Академический Документы
Профессиональный Документы
Культура Документы
Rate of Reaction Experiment: Effect of Temperature
Загружено:
ryder1man6433Оригинальное название
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
Rate of Reaction Experiment: Effect of Temperature
Загружено:
ryder1man6433Авторское право:
Доступные форматы
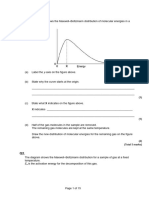
RATE OF REACTION (Kedah 2012) 1.
Table 6 shows the result of an experiment to study the effect of temperature on the rate of reaction between 50cm3 1.0 moldm-3 hydrochloric acid solution, HCl and 50cm3 2.0 moldm-3 sodium thiosulphate solution, Na2S2O3.
(a) Balance the chemical equation below for the reaction that occurs in this experiment.
m = ___________________________ n = ___________________________ [2 marks] (b) Draw the set-up of apparatus used to measure the time taken for X mark to disappear from sight.
[2 marks] (c) Name the substance that is responsible for the X mark to disappear from sight. ________________________________________________________________________________ [1 mark] (d) Plot a graph of temperature against time taken for the X mark to disappear from sight. [4 marks] (e) Based on the graph in (d), state the relationship between temperature and time. ________________________________________________________________________________ [1 mark]
(Johor 2011) 2. An experiment is carried out to investigate a factor that influences the rate of reaction. The experiment is repeated five times at different temperatures. Table below shows the temperature and time taken for X mark to disappear from view when observed vertically from the top. Experiment 1 2 3 4 5 Temperature / oC 30.0 40.0 50.0 60.0 70.0 Time/s 55.5 33.0 23.0 17.0 13.0
(a) Compare the rate of reaction between Experiment 1 and Experiment 5. _____________________________________________________________________________ [1 mark] (b) Explain your answer in (a) using the collision theory. _____________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ [4 marks]
(c) Diagram 6.2 shows a conversation between Ummu and Qistina.
Paper 3 (Terengganu 2012) 1. Fresh milk stored in the refrigerator lasts longer than the milk that is placed on the table. Referring to the above situation, plan a laboratory experiment to investigate the effect of temperature of sodium thiosulphate solution on the rate of reaction when reacts with dilute hydrochloric acid. Your planning must include the following items: (a) Problem statement (b) All the variables (c) Hypothesis (d) List of materials and apparatus (e) Procedure (f) Tabulation of data [17 marks]
Qs. 8 (SBP 2012) (a) The smaller sized potatoes will cook faster than the bigger sized ones. Explain why. [4 marks] (b) A group of students carried out two experiments to investigate the factor affecting the rate of reaction between zinc powder and hydrochloric acid, HCl. Diagram 8 shows the set-up of
apparatus used in the experiments.
(i) In the presence of substance X in experiment II, the higher volume of gas is released in the first two minutes compared to experiment I. State one substance that can be used as substance X. [1 mark] (ii) Calculate the average rate of reaction of in Experiment I and Experiment II in the first two minutes. [2 marks] (iii) Compare the rate of reaction between Experiment I and Experiment II. Explain your answer based on collision theory. [5 marks] (iv) Sketch a graph of volume of gas released against time for both sets of experiments for the first two minutes. [2 marks] (v) Write an ionic equation for the reaction between zinc powder and hydrochloric acid. [2 marks] (vi) Hydrochloric acid in experiment I is replaced with sulphuric acid with the same volume and concentration. Compare the rate of reaction and the maximum volume of hydrogen gas released between these two experiments. Explain your answer. [4 marks]
Вам также может понравиться
- Rate of Reaction FactorsДокумент8 страницRate of Reaction FactorsAmirah Noor AffandiОценок пока нет
- PPT, Form 4 (Chemistry) - 2014Документ19 страницPPT, Form 4 (Chemistry) - 2014Nur HafezaОценок пока нет
- Kadar Tindak Balas.K 2 & K3Документ16 страницKadar Tindak Balas.K 2 & K3Narah NasОценок пока нет
- SPM Kimia Tingkatan, 5 Rate of Reaction ExerciseДокумент7 страницSPM Kimia Tingkatan, 5 Rate of Reaction Exerciseryder1man6433Оценок пока нет
- Chapter 1Документ11 страницChapter 1kenenathОценок пока нет
- Chapter 1 Rate of ReactionДокумент4 страницыChapter 1 Rate of ReactionMacai OperasiОценок пока нет
- 4541/3 Chemistry Paper 3 Mei 2014 1 HourДокумент10 страниц4541/3 Chemistry Paper 3 Mei 2014 1 HourndianaoОценок пока нет
- RateДокумент29 страницRateapi-422428700Оценок пока нет
- Rate of Reaction QPДокумент21 страницаRate of Reaction QPHina Saeed ChОценок пока нет
- Chemistry and Climate ChangeДокумент171 страницаChemistry and Climate ChangeNazima MunirОценок пока нет
- CHEM 2OA3 Fall 2013 Assignment 3Документ14 страницCHEM 2OA3 Fall 2013 Assignment 3klibzyОценок пока нет
- Practicals Pack 2Документ13 страницPracticals Pack 2bilaalquadriОценок пока нет
- Physical Science Paper 4 November 2009Документ12 страницPhysical Science Paper 4 November 2009BRANDON TINASHEОценок пока нет
- Year 10 Chemistry Time: 2 HoursДокумент12 страницYear 10 Chemistry Time: 2 HoursAdrianHedleyОценок пока нет
- 2006 Paper 3-BIДокумент3 страницы2006 Paper 3-BIAfrina Lee DonghaeОценок пока нет
- Chemistry Paper 3Документ6 страницChemistry Paper 3NasaОценок пока нет
- Rate of Reaction FactorsДокумент8 страницRate of Reaction FactorssmcmasaiОценок пока нет
- How FastДокумент54 страницыHow FastKaushal Silva RanpatabendigeОценок пока нет
- 20-21 F.1 Science Mid Year ExamДокумент12 страниц20-21 F.1 Science Mid Year ExamkittyluluyyОценок пока нет
- Rates of Reaction - Mini LabsДокумент7 страницRates of Reaction - Mini LabsPolly LeungОценок пока нет
- 6.1 Rate of Reaction QP-compressedДокумент34 страницы6.1 Rate of Reaction QP-compressedradhaОценок пока нет
- Year 11 Chemistry Time: 2 HoursДокумент12 страницYear 11 Chemistry Time: 2 HoursAdrianHedleyОценок пока нет
- 11C1 Review QuestionsДокумент10 страниц11C1 Review QuestionsfuazafrimpongОценок пока нет
- Responses To Chemistry Past Paper - January 2016Документ6 страницResponses To Chemistry Past Paper - January 2016yar.sugar.mamiiОценок пока нет
- Kinetics QPДокумент22 страницыKinetics QPdovidОценок пока нет
- CSEC Chemistry January 2011 P2Документ18 страницCSEC Chemistry January 2011 P2AshleyОценок пока нет
- Rates of Reaction L11 HLДокумент16 страницRates of Reaction L11 HLdani.castillo1307Оценок пока нет
- CRE 3 Exam 2011 Q FINAL - Sven - Post Exam Panel Comments Post External B1Документ5 страницCRE 3 Exam 2011 Q FINAL - Sven - Post Exam Panel Comments Post External B1Deepro BhattacharyaОценок пока нет
- Rates Practice Exam QuestionsДокумент18 страницRates Practice Exam QuestionsisheanesuОценок пока нет
- Year 10 Chemistry Time: 2 HoursДокумент9 страницYear 10 Chemistry Time: 2 HoursAdrianHedleyОценок пока нет
- Sep 2017Документ32 страницыSep 2017Dylan EllulОценок пока нет
- Chemistry Mcse PiДокумент10 страницChemistry Mcse PiMoses SamalaniОценок пока нет
- f5 Chapter 1 Essay QДокумент4 страницыf5 Chapter 1 Essay Qzhen1998Оценок пока нет
- Program Pecutan Akhir Chemistry SPM 2012 2Документ14 страницProgram Pecutan Akhir Chemistry SPM 2012 2Zuliana ZolkafliОценок пока нет
- PP1 Kimia F5 K2Документ27 страницPP1 Kimia F5 K2Zul Adli AliОценок пока нет
- Chapter 1: Rate of Reaction: Larning Task 1.2 Problem SolvingДокумент29 страницChapter 1: Rate of Reaction: Larning Task 1.2 Problem Solvingamin_zamanОценок пока нет
- Review Booklet 2Документ10 страницReview Booklet 2Mirjeta ZymeriОценок пока нет
- Solaf Chemistry SPM 2014: Chapter 1 Form 5: Rate of ReactionДокумент18 страницSolaf Chemistry SPM 2014: Chapter 1 Form 5: Rate of ReactionNik Diana Hartika Nik Husain100% (1)
- INSTRUCTIONS FOR THIS TEST (Reading This Is Not Included in The 5-Minute Reading Time)Документ6 страницINSTRUCTIONS FOR THIS TEST (Reading This Is Not Included in The 5-Minute Reading Time)Isabella Martins AndersenОценок пока нет
- Quantititive Chemistry - Paper 1 TES - 4Документ37 страницQuantititive Chemistry - Paper 1 TES - 4KshitijОценок пока нет
- UntitledДокумент16 страницUntitledMichel ElizeeОценок пока нет
- Physical Sciene Paper 4 November 2010Документ8 страницPhysical Sciene Paper 4 November 2010BRANDON TINASHEОценок пока нет
- Chem 11 Total Review With Answers Key UpdateДокумент28 страницChem 11 Total Review With Answers Key Updatemelissa.figueroamoralesОценок пока нет
- Module 14 ElectrolysisДокумент32 страницыModule 14 ElectrolysisTan AylinОценок пока нет
- Chemistry F5C1Документ9 страницChemistry F5C1Mohammad Nur SyafiqОценок пока нет
- Worksheet 1 - 1 New Key PDFДокумент6 страницWorksheet 1 - 1 New Key PDFThaarvena Retina100% (1)
- Chemistry Paper 4 October 2004Документ11 страницChemistry Paper 4 October 2004Dean DambazaОценок пока нет
- Revision 7 Rates of ReactionДокумент15 страницRevision 7 Rates of Reactionkhadijahussain107Оценок пока нет
- ALevel Physical Chemistry Past PapersДокумент24 страницыALevel Physical Chemistry Past PaperssabihagailaniОценок пока нет
- GR 9, Chem P-1Документ23 страницыGR 9, Chem P-1kashif mohammedОценок пока нет
- Chemistry SPM Trial Paper 3Документ7 страницChemistry SPM Trial Paper 3Kaneson IyarooОценок пока нет
- Mid Year Exam Chemistry Form 4Документ13 страницMid Year Exam Chemistry Form 4Wan Shuhaimi73% (11)
- 2022 Chemistry Paper I (Theory) MJ CLUSTERДокумент11 страниц2022 Chemistry Paper I (Theory) MJ CLUSTERishmaeljonas681Оценок пока нет
- VVДокумент5 страницVVLisaam De YesteОценок пока нет
- Quiz 8Документ5 страницQuiz 8cikgu_aminОценок пока нет
- WS Grade 10 IG Chemistry 23-24 - Revision For UT1Документ4 страницыWS Grade 10 IG Chemistry 23-24 - Revision For UT1SiyaОценок пока нет
- Paper IIIДокумент4 страницыPaper IIIyokecheng20003826Оценок пока нет
- 2019f5s9ex7chemistry 2Документ10 страниц2019f5s9ex7chemistry 2AlyciaLeeОценок пока нет
- O Level Biology Practice Questions And Answers EnzymesОт EverandO Level Biology Practice Questions And Answers EnzymesРейтинг: 5 из 5 звезд5/5 (1)
- Collision TheoryДокумент4 страницыCollision Theoryryder1man6433Оценок пока нет
- Redox ExperimentsДокумент12 страницRedox Experimentsryder1man6433100% (1)
- Redox ExperimentsДокумент12 страницRedox Experimentsryder1man6433100% (1)
- Form 4 Chemistry Exam QuestionsДокумент8 страницForm 4 Chemistry Exam Questionsryder1man643367% (6)
- Natural RubberДокумент3 страницыNatural Rubberryder1man6433Оценок пока нет
- Please Choose One Item From The List AboveДокумент3 страницыPlease Choose One Item From The List Aboveryder1man6433Оценок пока нет
- Chapter 3: Oxidation and Reduction Form 5 Chemistry Title: Rusting As A Redox ReactionДокумент3 страницыChapter 3: Oxidation and Reduction Form 5 Chemistry Title: Rusting As A Redox Reactionryder1man6433Оценок пока нет
- Chemistry Tpical Essay QuestionДокумент15 страницChemistry Tpical Essay Questionryder1man6433Оценок пока нет
- Diffusion and Melting PointДокумент6 страницDiffusion and Melting Pointryder1man6433Оценок пока нет
- Redox and ElectrochemistryДокумент44 страницыRedox and Electrochemistryryder1man6433Оценок пока нет
- Natural RubberДокумент3 страницыNatural Rubberryder1man6433Оценок пока нет
- Chemical BondsДокумент5 страницChemical Bondsryder1man6433Оценок пока нет
- Group 14 Periodic TableДокумент15 страницGroup 14 Periodic Tableryder1man6433Оценок пока нет
- 64.existence of ChemicalsДокумент5 страниц64.existence of Chemicalsryder1man6433Оценок пока нет
- Isotopes and Their ImportanceДокумент7 страницIsotopes and Their Importanceryder1man6433Оценок пока нет
- Ulangkaji Akhir Menjelang SPMДокумент32 страницыUlangkaji Akhir Menjelang SPMThivagar RajasekaranОценок пока нет
- Bengkel Bimbingan Berkala Acids and BasesДокумент12 страницBengkel Bimbingan Berkala Acids and BasesReneelda HassanОценок пока нет
- Chemistry Form 6 Chap 01 PDFДокумент44 страницыChemistry Form 6 Chap 01 PDFryder1man6433Оценок пока нет
- Chemistry QuestionДокумент16 страницChemistry Questionryder1man6433Оценок пока нет
- Chemistry Formulas And NomenclatureДокумент51 страницаChemistry Formulas And Nomenclatureryder1man6433Оценок пока нет
- Sem 1 Trial 2013Документ8 страницSem 1 Trial 2013ryder1man6433Оценок пока нет
- Hybridisation in ChemistryДокумент22 страницыHybridisation in Chemistryryder1man6433Оценок пока нет
- Answer Gerak Gempur Chemistry 2013Документ11 страницAnswer Gerak Gempur Chemistry 2013ryder1man6433Оценок пока нет
- CBSE Class X (Science) Page 1 30Документ30 страницCBSE Class X (Science) Page 1 30Nitesh Bhardwaj0% (1)
- CHP 1 Scientific MethodДокумент13 страницCHP 1 Scientific Methodryder1man6433Оценок пока нет
- The hypothesis is that ionic compounds conduct electricity in the molten or dissolved state while covalent compounds do notДокумент10 страницThe hypothesis is that ionic compounds conduct electricity in the molten or dissolved state while covalent compounds do notryder1man64330% (1)
- 1 Matter & The Atomic Structure ModulДокумент43 страницы1 Matter & The Atomic Structure Modulryder1man6433100% (1)
- Chemical Bond f4 Exercise ModuleДокумент5 страницChemical Bond f4 Exercise Moduleryder1man6433Оценок пока нет
- Trial Kedah Chemistry SPM 2013 K2 SKEMAДокумент12 страницTrial Kedah Chemistry SPM 2013 K2 SKEMACikgu Faizal100% (2)
- BS en 00437-2003 + A1-2009 PDFДокумент46 страницBS en 00437-2003 + A1-2009 PDFShan Sandaruwan AbeywardeneОценок пока нет
- FASID Table Provides MET Service Requirements for AerodromesДокумент31 страницаFASID Table Provides MET Service Requirements for Aerodromesdenys galvanОценок пока нет
- Dynamic Architecture: University of Duhok Architectural Department Stage: 4th Advance Building TechnologyДокумент28 страницDynamic Architecture: University of Duhok Architectural Department Stage: 4th Advance Building Technologyأحمد خيرالدين عليОценок пока нет
- Decimal Fractions: Important Concepts and FormulaeДокумент12 страницDecimal Fractions: Important Concepts and FormulaechaostheoristОценок пока нет
- Denise Riley Preface - ApДокумент9 страницDenise Riley Preface - ApAdam PietteОценок пока нет
- 01 English As A Global LanguageДокумент11 страниц01 English As A Global Languagemaite fpОценок пока нет
- Startup IndiaДокумент32 страницыStartup IndiaNaveen JОценок пока нет
- A Rhetoric of ArgumentДокумент398 страницA Rhetoric of ArgumentAbdelmajid Said100% (9)
- Indigenous Reptilian, UFOs, MARS, Reptoid Predation Found On Mars, Benevolent Hybrid Reptilian HumansДокумент554 страницыIndigenous Reptilian, UFOs, MARS, Reptoid Predation Found On Mars, Benevolent Hybrid Reptilian HumansMindSpaceApocalypse100% (4)
- Crash 2023 06 14 - 16.32.10 FMLДокумент5 страницCrash 2023 06 14 - 16.32.10 FMLEspeciales ???Оценок пока нет
- International Business Management TopicsДокумент8 страницInternational Business Management Topicsashishkumarsharma123Оценок пока нет
- PH127 Lectures Nov. 2022 4Документ212 страницPH127 Lectures Nov. 2022 4dicksonjohnxpОценок пока нет
- Caterpillar Cat M322F Wheeled Excavator (Prefix FBW) Service Repair Manual (FBW00001 and Up) PDFДокумент21 страницаCaterpillar Cat M322F Wheeled Excavator (Prefix FBW) Service Repair Manual (FBW00001 and Up) PDFfkdmmaОценок пока нет
- Law of Delict - ConductДокумент11 страницLaw of Delict - ConductJulinah HlongwaneОценок пока нет
- Full Download Ebook Ebook PDF Media and Communication in Canada Networks Culture Technology Audience Ninth 9th Edition PDFДокумент42 страницыFull Download Ebook Ebook PDF Media and Communication in Canada Networks Culture Technology Audience Ninth 9th Edition PDFlillie.christopher194100% (38)
- Bipolar Transistor BiasingДокумент29 страницBipolar Transistor Biasingmoin_mohdОценок пока нет
- Rugged high quality cylindersДокумент2 страницыRugged high quality cylindersD Rider CasanovaОценок пока нет
- AKU B.Tech 5th Sem Result 2021Документ1 страницаAKU B.Tech 5th Sem Result 2021Affan AkbarОценок пока нет
- Electrical SystemДокумент84 страницыElectrical SystemFELIX AGUILARОценок пока нет
- Open Source Used in Cisco Packet Tracer Mobile iOS 2.1Документ39 страницOpen Source Used in Cisco Packet Tracer Mobile iOS 2.1Fernandö AcОценок пока нет
- As 1833-2002 Austenitic Cast IronДокумент6 страницAs 1833-2002 Austenitic Cast IronSAI Global - APACОценок пока нет
- User Manual Operation Maintenance Repair: HY-404-En 04-08Документ35 страницUser Manual Operation Maintenance Repair: HY-404-En 04-08phankhoa83-1Оценок пока нет
- ModelsGuidelines v5Документ37 страницModelsGuidelines v5Martin NolanОценок пока нет
- Programming Languages For PLC: International Standard IEC61131-3 (Part One)Документ32 страницыProgramming Languages For PLC: International Standard IEC61131-3 (Part One)RaminptОценок пока нет
- Persepolis EssayДокумент3 страницыPersepolis EssayMichaelОценок пока нет
- Psychrometric Chart Analysis of Air-Water SampleДокумент4 страницыPsychrometric Chart Analysis of Air-Water SampleJanry EfriyantoОценок пока нет
- Programming in C Data Structures 15pcd13 NotesДокумент112 страницProgramming in C Data Structures 15pcd13 Noteshavyas100% (1)
- DLL Music Q4 W1Документ5 страницDLL Music Q4 W1ARJAY BORJEОценок пока нет
- Bapi Goodsmvt Create SCNДокумент3 страницыBapi Goodsmvt Create SCNJohnbrocklyОценок пока нет
- Design 10-Ton Crane GirderДокумент13 страницDesign 10-Ton Crane GirderDavid_PuenОценок пока нет