyРейтинг: 3.5 из 5 звезд3.5/5 (2219)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreОт EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreРейтинг: 4 из 5 звезд4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)От EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Рейтинг: 4.5 из 5 звезд4.5/5 (119)
- DETERMINING THE ACTIVATION ENERGY OF THE IODINATION OF ACETONEДокумент26 страницDETERMINING THE ACTIVATION ENERGY OF THE IODINATION OF ACETONEjoebidenyyzОценок пока нет
- Study of The Kinetics of Carboxymethylcellulose Synthesis in A Screw ReactorДокумент6 страницStudy of The Kinetics of Carboxymethylcellulose Synthesis in A Screw ReactorElna PurwantiОценок пока нет
- Chemical Kinetics MCQ (Class 12) : - Eart EartДокумент4 страницыChemical Kinetics MCQ (Class 12) : - Eart EartBuri MtmОценок пока нет
- Test On Chemical KineticsДокумент4 страницыTest On Chemical Kineticsdevansh dewanОценок пока нет
- General Chemistry II Kinetics Practice ProblemsДокумент15 страницGeneral Chemistry II Kinetics Practice ProblemsVinoth KumarОценок пока нет
- 4.3 How Fast - RatesДокумент26 страниц4.3 How Fast - Ratescrazieeiraqi100% (1)
- Abdel Jawad 2005Документ8 страницAbdel Jawad 2005Alberto Tupa OrtizОценок пока нет
- CATALYSISДокумент7 страницCATALYSISJudy Ann Leyco BartolataОценок пока нет
- Why Chemical Kinetics MatterДокумент90 страницWhy Chemical Kinetics MatterVikas MishraОценок пока нет
- General Aging Theory and Simplified Protocol For Accelerated Aging of Medical DevicesДокумент6 страницGeneral Aging Theory and Simplified Protocol For Accelerated Aging of Medical Devicesbaltazarme100% (1)
- Introduction To Heterogeneous Catalysis - StoltzeДокумент123 страницыIntroduction To Heterogeneous Catalysis - StoltzeMichael J. FinkОценок пока нет
- Physical ScienceДокумент15 страницPhysical ScienceemОценок пока нет
- Reaction Kinetics and The Kinetics-Based Interpretation of EquilibriumДокумент54 страницыReaction Kinetics and The Kinetics-Based Interpretation of EquilibriumwastequestОценок пока нет
- Maximizing concentrations in mixed flow reactorsДокумент2 страницыMaximizing concentrations in mixed flow reactorsSHOURYA SINGHОценок пока нет
- Quizlet Chapter 8 2Документ12 страницQuizlet Chapter 8 2EUNAH LimОценок пока нет
- Solvation Effects On Quantum Tunneling ReactionsДокумент11 страницSolvation Effects On Quantum Tunneling ReactionsZdeněk ChvalОценок пока нет
- Physical Science Handout Week5-6Документ19 страницPhysical Science Handout Week5-6Jhay Lorraine Sadian PalacpacОценок пока нет
- Chapter 2 Kinetics of Homogeneous Reactions (Part 2 of 3)Документ19 страницChapter 2 Kinetics of Homogeneous Reactions (Part 2 of 3)Molike HononoОценок пока нет
- Kinetics Assign 2020Документ7 страницKinetics Assign 2020SabaОценок пока нет
- CHEMICAL KINETICS RATE EQUATIONSДокумент25 страницCHEMICAL KINETICS RATE EQUATIONSManan SethiОценок пока нет
- Collision-Theory WebquestДокумент3 страницыCollision-Theory Webquestapi-252514594Оценок пока нет
- Q1. (A) State What Is Meant by The Term Activation Energy of A ReactionДокумент85 страницQ1. (A) State What Is Meant by The Term Activation Energy of A ReactionDaniyal HemaniОценок пока нет
- Deodhar Classes Edited PDF 12Документ6 страницDeodhar Classes Edited PDF 12Aditya MoreОценок пока нет
- CHEM 204 Problem Set 2 Rate Constants and KineticsДокумент2 страницыCHEM 204 Problem Set 2 Rate Constants and KineticsmyriamОценок пока нет
- Activation EnergyДокумент2 страницыActivation EnergyJohn Stalin Gadvay VillegasОценок пока нет
- SPM Rate of ReactionДокумент2 страницыSPM Rate of ReactionAfida HamsaniОценок пока нет
- Kinetics 2 PPTДокумент75 страницKinetics 2 PPTFatma MoustafaОценок пока нет
- CHEMICAL KINETICS - 03-Assignments (New)Документ19 страницCHEMICAL KINETICS - 03-Assignments (New)Raju SinghОценок пока нет
- Understanding Vacancy Formation and Concentration Using Arrhenius TheoryДокумент7 страницUnderstanding Vacancy Formation and Concentration Using Arrhenius TheoryEmad Suliman AbusittaОценок пока нет
- Chemical KineticsДокумент41 страницаChemical Kineticskishangopi1230% (1)