Академический Документы
Профессиональный Документы
Культура Документы
Prenatal Development of The Eye and Its Adnexa
Загружено:
mumunooИсходное описание:
Оригинальное название
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
Prenatal Development of The Eye and Its Adnexa
Загружено:
mumunooАвторское право:
Доступные форматы
Prenatal Development of the Eye and Its Adnexa CYNTHIA S. COOK, VICTORIA OZANICS and FREDERICK A.
JAKOBIEC Main Menu Table Of Contents EARLY MORPHOGENESIS LENS INDUCTION AND DIFFERENTIATION CONNECTIVE TISSUE COATS STRUCTURES OF THE AQUEOUS OUTFLOW PATHWAYS UVEA NEUROECTODERMAL LAYERS BRUCH'S MEMBRANE OPTIC NERVE AND DISC VITREOUS AND HYALOID SYSTEM ADNEXA CONCLUSIONS ACKNOWLEDGMENTS REFERENCES
Search
In this text, we attempt to provide an overview of ocular embryology by describing essential developmental events in a concise fashion. Fine structural data on human and primate eye components have become available since the appearance of standard publications on ocular embryology by Mann,1 Barber,2 Dejean and coworkers,3 and Duke-Elder and associates.41 These observations aid in reconfirming or reevaluating the functional development of ocular structures as expressed by morphologic changes. Our descriptions are based on mammalian tissues, including both humans and other species that serve to model human development. Comparisons have demonstrated that the sequence of developmental events is similar across species. Factors that must be taken into consideration when making interspecies comparisons include: duration of gestation; differences in anatomic endpoint (such as the absence in other species of a macula, Schlemm's canal, or Bowman's membrane); and when eyelid fusion breaks (during the sixth month of gestation in the human versus 2 weeks postnatally in the mouse. Within the limits of these species variation, mice have proven to be a valuable model in the study of normal and abnormal ocular morphogenesis. In particular, the study of effects of acute exposure to teratogens during development has provided valuable information about the specific timing of events leading to malformations. In development of the eye, as in other organs, the multiplication of cells as well as directional change in shape, structure, and function of the cells govern growth. Gene determination decides the direction in which a change can occur, whereas the reciprocal demands of the individual cells or parts determine how far that direction must be followed. Fundamentally, the
process consists of these two activities: change in structure and shape due to relatively different rates of growth and also change in structure and function due to differentiation and functional specialization. Induction of one ocular tissue by another and interrelations between these developing tissues have been extensively reinvestigated in many laboratories using various experimental techniques.521 One example is the lens, which arises in direct response to induction by the optic vesicle. The developing lens, in turn, promotes normal morphogenesis of neural ectodermal and mesenchymal elements in the eye. It has an inducing influence on corneal differentiation and promotes vitreous growth. Moreover, a strong organogenetic connection exists between lens and iris. The reciprocal interactions between optic cup and lens bring about the functional adjustment of the ocular axes. Although the neural retina grows and differentiates independently of the lens, the presence of the lens may influence the normal growth and change in shape of the pigment epithelium, choroid, and sclera. The pigment epithelium, however, directs the deposition of the mesenchyme around it; subsequently, all three layers grow in unison. The pigment epithelium also depends on the vitreous body for increase in its area. Back to Top EARLY MORPHOGENESIS Although events occurring during the first few weeks after fertilization, before the appearance of identifiable ocular primordia, may seem to have little significance to the clinical ophthalmologist, evidence indicates that abnormalities that originate during this period may be responsible for many ocular malformations that occur in humans. Gastrulation (formation of the mesodermal germ layer) occurs early in gestation (day 7 in mice, day 20 in humans). The primitive streak forms as a longitudinal groove within the epiblast (future ectoderm) of the bilaminar embryonic disc. Epiblast cells migrate medially toward the primitive streak where they invaginate to form the mesodermal layer (Fig. 1). This forms the classic three germ layers: ectoderm, mesoderm, and endoderm. Gastrulation progresses in a cranial to caudal direction. Concurrently, cranial surface ectoderm proliferates forming bilateral elevations called neural folds (Fig. 2). Columnar surface ectoderm in this area now becomes neural ectoderm.
Fig. 1. A. Drawing of a 17-day-old embryo in gastrulation stage, dorsal view, with the amnion removed. B. Crosssection of a 17-day-old embryo through the primitive streak. The primitive streak represents invagination of epiblast cells between the epiblast and hypoblast layers. Note that the epiblast cells filling the middle area form the mesodermal layer. C. Cross-section of the embryo at the end of the third week showing the three definitive germ layers: ectoderm, mesoderm, and endoderm. (Cook CS, Sulik KK, Wright KW: Embryology. In Wright KW [ed]: Pediatric Ophthalmology and Strabismus, pp 343. St Louis: Mosby, 1995. Fig. 2. A. Drawing of dorsal view of a human embryo at 19 to 20 days' gestation. The neural plate transforms into two neural folds on each side of the neural groove. The neural groove in the middle of the embryo is shaded to represent neural ectoderm; the unshaded surface of the embryo is surface ectoderm. B. Cross-section of same embryo through the neural plate. Ectoderm in the area of the neural groove (shaded cells) has differentiated into neural ectoderm, whereas the ectoderm on each side of the neural groove is surface ectoderm (clear white cells) (Cook CS, Sulik KK, Wright KW: Embryology. In Wright KW (ed): Pediatric Ophthalmology and Strabismus pp 343. St Louis: Mosby, 1995.) Experimental studies in mice using acute exposure to teratogens have demonstrated the significance of the period of gastrulation to later ocular development. Exposure to ethanol or retinoic acid during a short period equivalent to the third week of human gestation causes primary damage to the forebrain neural ectoderm.2224 This results in a spectrum of malformations including microphthalmia, anterior segment dysgenesis (Peters' anomaly), iris and optic nerve colobomas, and persistent hyperplastic primary vitreous.25,26 As the neural folds elevate and approach each other (neurulation), a specialized population of mesenchymal cells, the neural crest, emigrates from the neural ectoderm at its junction with the surface ectoderm. In the development of the eye, the neural ectoderm (deriving from the neural plate and neural folds), the surface ectoderm, the neural crest, and, to a lesser extent, the mesoderm are of importance (Table 1).
TABLE 1. Embryonic Origins of Ocular Tissues Neural ectoderm (optic cup) Neural retina
Retinal pigment epithelium Pupillary sphincter and dilator muscles Posterior iris epithelium Ciliary body epithelium Optic nerve
Neural crest (connective tissue) Corneal endothelium Trabecular meshwork Stroma of cornea, iris, and ciliary body Ciliary muscle Choroid and sclera Perivascular connective tissue and smooth muscle cells Meninges of optic nerve Orbital cartilage and bone Connective tissue of the extrinsic ocular muscles Secondary vitreous Zonules
Surface ectoderm (epithelium) Corneal and conjunctival epithelium Lens Lacrimal gland Eyelid epidermis Eyelid cilia Epithelium of adnexa glands Epithelium of nasolacrimal duct
Mesoderm (muscle and vascular endothelium) Extraocular muscle cells Vascular endothelia Schlemm's canal endothelium Blood
The cranial neural crest contributes most of the connective tissues of the eye and its adnexal structures.14,19,2741 The hyaluronic acid-rich extracellular matrix influences migration and
differentiation of the neural crest cells. This acellular matrix is secreted by the surface epithelium as well as the neural crest cells and forms a space through which crest cells migrate. Fibronectin secreted by the noncrest cells forms the limits of the mesenchymal migration. Interactions between the migrating neural crest and the associated mesoderm appear to be essential for normal crest differentiation. Many congenital malformations of the anterior segment and cornea probably arise from derangements in the axial migration of ocular neural crest. Experimental embryologic studies have shown that the mesoderm actually contributes little to head and neck mesenchyme. The cranial correlates to the paired paraxial somites are called somitomeres. Seven pairs of cranial somitomeres have been identified in the mouse.33,40,4251 In the eye, the mesoderm contributes only to the striated extraocular muscles and vascular endothelia. To these limited primary mesodermal elements come associated neural crest satellite cells (surrounding the striated muscles) and pericytes (surrounding the vascular endothelium). Circulating blood elements originate from mesoderm. The term mesenchyme broadly refers to any embryonic connective tissue and should not be confused with mesoderm. With respect to the head and neck, most of this connective tissue derives from the cranial neural crest, with the exceptions mentioned. The optic primordium is a thickened zone in the differentiating central nervous system that forms the neural folds of the early embryo. Some of the neuroepithelium composing the optic primordium becomes the future optic cup and stalk; some cells may delaminate to contribute to the neural crest.27 The optic sulcus or groove arises in the primordium at the time when the neural folds are still open in the forebrain (8 to 15 somite pairs, approximately 2 to 3.5 mm) (Figs. 3 and 4A). With enlargement of the sulcus, the optic evaginations and, later, the optic pits appear in the region of the future forebrain (see Fig. 4B). The portion of the evaginations adjacent to the midbrain contacts the mesencephalic neural crest cells, which will form the mesenchymal envelope isolating neural from surface ectoderm (see Fig. 4C). Fig. 3. Drawing of 23-day-old embryo, dorsal view, showing partial fusion of the neural folds. Brain vesicles have divided into three regions: forebrain, midbrain, and hindbrain. Facing surfaces of the forebrain are lined with neural ectoderm (shaded cells), but the most of the embryo is now lined with surface ectoderm (clear white) because the neural groove has closed. On the inside of both forebrain vesicles is the site of the optic sulci. (Cook CS, Sulik KK, Wright KW: Embryology. In Wright KW [ed]: Pediatric Ophthalmology and Strabismus, pp 343. St Louis: Mosby, 1995.)
Fig. 4. Formation of optic sulcus and vesicle. Mouse embryos are illustrated and follow a sequence nearly identical to that observed in human embryos. A. A scanning electron micrograph of a mouse embryo at day 8 (6 somite pairs) equivalent to the human at 4 weeks' gestation. The amnion has been removed. Arrowhead indicates the optic sulcus, an evagination of the forebrain (FB). The entrance to the foregut is indicated by the curved arrow ( 165). B. Optic sulcus continues to deepen through a process of microtubule-mediated cell elongation and microfilament-mediated apical constriction ( 387). C. Embryo fractured through the midbrain in a plane indicated by the horizontal line in Figure 2A. Note the junction between the columnar neural ectoderm (NE) and the flattened surface ectoderm (SE). This is the area from which the mesenchymal neural crest cells emigrate (arrows) to join the underlying mesoderm. These mesencephalic neural crest cells will populate the area around the optic primordia and give rise to most of the ocular connective tissue ( 1071). B. Embryo of 25 somite pairs (fifth week in a human), the bulge of the enlarging optic vesicle (arrows) can be appreciated externally. Mx, maxillary prominence of the first visceral arch: Mn, mandibular prominence of the first visceral arch; II, second visceral arch ( 447). E. Frontal fracture at the level of the optic vesicle (OV). The optic vesicle is lined by the columnar neural ectoderm (N) and enlarges, approaching the surface ectoderm (E). The optic stalk (OS) is continuous with the cavity of the forebrain ( 440). F. Removal of the surface ectoderm (E) from an embryo of 25 somite pairs reveals the exposed basal lamina of the optic vesicle (arrows). Enlargement of the optic vesicle has displaced the adjacent mesenchyme (M) so that the basal lamina of the surface ectoderm (E) is in direct contact with that of othe optic vesicle ( 214). At about the 24th day (2 to 4 mm) with the closure of the neural tube, the optic pits are pushed outward away from the central nervous system and toward the surface ectoderm. The two lateral bulges, caused by the outward extension of the growing optic pits, become pouch-shaped vesicles at about the 25th day of development (20 somite pairs) (Fig. 5; see Fig. 4D and E).
Fig. 5. A. Drawing of a cross-section through forebrain and optic sulci of 24-day-old embryo. Note that the neural tube is still open. The optic sulci are lined by neural ectoderm (shaded cells), while the surface of the forebrain is covered with surface ectoderm (clear white cells). As the optic sulci (neural ectoderm) evaginate toward the surface ectoderm (hollow arrows), the edges of the brain vesicles move together to fuse, thus closing the neural tube (solid arrows). B. Drawing of a cross-section through a 26-day-old embryo at the level of the optic vesicle. Note that neural tube is closed, the surface ectoderm now lines the surface of the forebrain, and the neural ectoderm is completely internalized. The surface ectoderm cells overlying the optic vesicles enlarge to form the early lens placode. (Cook CS, Sulik KK, Wright KW: Embryology. In Wright KW [ed]: Pediatric Ophthalmology and Strabismus, pp 343. St Louis: Mosby, 1995.) The optic vesicles become sheathed with cells of neural crest origin27 that, except for a small region in the center of the bulge, separate them from the surface ectoderm (see Fig. 4E). The future primordium of the retina is present before closure of the neural tube, when the neural ectoderm is still open to the amniotic cavity. The optic stalk is formed by a constriction of the area between the vesicles and the future forebrain. At this time, all cells lining the inner surface of the vesicle's cavity are ciliated, and its outer surface, as well as the inner aspect of the surface ectoderm overlying it, is covered by a thin basal lamina. The next event is invagination of the optic vesicles by differential growth and buckling to form the optic cup (Figs. 6 to 9). The temporal and lower walls move inward against the upper and posterior walls. This process also involves the optic stalk so that the optic (choroid/embryonic/retinal) fissure is formed where the two laterally growing edges of the cup and stalk meet. Mesenchyme (primarily neural crest) penetrates immediately into the cup by filling up the fissure. Fig. 6. Drawing of a transection through a 28-day-old embryo showing invaginating lens placode that is pushing into the optic vesicle (arrows), thus creating the optic cup. Note the orientation of the eyes 180 degrees from each other. This is also illustrated in Figures 9B and C. (Cook CS, Sulik KK, Wright KW: Embryology. In Wright KW [ed]: Pediatric Ophthalmology and Strabismus, pp 343. St Louis: Mosby, 1995.)
Fig. 7. Drawing shows formation of the lens vesicle and optic cup. Note that the optic fissure is present because the optic cup is not fused inferiorly. Mesenchyme (M) surrounds the invaginating lens vesicle. Note that the optic cup and optic stalk are made of neural ectoderm. (Cook CS, Sulik KK, Wright KW: Embryology. In Wright KW [ed]: Pediatric Ophthalmology and Strabismus, pp 343. St Louis: Mosby, 1995.)
Fig. 8. Drawing of cross-section at approximately 5 weeks' gestation through optic cup and optic fissure. The lens vesicle is separated from the surface ectoderm. Mesenchyme (M) surrounds the developing lens vesicle and the hyaloid artery is seen with the optic fissure. See also Figure 9F. (Cook CS, Sulik KK, Wright KW: Embryology. In Wright KW [ed]: Pediatric Ophthalmology and Strabismus, pp 343. St Louis: Mosby, 1995.) Fig. 9. Invagination of the optic cup and lens vesicle. Mouse embryos are illustrated. A. Embryo of somite pairs (fifth week in a human). On external examination, the invaginating lens placode can be seen (arrow). Note its position relative to the maxillary (Mx) and mandibular (Mn) prominences of the first visceral arch ( 106). B. Embryo of the same age as in Figure 3A. Frontal fracture through the lens placode (arrow) illustrates the associated thickening of the surface ectoderm (E). Mesenchyme (M) of neural crest origin is present adjacent to the lens placode. Distal portion of the optic vesicle thickens concurrently, as the precursor of the neural retina (NR), whereas the proximal optic vesicle becomes a shorter, cuboidal layer that is the anlage of the retinal
pigmented epithelium (PE). The cavity of the optic vesicle (V) becomes progressively smaller ( 367). C. Epithelium of the lens placode continues to invaginate (L). There is an abrupt transition between the thicker epithelium of the placode and the adjacent surface ectoderm, which is not unlike the transition between the future neural retina (NR) and the future pigmented epithelium (PE). (Periodic acid-Schiff's stain; 443) D. As the lens vesicle enlarges during the eleventh day, the external opening, or lens pore (arrow), becomes progressively smaller. The lens epithelial cells at the posterior pole of the lens elongate to form the primary lens fibers (L). NR, anlage of the neural retina; PE, the anlage of the pigmented epithelium (now a very short cuboidal layer) ( 300). E. External view of the lens pore (arrow) and its relationship to the maxillary prominence (Mx)32 somite pairs ( 260). F. Frontal fracture reveals the optic fissure (*) where the two sides of the invaginating optic cup meet. This forms an opening in the cup allowing access to the hyaloid artery (H), which ramifies around the invaginating lens vesicle (L). The former cavity of the optic vesicle is obliterated except in the marginal sinus (S), at the transition between the neural retina (NR) and the pigmented epithelium. E, surface ectoderm ( 307). The optic vesicle and optic stalk invaginate through differential growth and infolding. Local apical contraction52 and physiologic cell death53 have been identified during invagination. This process progresses from inferior to superior so that the sides of the optic cup and stalk meet inferiorly in the optic fissure. The two lips of the optic fissure meet and initially fuse anterior to the optic stalk with fusion progressing anteriorly and posteriorly. Failure of normal closure of this fissure may result in inferiorly located defects (colobomas) in the iris, choroid, or optic nerve. Closure of the optic cup through fusion of the optic fissure allows establishment of intraocular pressure. Studies have demonstrated that, in the chick, the protein in the embryonic vitreous humor is derived from plasma proteins entering the eye by diffusion out of permeable vessels in the anterior segment.54 After optic fissure closure, protein content in the vitreous decreases, possibly through dilution by aqueous humor produced by developing ciliary epithelium. Table 2 lists the chronologic sequence of ocular development and comparative body-eye measurements in relationship to embryonic time intervals.
TABLE TWO. Revised Sequence of Human Ocular Development
Neuroectoderm al Derivatives Posterior iris epithelium, ciliary body epithelium, pupillary muscles, neural retina, retinal pigment epithelium CR (RPE), Lengt secondary Mont Week(s Day(s h vitreous, and h ) ) (mm) optic nerve 1 3 20 12 Neural plate thickens
Neural Crest Derivatives Corneal endothelium, stroma of cornea, iris, and ciliary body, ciliary muscle, trabecular meshwork, choroid, sclera, secondary vitreous, and orbit
Surface Ectoderm Derivatives Corneal and conjunctiva l epithelium, lens, eyelid epidermis, eyelid cilia and glands, lacrimal gland, nasolacrim al duct Gastrulation (formation of mesoderm)
Mesoderma l Derivatives Endotheliu m of Schlemm's canal, vascular (hyaloid, tunica vascula lentis (TVL) endotheliu m, extraocular muscles
22
23.5 Optic sulci present in forebrain 23 Neural tube closed Optic stalk formed Optic sulci converted into optic vesicles Optic vesicle contacts surface ectoderm Mesenchyme surrounds optic vesicle Lens placode begins to thicken Eyelid territory determined Lens pit forms as lens placode invaginates Cord of ectoderm Hyaloid artery enters through the optic fissure
24
25
34
27
45
29
57
Optic vesicle begins to invaginate forming optic cup with optic fissure
buried by maxillary processes to later form nasolacrima l duct 33 79 Optic fissure closed Pigment in outer layer of optic cup (future RPE) Oculomotor nerve present Trochlear and abducens nerves appear Lens pit closed forming lens vesicle surrounded by intact basement membrane (lens capsule) Corneal epithelium formed
37
811 Ciliary ganglion Choriocapillaris Primary present formed around lens fibers the optic cup fill lens vesicle forming embryonal nucleus 11 14 Retina consists Corneal of: external endothelium limiting formed membrane (with zonula adherens), proliferative zone, primitive zone, marginal zone, and internal limiting membrane Retina consists of: inner neuroblastic layer, transient fiber layer of Secondary lens fibers form Lid folds present
40
42 45
13 17
Chievitz, proliferative zone, and outer neuroblastic layer 45 48 16 18 Ganglion cells Anterior give rise to chamber nerve fiber layer beginning to form First orbital bone formation (ethmoid) Optic sheath formation begins Optic cup measures 1 mm Optic fissure within the optic stalk closed Optic stalk cavity obliterated by optic nerve fibers which now reach the brain Transient fiber layer of Chievitz disappears, except in macula Secondary vitreous forming Acellular corneal stroma present Levator muscle forming
48 54
18 22
54 57
23 31
Cellular corneal stroma forming (57 layers) Descemet's membrane present (not continuous) Pupillary membrane formed Scleral condensation present
Epithelial buds of lacrimal gland present
10
63
43 48
Tenon's capsule Eyelids fuse Hyaloid present vasculature reaches maximal
developmen t 11 71 77 505 Inner plexiform layer formed Cilia within developing inner segments 60 80 Outer plexiform layer separates horizontal and bipolar nuclei from rudimentary rods and cones Synapses develop between photoreceptors, ganglion cells, and bipolar cells in central retina First indication of ciliary processes Lamina cribrosa formation begins Marginal bundle of Drualt/vitreous base present Conjunctiva l goblet cells present
1214 78 90
Glands of Moll, meibomian glands present
Rectus muscle tendons fuse with sclera Branches of ophthalmic artery accompany hyaloid artery Iridal major arterial circle formed
15
90 100
Orbital axis 105 Ciliary muscle appears
Glands of Zeiss present Schlemm's canal present Tunica vasculosa lentis begins to atrophy
16
100 Mitosis ceases in Corneal 120 the neural retina endothelium exhibits zonulae occludentes Aqueous humor formation begins Regression of corneal endothelium covering iridocorneal angle recess 120 Pupillary 130 sphincter develops Scleral spur developing Bowman's
Short eyelashes appear
Hyaloid artery begins to
membrane present
atrophy to the disc; branches of the central retinal artery form
120 Outer segments 180 formation begins Differentiation of macula begins 175 Pupillary dilator 230 muscle develops Ora serrata distinct nasally
Layers of the choroid complete Cloquet's canal formed Pupillary membrane begins to atrophy axially Capsulohyaloid al ligament present Iris pigmentation present Lamina cribrosa mature Myelination begins at the chiasm and progresses to the lamina cribrosa Retinal vessels reach the ora serrata Lacrimal duct canalized Eyelids begin to open, light perception
220 260
240 Retinal layers Regression of 280 developed pupillary except at macula membrane nearly complete 310 Orbital axis 71 350
9 term
Back to Top LENS INDUCTION AND DIFFERENTIATION As the optic vesicles enlarges, it contacts the overlying surface ectoderm. The first manifestation of lens induction is the appearance of a disc-shaped
thickening of surface epithelial cells (27 days' gestation) (see Figs. 5B, 6, and 9A and B). A tight, extracellular matrix-mediated adhesion between the optic vesicle and the surface ectoderm has been described. This anchoring effect on the mitotically active ectoderm results in cell crowding and elongation and formation of a thickened placode. Adhesion between the optic vesicle and lens placode serves to ensure alignment of the lens and retina in the visual axis. Although adhesion between the optic vesicle and surface ectoderm exists, the respective basement membranes remain separate and intact throughout the contact period (see Fig. 4F). Inductors for lens formation may act on the regulation of structural genes, or they may act directly on the cell cytoplasm. Lens induction thus may involve transfer of inductor substances from the optic cup to the surface cells across both basement membranes. Invagination of the lens placode (29 days) is accomplished by a synergistic elongation of the placode cells with contraction of their apical cytoplasm and terminal bar system (see Figs. 7 and 9C). The processes of differentiation into a lens pit, cup, and then a vesicle have been studied in detail.6171 As the lens placode invaginates, it forms a hollow vesicle (see Figs. 8 and 9D). The area of contact of the optic vesicle and the surface ectoderm determines the size of the lens vesicle, orbit, and palpebral fissure. The lens separates from the surface epithelium at about 33 days' gestation (7 to 9 mm; see Fig. 9D). The vesicle consists of a single layer of cells, covered by a basal lamina. Through appositional growth to its epithelial surface, the basal lamina acquires more layers that become the lens capsule. At first, the posterior capsule is more prominent than the anterior; the outer layers may have components from the mesodermal tissues forming the hyaloid vascular network.72 A zone of necrosis develops, displacing the lens placode from the surface ectoderm (see Fig. 9E and F). The process of lens vesicle detachment is accompanied by active migration of epithelial cells out of the keratolenticular stalk, cellular necrosis, and basement membrane breakdown.73,74 Cup formation is achieved by contraction of the apical filaments. The process of induction is thus localized. PRIMARY LENS FIBERS The hollow lens vesicle consists of a single layer of epithelial cells with cell apices directed toward the center. Following detachment from the surface ectoderm, the lens vesicle is surrounded by a basal lamina, the future lens capsule. The cells lengthen (Figs. 10 and 11A) until the lumen of the vesicle is filled (45 days, 17 mm). These constitute the primary lens fibers. The apical ends of the newly formed fibers become firmly attached to the apical surface of the anterior lens epithelium.
Fig. 10. Drawing showing formation of the embryonic lens nucleus and primary lens fibers at approximately 6 weeks. Neural crest mesenchyme (M) surrounds the optic cup. The posterior lens epithelial cells (located nearest the developing retina) elongate to form the primary lens fibers. The anterior epithelium remains cuboidal and becomes the anterior epithelium in the adult. The optic fissure is now closed. The hyaloid vessels are seen between the lens and retina. (Cook CS, Sulik KK, Wright KW: Embryology. In Wright KW [ed]: Pediatric Ophthalmology and Strabismus, pp 343. St Louis: Mosby, 1995.) Fig. 11. Form ation of the lens fibers ; early retina l differ entiat ion. A. Elon gation of the lens fibers located nearest to the neural retina forms the embryonal lens nucleus (L) and obliterates the lens vesicle cavity. The endothelial cells that form the tunica vasculosa lentis are indicated by arrows ( 392). B. Formation of the secondary lens fibers is apparent as elongation of the epithelial cells at the equatorial lens bow. C, cornea; NR, neural retina; L, lens ( 270). C. Electron micrograph evaluation of the developing lens (L). LE, anterior lens epithelium, E, surface ectoderm ( 298). D. Corneal endothelium (open arrow) and stroma (C) are completely formed but the anterior iridial stroma and iridocorneal angle (*) structures are still immature and covered by the endothelium. The outer, pigmented layer of the optic cup (O), which forms the pupillary sphincter and dilator muscles, is in apposition to the cornea in the area of the future aqueous outflow pathways (*). The arrowhead indicates the capillaries of the anterior tunica vasculosa lentis. L, lens ( 407). E and F. The retina has segregated into an inner neuroblastic layer (IN) containing the primitive ganglion cells the axons of which form the nerve fiber layer (arrow), and an
outer neuroblastic layer (ON) containing the primordia of the photoreceptors, retinal interneurons, and glial cells (E, 430; F, 316). PE, retinal pigmented epithelium. The retinal anlage promotes primary lens fiber formation in the adjacent lens epithelial cells. Surgical rotation of the lens vesicle in the chick's eye by 180 degrees results in elongation of the lens epithelial cells nearest the presumptive retina, regardless of the orientation of the transplanted lens.56 The retina thus develops independently from the lens, while the lens appears to rely on the retina for cytodifferentiation. This transformation of primary lens fibers is accompanied by ultrastructural changes in the nucleus and cytoplasm, decreased numbers of organelles, and increased numbers of fibrillar materials composed of the characteristic lens proteins.71 The primitive lens filled with primary lens fibers forms the embryonal nucleus, visible in the adult. This portion of the lens lacks sutures. SECONDARY LENS FIBERS The cells nearest the corneal primordium remain cuboidal and become the lens epithelium, which remains mitotic throughout life, giving rise to future lens fiber cells. Production of the secondary lens fibers is initiated by migration of the anterior epithelial cells toward the equator and their elongation at various degrees with a shift in their nuclear distribution, thus resulting in the lens bow (Fig. 12B, C, and F, and 13; see Figs. 11B and C). The basal ends of the fibers remain tightly attached to the basal lamina; their apical ends extend anteriorly to the center, thus forming the anterior suture. The tips of these secondary fibers are not yet tapered. A corresponding increase in cell volume and decrease in intercellular space within the lens accompany lens fiber elongation.61 The lens fibers exhibit surface interdigitations. They extend around the primary fibers beneath the capsule and meet in planes, the lens sutures, arranged essentially vertically to the surface. The basic anatomy of the lens is established after the first layer of secondary fibers has been placed (seventh week of gestation).75
Fig. 12. Form ation of the lens and irido corne al angle . A. Ante rior segm ent at 8 week s' gesta tion. The corne al stroma (C) and endothelium have formed. The dense pupillary membrane (arrow) fills much of the space within the anterior chamber. L, lens ( 100). B. Fractured lens at 7 weeks' gestation. Note embryonic nucleus (N) and anterior lens epithelium (arrow) ( 102). C. Higher magnification of (B) to illustrate secondary lens fibers and lens bow ( 376). D. Longitudinal view of lens fibers illustrating interdigitations ( 706). E. Cross-section of lens fibers illustrating tightly apposed hexagonal arrangement ( 1012). F. Light microscopic view of lens bow and close proximity of lens equator with anterior margin of optic cup. Note the hyaloid vasculature surrounding the lens (arrows) ( 220). G. At 8 weeks' gestation, following removal of the lens and the pupillary membrane, the anterior chamber can be visualized ( 103). H. Higher magnification of (G). The edge of the pupillary membrane can be seen (arrow) as well as the anterior margin of the optic cup (O) and the developing outflow pathways. The clefts visible in the limbal region canalize to form Schlemm's canal. C, cornea ( 220). I. At 13 weeks' gestation, there are immature ciliary processes located in the region of the future posterior iris (arrow). Differential growth with relative posterior movement of the inner optic cup, results in the ultimate mature conformations coinciding with exposure of the trabecular meshwork as described by Anderson ( 95). C, cornea; (B-E, courtesy of Dr. Kathy Sulik.)
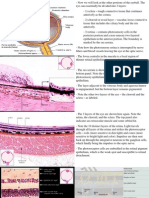
Fig. 13. Lens at 65 mm (12-week fetus) in transverse section. Posterior suture (arrow) extends from the surface to the central, primary lens fibers (location of the embryonic nucleus). The triangular anterior suture (thick arrow) is indicated by an assembly of transversely cut fibers at the anterior pole. Posterior vascular lens capsule is indicated by hollow arrow. The nucleated area is the location of the secondary lens fibers. Lens bow (Lb) is formed by anteriorly migrating nuclei of newly formed lens fibers. pm, vessels of the pupillary membrane; V, vitreous ( 40). LENS SUTURES Succeeding generations of cells extend anteriorly and posteriorly from the equator beneath the capsule. The anterior suture line is shaped like a Y that is inverted in the posterior aspect. The posterior suture is formed when the posterior central cells lose their nuclei, become separated from their basal lamina, and migrate inward.66 Curved lens fibers result, with the superficial ones being the longest. Linear and triradiate sutures form, representing different stages in lens development. MATURATION The shape of the lens and its orientation with respect to the optic axis continually adjust to the developing eye. This is partly regulated by the neural retina and peripheral mesenchyme.10 Through the third month of gestation, the anteroposterior diameter is greater than the equatorial. Mainly because of the continued generation of secondary fibers, the equatorial diameter increases rapidly, thus making the lens more and more ellipsoid. The lens, still somewhat spherical at birth, grows throughout life. A general structural densification occurs progressively during maturation. Fibrillar material is increased within the cytoplasm and cell organelles are decreased. The successive parallel layers of interdigitating, elongated lens fibers become tightly apposed (see Fig. 12D and E). Deeper nuclei become homogenous and dense. By the end of the third month, the innermost cells have lost their nuclei and simultaneously show disintegration of the chromatin and the ribosomes, leaving a finely filamentous cytoplasm. Back to Top CONNECTIVE TISSUE COATS CORNEA Among the many publications on the morphogenesis of the cornea (Fig. 14)
and the development of its constituents in various vertebrates, only a few can be cited in this general review. Fig. 14. Sch ema tic diag ram of the dev eloping corneacentral region. A. At 39 days, the two-layered epithelium rests on a basal lamina. It is separated from a two-to three-layered endothelium by a narrow, cellular space. B. At 7 weeks, mesenchyme from the periphery migrates into the space between epithelium and endothelium. It is the precursor of the future corneal stroma. C. The mesenchyme (fibroblasts) is arranged in four to five incomplete layers by 7 weeks and a few collagen fibrils appear among them. D. By 3 months, the epithelium has 2 to 3 layers of cells and the stroma about 25 to 30 layers of fibroblasts (keratoblasts) that are more regularly arranged in its posterior half. There is a thin, uneven Descemet's membrane between the most posterior keratoblasts and the monolayered endothelium. E. By midterm (4.5 months) some wing cells are forming above the basal epithelial cells and an indefinite, acellular Bowman's membrane emerges beneath the basal lamina. About one third of the anterior portion of the multilayered stroma has its keratoblasts ina disorganized formation. Descemet's membrane is well developed. F. At 7 months the cornea has its adult structure established. A few mostly superficial keratoblasts are still randomly oriented with respect to the corneal surface. The collagenous lamellae in the rest of the stroma are in parallel array with only a few spaces in the matrix lacking collagen fibrils. Breaks (near the bottom of E and F) indicate that the central portion of the stroma is not represented. Epithelium When the lens cup separates from the surface ectoderm in embryos at about 33 days' postfertilization (7 to 9 mm in length), development of the cornea can be said to have begun. The surface ectoderm becomes continuous covering the optic cup and lens vesicle and later develops into the corneal epithelium. Descemet's Membrane and Endothelium
During the next week, mesenchymal cells grow centrally between the basal laminae of the lens and corneal epithelium (Fig. 15; see 14A-C). Posterior to the basal lamina of the corneal epithelium, the mesenchyme has produced a double row of flattened cells, the future corneal endothelium (see Fig. 14A). Fig. 15. Corneal epithelium (Ep) and mesenchymal cells (Me) beneath the basal lamina are destined to form the endothelium. Section is from a monkey embryo at 34 days, comparable with that of a human at approximately 5.5 weeks ( 480). Le, lens. Descemet's membrane first appears at 8 weeks as a patchy accumulation resembling basement membrane material.91,92 The patches become confluent and thickened owing to the synthetic activity of the endothelial cells. Evidence of organization is seen early during the fourth month, when four or five superimposed lamellae interspersed with collagen fibrils appear on the stromal side of the endothelial basal lamina. The endothelium has zonulae occludentes at the cell apices by the middle of the fourth month of development. Their appearance corresponds to the onset of aqueous humor formation. Stroma Following formation of the corneal endothelium, mesenchyme (neural crest) continues to migrate axially over the rim of the optic cup during the seventh week (17 to 18 mm) (Fig. 16). At 8 weeks (18 to 22 mm), migrating mesenchymal cells from the periphery invade the space between epithelium and endothelium. This mesenchyme, as well as that which will give rise to the sclera and iris stroma, is of neural crest origin.30 The central portion of the future stroma is still acellular (see Fig. 14B). The endothelium merges with the stratified cells at the periphery over the lips of the optic cup. This mass of cells, in turn, is continuous with the cellular scleral condensation extending to the equator of the globe. The developing keratocytes begin to produce glycosaminoglycans.104
Fig. 16. Embryo at 22 mm (approximately 7 weeks) showing relation of the anterior segment components ( 260). The two arrows indicate blood channels in the mesenchyme around the rim of the cup. Peripheral part of the pupillary membrane running from the mesenchyme in front of the optic cup (mes) to the anterior lens capsule outlines the incipient anterior chamber lying between it and the posterior surface of the cornea (hollow arrows). Asterisk is placed at the peripheral limit of the anterior chamber. Curved arrows point to capsula perilenticularis fibrosa. C, cornea; LE, lens epithelium; V, primary vitreous; ov, tip of the neuroectodermal optic cup. In the early 8-week-old embryo, about 22 mm in length, the mesenchymal stroma consists centrally of five to eight rows of cells (Fig. 14C), within a fibrillar matrix containing collagen. Nerves have been identified within the corneal stroma and between epithelial cells at 3 months.105107 The most posterior layers of the corneal stroma are confluent peripherally with a condensed band of mesenchyme that is gradually spreading backward to enclose the eye. The mesenchyme destined to form the sclera is not distinct from that which will form the four oculomotor muscles. The cornea at 2 months (about 20 mm) consists of an epithelium of outer squamous and basal columnar cells. The middle polygonal or wing cells of the adult do not appear until the fourth or fifth month. The stroma has about 15 layers of cells with rapidly developing collagen fibrils, most in the posterior portion. At 3 months, the endothelium of the central area consists of a single row of flattened cells that seem to rest on an interrupted basal lamina, the first indication of a thin Descemet's membrane. With the exception of Bowman's membrane, all corneal components are present (see Fig. 14D). Bowman's Membrane Arising relatively late in gestation (see Fig. 14E and F), Bowman's membrane is observed by light microscopy during the fifth month, but somewhat earlier by electron microscopy. It is always acellular, presumably formed by the most anterior fibroblasts of the stroma, which move posteriorly as Bowman's fibers and the ground substance are synthesize. The epithelium may play a partial role in the local polymerization of the collagen precursors presumably produced by the most anterior stromal fibroblasts.108 Transparency Perhaps the most important and unique corneal characteristic is its
transparency, which also develops during fetal life. The early embryonic and fetal cornea is translucent rather than transparent and is more hydrated than in the adult.94 Condensation begins in the posterior stroma during fetal maturation.95 At about the time that the most anterior stromal lamellae are formed, corneal transparency reaches adult quality. During this development, the water content of the cornea is being reduced so that the adult level of corneal hydration is attained at the same time as transparency. SCLERA The sclera forms first anteriorly, by mesenchymal condensation at the limbus near the future insertion of the rectus muscles and grows gradually posteriorly. Fibrocytes are involved in the synthesis of the elastic foci in the sclera.109 In contrast, the cornea lacks elastic components. Inspection of the sclera at 60 to 65 mm or 12 weeks reveals it as a mesenchymal condensation that has reached the posterior pole of the eye and surrounds the optic nerve. Some cells have entered among the optic nerve fibers and are arranged transversely, forming the first stages of the connective tissue lamina cribrosa. The scleral spur appears at 4 months as circularly oriented fibers; at 5 months, it is visible behind the anterior chamber. At this time the sclera is well differentiated all around the eye. Although the corneal and scleral cells are derived from the same mass of mesenchyme surrounding the anterior part of the optic cup, they behave differently when in their definitive position. Corneal fibroblasts form collagen faster than the scleral cells and differ in the rate and amount of noncollagenous protein that they synthesize.110 Back to Top STRUCTURES OF THE AQUEOUS OUTFLOW PATHWAYS IRIDOCORNEAL ANGLE Light and scanning electron-microscopic studies reveal the anterior chamber angle of the human eye to have a continuous endothelial lining during the third and fourth months (Figs. 17 and 18). The tissues in the angle later differentiate into a loose reticulum with large enclosed spaces near the iris and ciliary body; outside of this trabecular tissue, a tighter aggregation of cells is oriented toward the sclera.111115 With the growth of surrounding structures, Schlemm's canal comes to lie at the level of the apex of the angle. Descemet's membrane and the corneal endothelium still cover a portion of the trabecular meshwork, but the endothelial lining of the chamber has become discontinuous (Figs. 19 and 20). The loose reticular tissue of the earlier stages now occurs only in the deepest part of the angle, where it has
large intercellular spaces (see Figs. 17C and 20). Fig. 17. Schematic diagram of the progressive deepening of the angle; its relation to the neighboring tissues. A. At 3 months, corneal endothelium extends nearly to the angle recess: an incipient Schlemm's canal (arrowhead) and a more posterior scleral spur condensation (hollow arrow) appear to its left. Pigment epithelium of the forward growing ectodermal optic is indented by blood vessels. The secondary vitreous fibrils run parallel to its surface (arrow). This is the faisceau isthmique or marginal bundle of Druault. B. At 4 months, the angle recess has deepened and the endothelial lining has receded somewhat. There is a small aggregate of differentiating sphincter muscle fibers near the tip of the optic cup. Arrowhead points to Schlemm's canal. The condensed tissue just posterior to Schlemm's canal is the developing scleral spur (hollow arrow). Arrow points to the developing tertiary vitreous or zonular fibers. They originate from the nonpigmented ciliary epithelium and pass at right angles through Druault's bundle toward the lens capsule. C. The iris has grown and only its ciliary portion is presented. The angle recess has deepened and is occupied by loose connective tissue separated by many spaces. The dilator muscle of the iris has reached its root, which is still thick. Arrowhead points to the major arterial circle. D. Sphincter muscle is fully developed and is separated by connective tissue septa into several groups of cells. The collarette is represented as a surface stromal bulge containing two blood vessels (curved hollow arrow). E. Schematic diagram of the developing iris dilator muscle at 6 months. During the sixth month, dilator muscle fibers (Dil. M) start to differentiate from extensions of the anterior epithelial cells (AE) into the stroma (ST). These cells are located peripherally to the developing von Michel's spur (MS), which itself is a pigmented projection of the anterior epithelium, demarcating the posterior limit of the sphincter muscle (SP). In the developing dilator muscle, myofilaments within the elongating processes become arranged parallel to the stromal axis. Some undifferentiated anterior epithelial cells (UN) are present. In the sphincter, which had originated earlier from the same layer of anterior epithelial cells, connective tissue septa and a capillary (CA) start to grow between clumps of cells, but connective tissue has not yet invaded between the muscle cells and the anterior pigment epithelium beneath it. Eventually, the sphincter muscle bundles become completely separated from the anterior epithelium,
whereas the dilator muscle sheet remains as the multilayered stromal projection of a part of this epithelium never separating from it. Therefore, the dilator muscle is not a separate cellular layer, but rather a partial myoid differentiation of cellular processes of the anterior neuroectodermal pigment epithelial cells. P, pupillary margin; PC, posterior chamber; PE, posterior epithelium; PM, pupillary membrane. Fig. 18. Excavation of the anterior chamber (AC) angle in a fetus at 75 mm (3 months) is at a level with the rim of the optic cup, which is well ahead of the lens bow. The corneal endothelium extends to the apex of the angle (hollow arrow). The location of the future trabecular meshwork is indicated by the arrow. On the side toward the lens, the angle is limited by the forward extension of the loosely woven mesenchyme over the optic cup margin. Blood vessels in the recesses of the pigment epithelium (solid arrow) precede its infolding. LE, lens epithelium; pm, pupillary membrane. Fig. 19. Angle at 7 months (approximately 225 mm). Apex of the wedge-shaped trabecular meshwork (Tr) is not in the illustration. The corneal endothelium (En) extends over one third of the trabecular lamellae. The loose tissue in the angle recess is isolated from the anterior chamber (AC) by processes of the reticular and mesenchymal cells (hollow arrows). There are large clefts (*), some of which are confluent, in the angle tissue. The angle recess extends beyond the level of the middle of the trabecular meshwork, and the immature Schlemm's canal (circled) is somewhat behind it. Ir, immature iris; Sc, sclera. (Smelser GK, Ozanics V: The development of the trabecular meshwork in primate eyes. Am J Ophthalmol 71:366, 1971.) Fig. 20. The angle in a fetus late in the ninth month (at approximately 37 weeks) extends somewhat beyond the posterior part of the trabecular meshwork, which has its apex at the termination of the corneal endothelium (En). The scleral spur (arrow) and the canal of Schlemm (arrowhead) are in front of the angle. Loose tissue in the angle is indicated by the hollow arrow. AC, anterior chamber; CM, ciliary muscle; cp, ciliary processes; C, cornea; Ir, iris; PC, posterior chamber; Sc, sclera. Anterior chamber angle formation seems to occur through a combination of processes. Differential growth of the vascular tunic results in posterior
movement of the iris and ciliary body relative to the trabecular meshwork and exposure of the outflow pathways.116 In addition, there is gradual cellular rearrangement and mesenchymal atrophy, as well as enlargement of numerous large spaces, until they become confluent with the anterior chamber.111 Following initial separation into corneoscleral and iridociliary trabecular regions at 15 weeks' gestation, the corneal trabeculae enlarge and there is regression of the corneal endothelium covering the angle recess. The discontinuity of the cellular layer covering the angle and the many lacunae present in late gestation may be correlated with the normal development of an increase in the outflow facility of aqueous humor. Outflow facility of fetal eyes under constant pressure reveals progressive increase with the age of the fetus (0.09 l/min/mmHg before 7 months to 0.3 l/min/mmHg at 8 months).117,118 It may be speculated that, if the splitting and rebuilding of the endothelial membrane lining of the early iridocorneal angle is arrested, a block to normal outflow may result. Persistence of the endothelial (Barkan's) membrane has been postulated to be of significance in the pathogenesis of congenital glaucoma. TRABECULAR MESHWORK Early during the fourth month, the primitive trabecular meshwork consists of a roughly triangular mass of undifferentiated mesenchymal cells with its apex between the corneal stroma and endothelium. The periphery of the corneal endothelium covers a portion of this primitive trabecular meshwork where it faces the anterior chamber (see Fig. 11D). SCHLEMM'S CANAL Studies using staining for neuron-specific enolase indicate that, although most structures of the iridocorneal angle are of neural crest origin, the endothelial lining of Schlemm's canal (like the vascular endothelia) is mesodermal.119 During the fourth month, a narrow Schlemm's canal is sometimes present (see Fig. 17A), possibly derived from extensions of a collector channel plexus, which will eventually become aqueous veins. Vacuolation of the endothelium around Schlemm's canal commences during the fourth month, and individual cells are connected by zonulae adherentes. During the following 3 months, the endothelium thins, with more vacuoles and tight junctions visible.120 Back to Top UVEA
The two layers of the optic cup (of neuroectodermal origin) consist of an inner nonpigmented layer and an outer pigmented layer. Both epithelial layers of the iris and ciliary body develop from the anterior aspect of the optic cup whereas the retina develops from the posterior optic cup. The optic vesicle is organized with all cell apices directed to the center of the vesicle. During optic cup invagination, the apices of the inner and outer epithelial layers become apposed. Thus, the cells of the optic cup are oriented apex to apex. A thin basement membrane lines the inner (vitreous) aspect of the nonpigmented epithelium and retina. Apical cilia projecting into the intercellular space are seen at 4.5 months. There is also increased prominence of Golgi complexes and associated vesicles within the ciliary epithelial cells. These changes and the presence of ciliary channels between apical surfaces probably represent the first production of aqueous humor.121 IRIS The iris develops by anterior growth of the optic cup. The iris stroma develops from the same population of mesenchyme (neural crest) that forms the corneal stroma, corneal endothelium, and pupillary membrane. The neuroectoderm of the optic cup differentiates into the pupillary sphincter and dilator muscles and posterior iris epithelium and induces differentiation of iris stroma. Closure of the optic fissure is normally completed by 33 to 35 days' gestation. Failure of fusion of the fissure may result in an inferior (typical) iris coloboma alone or with iris hypoplasia. Tunica Vasculosa Lentis, Pupillary Membrane, and Iris Stroma In the 17 to 18 mm embryo (7th week), vascular outgrowths are seen extending from the rim of the optic cup over the anterior lens surface (see Fig. 16). Mesenchyme migrating into the space between the lens epithelium and corneal endothelium becomes the pupillary membrane during the eighth week (21 to 26 mm; see Fig. 12A, G, and H, and Figs. 16 and 18).81 The anterior chamber is then bounded anteriorly by the avascular corneal endothelium and posteriorly toward the lens by a thin, vascularized mesenchyme, the anterior portion of the tunica vasculosa lentis. The anterior tunica vasculosa lentis is continuous with the pupillary membrane, which is supplied by means of branches of the long posterior ciliary arteries and the major arterial circle (54 to 75 mm; Figs. 21 to 23). By the end of the third month, there is a rapid forward growth of both walls of the optic cup between the folded region and its margin (see Fig. 17A and B and Fig. 22).
Fig. 21. Section through the anterior portion of the eye of a fetus of 54 mm (about 10.5 weeks). The rim of the optic cup extends anteriorly beyond the lens equator. Small vessels indent the outer (basal) surface of the pigment epithelium (arrowheads). The inner, nonpigmented wall of the optic cup is smooth. Dense arrays of vitreous fibers (Vf) attached to its inner surface form a faint condensation from the lens equator region to near the margin of the cup. This is the faiseau isthmique or marginal bundle of Druault. There is a narrow space between the tip of the optic cup and the artifactually detached lens epithelium. Pupillary membrane is indicated by hollow arrow. cj, conjunctival sac; C, cornea; R, retina. Anlage of ciliary muscle is marked by asterisks. Fig. 22. Portion of anterior segment of the eye at 75 mm (approximately 3 months). Blood vessels (large arrowheads) are adjacent to the indented portions of the pigment epithelium. The nonpigmented epithelium of the inner wall also starts to buckle (hollow arrow). Fiber strands (faisceau isthmique of Druault) or marginal bundle connect the vitreous with the mesoderm around the rim of the optic cup (small arrowheads). AC, anterior chamber; cap, capsulopupillary vessel of the hyaloid system; C, cornea; pm, pupillary membrane. Fig. 23. Region of angle of a fetus at 170 mm (approximately 5 months), showing thick iris root with a branch from the major arterial circle (arrowheads) passing into the primitive iris stroma (Ir), in which the vessels are layered at two levels (curved arrows). The pigment epithelium is indented by thin-walled vessels (astericks). AC, anterior chamber; ci, nonpigmented ciliary epithelium (oblique to the plane of section); C, cornea; cs, canal of Schlemm; Tr, trabecular metshwork. During the fifth month, a series of loops of vascular arcades reach centrally into the mesenchyme of the growing iris (see Fig. 23). These originate from branches of the long ciliary arteries. Immature tight junctions unite endothelial walls of the iris vasculature as soon as they are formed; there are no fenestrations.122 The arteriovenous loops of the pupillary membrane come to be arranged over the sphincter region and are the basis for the formation of the collarette. During the sixth month there is resorption of the axial (pupillary) portion of
the pupillary membrane with subsequent atrophy of the blood vessels. The rest of the pupillary membrane disappears during the seventh and eighth month, not so much by dissolution as by remodeling of its constituents. The mesenchymal frame of the pupillary membrane is incorporated into the prospective iris stroma. Programmed cell death and phagocytosis by macrophages are involved in regression of the pupillary membrane. Dysfunction of any of these processes may play a role in the persistence of the pupillary membrane.123,124 Neuroectodermal Constituents of the Iris The inner layer of anterior portion of the optic cup differentiates into the posterior iris epithelium (continuation of the nonpigmented ciliary epithelium; Fig. 24). Pigmentation proceeds gradually from the pupillary margin, beginning at midterm (Fig. 25), toward the ciliary region and is completed during the seventh month. Fig. 24. Section through a portion of the anterior segment at 85 mm (fourth month) ( 192). The immature ciliary processes (Cp) are at a level anterior to the angle. Pigmentation of the inner layer of the marginal sinus (ms) has reached its base. The future iridial portion of the cup (Ir) has an unpigmented cuboidal, basal lamina-lined epithelium (hollow arrow). Nonpigmented cells in the valleys of the primitive processes are columnar (arrowheads) and slanted in the direction of the fibers. These are attached into a basal lamina-like condensation and stretch partially over the gap between ciliary folds and lens. They are the primitive zonula fibers (zf). Arrow points to the fetal origin of the vitreous base. The atrophying capsulopupillary vessels are indicated by the asterisk. Curved arrow points to anlage of ciliary muscle. AC, anterior chamber; cim, major arterial circle; LE, lens epithelium; Sc, sclera. Fig. 25. Anterior segment of an eye of a fetus at 95 mm (estimated age, late fourth month) showing the short iridial portion (Ir) and a more developed ciliary portion (Cp) of the forward-growing neuroectodermal cup ( 69). Recognizable primordium of the ciliary muscle (cm) exists. Future ora serrata region is indicated by the arrowhead. Sphincter muscle starts to differentiate in the area shown in the square. cj, conjunctival stroma; C, cornea; Li, eyelid; R, retina; Sc, sclera; Vi, vitreous.
The smooth muscles of the pupillary sphincter and dilator muscles represent the only muscles in the body of neural ectodermal origin. In avian species, however, the pupillary muscles are striated and originate from stromal mesenchymal (neural crest) cells that migrate into the muscle bundles to become skeletal muscle cells.125 The first sign of differentiation of the sphincter muscle is the appearance of basal infoldings in the anterior epithelial layer (continuation of pigment epithelium) near the rim of the optic cup (see Figs. 17B and 25). This change is followed by reduced melanogenesis. At 3 months' gestation, fine fibrillar material is present in the basal part of these cells. In the sixth month, connective tissue septa and capillaries invade the muscle bundles and separate them from their origin, except at the pupillary edge. The muscle comes to lie free in the posterior mesenchymal layer (see Fig. 17D).126,127 The dilator muscle develops later than the sphincter with fibers identified in the sixth month. The first sign of their differentiation is the appearance of fine fibrils in the columnar cells of the anterior epithelium (see Fig. 17E). The myoepithelial cells have a spindle shape, are contractile, but remain attached to their anterior epithelial site of origin. A basal lamina covers cell surfaces facing the stroma. Capillaries or mesenchymal septa do not invade the sheet of the partially differentiated muscle, which continues to develop after birth.127,128 Melanogenesis Pigmentation varies according to individual or racial coloration. In the macaque, chromatophores are noted in the iris stroma until term, in contrast to the human in whom pigmentation occurs between 6 and 7 months' gestation.129 Melanosomes in the human iris are mature at term.130 Most chromatophores, as seen with the optic microscope, appear to develop postnatally. Pigmentation in the anterior border layer is insignificant. If the stroma has a scant collagen fibril content and is thin, it allows the pigment epithelium to peek through and a brownish color is noticeable. Blue irides have a transparent anterior border layer allowing interference or double refraction in the region of the stromal collagen. In the newborn, the superficially flat iris is not fully developed. The stroma is very thin and delicate with poorly formed connective tissue sheaths around the vessels. The collarette is nearer the pupil, but the anterior leaf is more completely developed around the pupil and not so transparent. Collagen formation is enhanced during the ninth month of gestation; it occurs first in the anterior stromal layers near the sphincter and then proceeds peripherally. In the newborn, however, the anterior leaf remains narrow; it grows toward the periphery but does not reach the iris root, where most of the obliterated
vessels end. CILIARY BODY The Ciliary Epithelium The anterior margin of the two-layered neuroectodermal optic cup lags behind the retina in differentiation (Fig. 26). Some evidence suggests proximity to the lens is required for differentiation of iris and ciliary body. Late in the third month (at 50 to 54 mm), longitudinally oriented interdigitations commence in the outer, pigmented layer of the anterior portion of the forward-growing cup, behind the advancing margin (see Figs. 17A and 21). By 12 weeks (at 65 mm), the outer (pigmented) layer starts to form meridional ridges; to adhere to the inner nonpigmented layer and to fold with it (see Figs 17B and 22). These 70 to 75 radial folds and ridges are the precursors of the ciliary processes. The growing tip extends forward, carrying with it the folded portion, which increases in complexity. A smooth region (the future pars plana) involving both epithelial layers comes to lie equatorial to these primitive ciliary processes (Fig. 27). Fig. 26. General view of the eye at approximately 20 mm (45 days). Ciliary portion of the neural cup (Ci) is relatively undifferentiated and extends to about the level of the lens equator. The mesenchyme around and anteriorly to the margin of the cup shows at least two different degrees of condensation, separated by an interface (arrow). Mesenchymal cells do not yet fill the center of the space between corneal epithelium and endothelium. Anlagen of the extraocular muscles (mu) are recognizable. Upper and lower lids (Li) are undifferentiated skin folds. An anterior chamber (AC) is delineated by the pupillary membrane at the arrow. Major components of the hyaloid vasculature (Hy) are represented. (Smelser GK: Embryology and morphology of the lens. Invest Ophthalmol 4:398, 1965.) Fig. 27. Schematic diagram of the developing ciliary body and iris; their relation to the positions of Schlemm's canal and ora serrata. Drawn after sagittal sections of celloidin-embedded eyes. A. At 4 months, a rough triangular anlage of the meridional ciliary muscles fibers (shaded) is present behind the angle recess. Arrow points to the incipient ora serrata behind the most posterior ciliary fold. Ectodermal iris is short and the canal of Schlemm (arrowhead) is behind the bottom of the angle. B. At 6 months, the meridional fibers
(shaded) are connected to the scleral spur, behind the angle. Some circular ciliary muscle fibers begin to differentiate and the pars plana and ectodermal iris lengthen. The canal of Schlemm (arrowhead) is mostly behind the level of the deep portion of the angle. The ora serrata is located over the middle portion of the ciliary muscle and is indicated by the arrow. C. At 7 months, one third of the pars plana covers the meridional ciliary muscle and the circular fibers (shaded) are well established. The angle has deepened so that the canal of Schlemm (arrowhead) is at its level. The ora serrata is indicated by the arrow. D. At 9 months, the pars plana lengthens and is over two thirds of the meridional fibers (shaded). The ora serrata is marked by the arrow. The iris is nearly fully developed but still has a thick root. The canal of Schlemm (arrowhead) is in its definitive location anterior to the angle recess. Stromal (and Vascular) Components of Ciliary Body and Processes With the accumulation of mesenchyme between the growing margins of the optic cup and surface ectoderm, differentiation of the stromal elements of the ciliary body begins (see Fig. 24). Primitive ciliary muscle fibers are visible in the mesenchyme between the infolding region and the scleral condensations late in the third month.132 Parallel vessels surround the anterior part of the optic cup and give rise to an irregular capillary-venous network (Fig. 28). During the fourth month, branches penetrate the mesenchyme that forms the core of the growing ciliary processes. The invading buds consist of endothelial ridges that develop lumina arising from the confluence of their intracytoplasmic vesicles with intercellular spaces.133 As soon as canalization is accomplished, pores appear in the endothelial wall, but the basal laminae are intermittent. Thus, each primitive ciliary process has a vascular branch connected to the capillary net in the associated mesenchyme. This predominantly venous network is formed from branches of the parallel vessels continuing forward from the anterior portion of the choroidal vascular investment. The small twigs within each process make an elaborate, mostly venous, tufted plexus. Fig. 28. Schematic diagram of the development of choroidal vasculature. A. During the second month, primitive vascular meshwork in the mesenchyme around the pigment epithelium connects with small arterial branches of the precursors of the short posterior ciliary arteries (SPCA) that arose from the ophthalmic as two trunks together with the long posterior ciliary arteries (LPCA). These two long arteries run anteriorly through the meshwork,
which is drained by tributaries of the infra- and supra-orbital venous plexuses (VV). B. Several future vortex veins (curved arrow) are anterior to the equator of the globe. The two long posterior ciliary arteries bifurcate and start to encircle the region of the future ciliary body. Two to three short posterior ciliary arteries send twigs to the scleral condensation surrounding the optic nerve (CZ). These are the precursors of the circle of Haller-Zinn. In the peripheral choroid the primitive capillary net still has a palisade-like arrangement. C. During the fourth month, a layer of larger vessels form. They are mainly tributaries of the vortex veins (left side). Medium-sized branches of the short posterior ciliary arteries become intercalated between the choriocapillaries and the large venous channels in the posterior choroid (asterisk). Anterior part of the choroidal vasculature, mostly venous, has parallel channels that break into a network in the emerging ciliary region. The long posterior ciliary arteries form the major arterial circle. Interarterial anastomoses are present. (Adapted and redrawn from Heimann K: The development of the choroid in man. Ophthalm Res 3:257, 1972.) During the fourth month, the long ciliary arteries have formed the major arterial circle (see Fig. 24), and by the end of the fifth month, recurrent branches from it are seen in the ciliary body region. Each of these processes, however, receives one arterial branch only during the eighth month. Anastomosis with vessels of the arterial layer of the choroid is then established. In general, ultrastructural expression of physiologic barriers, such as the blood-aqueous and blood-retinal barriers, is established early in gestation, almost simultaneously with the recognizable differentiation of the cells with which this concept is associated (i.e., tight junctions in the retinal or iris capillary endothelia and the pigment epithelium). Fenestration of the choriocapillaris and capillary endothelium of the ciliary processes is observable soon after lumina occur in these channels, thus providing the basis for their permeability. Fine Structure of Ciliary Epithelia In the early fetus, the inner (vitreal) surface of the nonpigmented ciliary epithelium exhibits irregularities and conical filaments (see Fig. 24) covered by a basal lamina. Ciliary channels have been observed in human fetuses between the fourth and sixth months.131,133 They are enlargements of the intercellular spaces between the apposed apical surfaces of the pigmented and nonpigmented epithelial cells of the ciliary processes. These channels are presumed to correlate with the onset of aqueous secretion and to constitute a primary reservoir for the aqueous humor. Basal infoldings into the vitreal aspect of
the nonpigmented epithelium facing the posterior chamber are noted prenatally in the nonhuman primates134 and in the ninth month in humans. Ciliary Muscle The ciliary muscle (see Fig. 27) develops in situ and, during the fourth month, organizes into fibers and strands. The triangular meridional portions differentiate in the fifth month. The anterior ends of the fibers are continuous with the developing scleral spur (see Fig. 25), although the tendons are not formed until 7.5 months. Circular fibers appear on the inner anterior aspect of the meridional muscle. The bundles increase in size and organization during the seventh month but are still incompletely formed at birth. The muscle then consists of slender bundles no thicker than one or two cell layers, whereas the meridional part adjacent to the sclera is more fully developed. Muscle fibers increase during the first year of life, but the connective tissue between the bundles and the amount of stroma do not grow much. With growth of the eyeball, the pars plana region elongates (see Fig. 27) so that the ora serrata, which is even with the midpoint of the ciliary muscle at 7 months' gestation, comes to lie on a level with its posterior third during the ninth month. Muscle capillaries are lined by continuous endothelia interconnected by tight junctions from the time of their formation. Unlike those of the ciliary processes, they are not fenestrated. CHOROID The stroma of the future choroid is wide and of a loose texture, surrounded by denser scleral mesenchyme by the end of the third month of gestation. Collagen fibrils have developed, and the fibroblasts are abundant with distended endoplasmic reticulum indicative of active protein synthesis.135 Experimental studies have demonstrated that the neural tube is essential for the appearance of choroidal melanoblasts. At a later age, after the elements of the neural crest have migrated and have reached the periocular tissue, this mesenchyme is capable of determining the choroidal pigmentation.136 Uveal melanocytes have the same neural crest origin as dermal melanocytes, differing in this respect from the pigment epithelium, which is strictly neural ectodermal in origin. However, the method by which melanosomes develop is identical in both choroid melanocytes and pigment epithelium.137 The structural foundation of the choroid is its vasculature (Fig. 28). Vessels originating from endothelial blood spaces appear early in the mesenchymal tissue in close proximity to the outer, pigmented layer of the newly formed optic cup.138 Their channels coalesce to form the annular vessel at the rim of the optic cup. They drain into the two main blood spaces, the supraorbital and infraorbital venous plexuses. During the second month (10 mm), the embryonic choriocapillaris forms around the developing pigmented epithelium, continuous with a plexus around the rim of the neuroectodermal
cup (see Fig. 28A). Near the end of the month, some larger channels of these sinusoids connect with small twigs from a few short precursors of the posterior ciliary arteries that reach the vascular choroid by 30 mm. Rudimentary vortex veins are formed by the confluence of collecting channels that drain the plexuses (see Fig. 28B). Arteries have narrow lumina and walls with two or more cell layers; veins are enclosed only by endothelium. With growth during the third month, the capillary bed stretches, some components enlarge and form the outline of a second venous layer. The capillaries situated beneath them become closed and a definitive choriocapillaris emerges. Normal choriocapillaries develop only from mesoderm that has been in contact with pigment epithelium. Extensions from the short posterior ciliary arteries radiate into this vascular bed, branch repeatedly, and empty directly into the choriocapillaris, which thus contains both arterial and venous components and reaches from papilla to equator. More anteriorly, only the primitive venous choriocapillary system exists at this period of development (see Fig. 28C). Back to Top NEUROECTODERMAL LAYERS RETINA Retinal morphogenesis in humans and other species has been the subject of many investigations.139158 Early Differentiation The primordium of the retina is present at the optic pit stage early during the third week of gestation even before closure of the neural tube (see Fig. 4A and B). The anterior part of the optic vesicle, the retinal disc, is the future neural retina, and has a marginal nonnucleated layer in contact with the lens placode. The sides of the invaginating vesicle are destined to become the pigment epithelium (see Fig. 9C and D). Following vesicle invagination to form the optic cup, the inner layer has an outer nuclear zone and an inner anuclear marginal zone. The outermost layer of cells of the nuclear zone (the germinating, or proliferative layer) projects cilia to the surface of the contacting outer layer, or future pigment epithelium. These cilia disappear during the seventh week. They are replaced by the precursors of the photoreceptor outer segments during the fourth month. The outer layer of the cup has two to three layers of pseudostratified columnar cells that enclose pigment granules at 33 days' gestation (7 to 9
mm). This layer produces the earliest pigmentation in the body. Punctate tight junctions near their apical ends join the cells. The basal lamina that originally surrounded the optic vesicle remains continuous over the inner (vitreal) and outer surfaces of the optic cup. The primitive retinal cells rest on a basement membrane that faces the inner future vitreal aspect and extend their apices toward the pigmented epithelial cells. In general, mitotic figures occur in the outer zone and prevail longest in the outer surface layer adjacent to the space representing the remnant of the primary optic cavity; and at the margin of the optic cup (future ciliary body-iris region). Mitosis first ceases in the central area; growth goes on longer in the periphery. Most cell division in the presumptive retina occurs before 120 mm (approximately 15 weeks). It is not established when mitosis ceases in the pigment epithelium. It is probably limited to the periphery in late fetal life. Formation of Layers Retinal differentiation commences when mitosis has practically stopped. It spreads from areas facing the future vitreous (marginal zone) toward the primary optic cavity, and from the center of the base of the optic cup (inner neuroblastic layer) toward its edge.159 Retinal ganglion cells and Mller's cells generally develop almost simultaneously. Here also, however, a gradient exists, given that axons and dendrites of ganglion cells near the optic nervehead differentiate earlier than those situated at the periphery. By proliferation and migration of cells, the neural epithelium separates into inner and outer neuroblastic layers in the seventh week of gestation (13 to 17 mm; see Fig. 11E and F). A few days later, a definite narrow nerve fiber layer is established, occasionally traversed by the radial fibers of the Mller cells. Immature ganglion cell bodies move into the inner neuroblastic layer along with other less mature cells, presumably future amacrines, creating in their wake a nuclei-free entanglement of processes, the transient fiber layer of Chievitz (Figs. 29A and 30A). With further realignment of cells, this layer is mostly obliterated by 8 to 10 weeks' gestation. At this period, the cells of the inner and outer neuroblastic layers intermingle by means of their cytoplasmic extensions. They fill up the previously acellular Chievitz layer; cell bodies shift positions, establishing a new, comparatively cell-free zone of intertwined processes, the inner plexiform layer (50 to 55 mm, approximately 10.5 weeks) (see Figs. 29B and 30B). With the emergence of the inner plexiform layer, an inner nucleated layer, consisting mostly of the cell bodies of ganglion cells, becomes separated from an outer neuroblastic zone. The cell bodies of the Mller's cells and the developing amacrines are located near the inner border of the outer neuroblastic zone. Bipolar cells differentiate mostly from the middle portion of this outer zone, whereas
horizontal cells and photoreceptors arise from its outermost region (see Fig. 29B and C, and Fig. 30B and C). These developmental processes are well under way by 10 weeks to 12 weeks (approximately 60 to 80 mm), when an identifiable outer plexiform layer separates the immature horizontal and bipolar cell nuclei from those of the photoreceptors. Fig. 29. Schematic diagram of the developing retina. Region of the posterior pole is represented in sagittal section in every diagram. A. At 2 months, transient fiber layer of Chievitz, which separated the inner from the outer neuroblastic layers of the primitive retina, is slowly being obliterated by shifting of the nuclear elements and realigning of their processes. Uppermost cells, lying vitread, are differentiating into ganglion cells. Those below the uneven transient layer of Chievitz (*) are immature, but destined to differentiate into amacrine and Mller cells. The future inner plexiform layer will be located between the shifted nuclei of the latter and those of the ganglion cells. The outer neuroblastic layer contains photoreceptor, bipolar and horizontal cell elements. B. At midterm (4 months), retinal lamination is essentially complete. The ganglion cells have a multilayered arrangement. The inner plexiform layer, composed of fibers of bipolar, ganglion and amacrine cells supported by mllerian fibers, has established sites of primitive conventional and ribbon synapses. In the inner nuclear layer, the still undifferentiated cellular components are recognizable by shape and position. In the outer nuclear layer, large cone nuclei are aligned adjacent to the pigment epithelium and the smaller rod nuclei are positioned more vitread. The outer plexiform layer has primitive lamellar synapses between bipolar cell dendrites and cone pedicles (not indicated). Photoreceptor outer segments are not yet present. C. At 5.5 months, the ganglion cells have thinned out to one to two layers (except in the macular area). The cellular components of the inner nuclear layer include amacrine cells with large pale nuclei in the innermost (vitread) zone of this layer; and pleomorphic, dark-staining mllerian cell nuclei; both these types originally came from the inner neuroblastic layer. Also included are the smaller bipolar cells and large, pale-staining horizontal cells that are in an irregular arrangement sclerad. These two cell types are derived from the outer neuroblastic layer, together with the photoreceptors. The outer plexiform layer has a linear arrangement of synapses between bipolar cells and rod spherules (key symbol). The outer nuclear layer consists of six to seven layers of nuclei; the outermost are cones aligned to the external limiting membrane. Growing photoreceptor outer segments projected into the space between
pigment epithelium and external limiting membrane (arrowhead). Cell death is represented by the round dark-centered symbols. D. Newborn retina has the adult configuration with vascularization (arrowheads) reaching the outer limits of the inner nuclear layer. Outer plexiform layer is thinner than that in the adult, but the line of synapses is well established (key symbol). Rod and cone inner and outer segments are fully developed and the tips of the outer segments contact the pigment epithelium. Fig. 30. A. Portion of retina at the fundus of a fetus at 50 to 55 mm (about 10 weeks). The inner neuroblastic layer (1) is separated from the outer neuroblastic layer (2) by a slowly disappearing layer of Chievitz (*). The pigment epithelium (PE) has a single layer of cells. NF, nerve fiber layer ( 560). B. Portion of the central area from a monkey retina at 76 days, comparable with that of human at approximately 3.5 to 4 months). 1, nerve fiber layer; 2, ganglion cell layer; 3, inner plexiform layer; 4, inner nuclear layer; 5, narrow outer plexiform layer. Cone nuclei (co) are aligned next to the external limiting membrane (arrow) ( 752). C. Section through a portion of the retinal fundus of a macaque fetus at 86 days (comparable with that of human at midterm). 1, nerve fiber layer; 2, ganglion cell layer; 3, inner plexiform layer; 4, inner nuclear layer; 5, outer plexiform layer; 6, outer nuclear layer. D. Section through the fundus of a retina of a fetus at 190 mm (estimated age, 5.5 months). The numbering of the layers is as in C. Double-headed arrow indicates blood vessels in the ganglion cell layer. Arrowhead on the bottom points to photoreceptor inner segments protruding into the extracellular space beyond the external limiting membrane ( 650). (B and C from Smelser GK, Ozanics V, Rayborn M, Sagun D: Retinal synaptogenesis in the primate. Invest Ophthalmol 13:340361, 1974.) Synaptogenesis precedes development of photoreceptor inner and outer segments by almost 2 months. Lamellar synapses start to form early in the fourth month in cone axons and bipolar terminals, as well as conventional synaptic complexes associated with amacrine cells; when these phenomena are operable, the cells are still immature. The newborn's retina has configuration and layers of the adult's. The photoreceptor outer segments are well developed and in contact with the pigment epithelium. Synapses of the outer plexiform layer are apparent (see Figs. 29D and 30D).
Early in retinal morphogenesis, limiting membranes are established. Junctions of the zonula adherens type, representing the external limiting membrane of the retina are present in the fifth week between the outer plasma membranes of adjacent neuroblasts. A thin basal lamina exists over the inner surface of the marginal layer even before the lens vesicle formation. Contribution of basal laminalike material from developing Mller's cell processes combines with it to form the primitive internal limiting membrane. Photoreceptors The first indication of inner segment differentiation is the appearance of cilia in the outer cells of the external nuclear layer of the 10-week-old fetus. Later, the cell membrane becomes involuted, envelops the centriole, and forms a cylindric cytoplasmic process facing the apical portion of the developing pigment epithelium. Outer segment formation commences at 5 months. Outer segments start to develop when the ciliary filaments provoke infolding of the plasma membrane. These folds multiply, entubate, and then separate from the plasma membrane to be randomly distributed within the cytoplasm. Finally, they flatten and rearrange themselves to assume a stepladder architecture as lamellar sacs.142,160 Although the major retinal constituents are laid down by the beginning of the fourth month, horizontal cells are distinguishable only as an irregular row during the fifth month, paralleling the development of the incipient photoreceptor inner and outer segments. The amacrine and ganglion cells are in their definitive locations and are more differentiated at the same time. Macular Development Differentiation of this specialized area of the retina, the macula, commences relatively late and involves accumulation and redistribution of the neuronal elements. This subject has been reinvestigated in research in a series of monkey fetuses and neonates.161 The earliest evidence of maculogenesis is the localized increase of superimposed nuclei in the ganglion cell layer at the posterior pole, temporal to the disc, during the fifth month. At 6 months' gestation, the center of the macula has eight to nine rows of nuclei and bulges slightly above the inner surface of the retina surrounding it. Deep to the thickened ganglion cell layer, the layer of Chievitz is present, which persists until after birth, in the macular region. The wider outer nuclear layer consists mainly of immature cones. During the seventh month, peripheral displacement occurs in ganglion cells. The thin layer within the incipient foveal depression combines with elements of the inner nuclear layer. The synaptic contact established among photoreceptors, bipolar cells, and ganglion cells in the human central retina between the 10th to 15th weeks is maintained despite this shifting of nuclei.
The cones develop long basal axons to accommodate these displacements.161 Changes in the shape of the foveal cones progress until after birth, involving a decrease in the width of the inner segments and lengthening of the outer segments, thus allowing an increase in foveal cone density. In the 8-month fetus, there are two layers of ganglion cells over the slightly depressed central area; these are reduced to a single layer in the newborn (Fig. 31). A thin inner nuclear layer is still present. By 4 months' postpartum, both layers have retreated to the fovea slopes, leaving the cone nuclei practically uncovered in the center of the depression. Fig. 31. Fovea of Macaca mulatta just prior to birth (159 days; term at 162 to 165 days). One interrupted row of ganglion cells and one to two layers of bipolar cells still extend across the foveal depression. A wide and welldeveloped horizontal outer plexiform layer of Henle (asterisks) and elongated cone inner and outer segments are present. The parafoveal area has the large accumulation of cells in the ganglion (G) and inner nuclear layer (IN) characteristic of the mature macula ( 95). Retinal Periphery Between 4 and 6 months, the ciliary body and retinal regions become distinct with a well-delineated ora serrata nasally (see Figs. 24, 25, and 27). At this time, a thin nerve fiber layer is present in the peripheral retina. The formation of the ora serrata goes on concurrently with that of ciliary process development. At 8 to 9 months, the temporal ora is complete (see Fig. 27D). Scalloping at the ora is increased after birth, presumably because of disproportionate postnatal growths of the pars plana and ora serrata zones compared with parallel growths during fetal life. The region from the ora to the equator of the retina continues to enlarge until 2 years of age. The surface area of the newborn retina is approximately 589 mm2.162 Retinal Vascularization Retinal angiogenesis has been extensively studied in laboratory animals163 177 and reexamined in humans.178,179 In humans, at approximately the 5 mm stage, the terminal portion of the primitive ophthalmic artery, a branch of the internal carotid artery, invades the optic fissure from below. After closure of the fissure between the fourth and fifth weeks, the vessel remains within the cavity of the optic cup (see Fig. 9F). It is now termed the hyaloid artery and its intraocular branches soon spread between the marginal zone of the primitive retina and the lens vesicle. The hyaloid artery supplies the nutritive
requirements of both the lens and the growing retina before the latter acquires its own vasculature. At 65 to 70 mm (approximately 12 weeks), vessels derived from the ophthalmic artery accompany the hyaloid artery for some distance. As the hyaloid artery regresses during the fourth and fifth months, retinal vessels develop. Primitive retinal vessels emerge near the hyaloid artery as it enters the optic disc.180 Spindle-shaped mesenchymal cells, apparently derived from the wall of the two venous channels at the disc, form aggregations around the hyaloid vessels.179,181 Buds or strands of cells thereafter push into the nerve fiber layer (Fig. 32). The proximal intraneural portions of the hyaloid vessels subsequently become the central retinal artery and vein. During the fifth and sixth months, lumina with occasional red blood cells appear within the solid cords. These may anastomose with adjoining cords, or primitive vessels, thus forming a polygonal network. Branches spread in depth to the outer border of the inner nuclear layer by the ninth month (see Fig. 29D). Fig. 32. Schema of the growing retinal vessels at approximately 4 months. Main branches of the central retinal artery and those of the central retinal vein extend a short distance in the nerve fiber layer toward the equator. In the center, the regressing hyaloid artery is enveloped by a mantle of glia (arrow). Within the anterior half of the nerve, the central retinal artery is usually flanked by two accompanying veins that eventually merge to form the central retinal vein (key symbol). As the primitive capillaries push toward the retinal periphery, they reach the ora serrata by the eighth month of gestation.180 From the fourth to the seventh months, the growth rate of these new vessels is about 0.1 mm/day. Because the mature pattern is attained at approximately 3 months after birth, the retinal vasculature is sensitive to postnatal developmental disturbances. In tissue culture experiments, it has been demonstrated that the retinal capillary endothelial cells retain their embryonic potential and can revert to more primitive cell types that can then redifferentiate into endothelial, fibroblastic, or muscle cells. Therefore, it is possible that endothelial cells, pericytes, and muscle cells may have a common origin. Late in gestation, the vascular endothelium of the retina is continuous, with single or multiple points of fusion between the cells. The effect of oxygen on developing retinal vessels is contingent on the
developmental sequence in angiogenesis. A relatively large capillary-free zone lies immediately adjacent to the retinal arteries and a similar zone, although much less evident, surrounds the veins. This capillary-free zone results from retraction and atrophy of the channels adjacent to the growing vessels, which is a more active process near the arteries. Such zones are found in fetuses at 6 to 9 months. This periarterial capillary-free space can be caused to widen by increased oxygen concentration in kittens,170 thus accelerating capillary retraction and atrophy. Anoxia has the reverse effect. PIGMENT EPITHELIUM The outer wall of the optic cup is composed of a mitotically active pseudostratified columnar epithelium. The inner surface bears cilia that disappear with the advance of melanogenesis at 33 days (7 to 9 mm). From between 6 to 6.5 weeks, the prospective pigment epithelium is a monolayer of cuboidal cells (see Fig. 30A) the apical surfaces of which are reflected into short projections against the future photoreceptor outer segments. Its total surface area is 240 mm2 at the fourth month, which increases to 800 mm2 by 2 years of age. On surface view, the cells are hexagonal. This is the first tissue in the body to exhibit melanogenesis. Pigment granule formation is similar to that occurring within the epidermal melanocytes. The melanin is deposited on the folded inner membranes of vesicles that are probably of Golgi body or outer nuclear membrane origin.147 The common origin of the inner and outer layers of the optic cup is demonstrated in mouse mutants exhibiting dysplasia of the retinal pigment epithelium resulting in formation of a second layer of neurosensory retina.182 The sequence of pigment epithelial differentiation is inferred from the cytoplasmic organelles seen at various stages. These indicate involvement initially with protein synthesis (ribosomes), then membrane and polysaccharide synthesis.183185 The deeper lateral and basal infoldings, the latter associated with transport, also become prominent during gestation.186 There is a continuous, although at a slower rate, addition of new pigment epithelial cells during fetal life. No mitotic figures are observed in the postnatal retinal pigment epithelium. The individual cells simply enlarge (hypertrophy rather than hyperplasia) to cover the large area created by further growth of the eyeball.187 Back to Top BRUCH'S MEMBRANE In the optic cup stage, the basement membrane lamella of the pigment epithelium is very well formed, but that of the choriocapillaris is either lacking or very delicate. The numerous fibroblasts in this region diminish later, leaving behind these collagenous fibrils. The interstitial spaces between
the choriocapillaris channels are wide, and Bruch's membrane is bordered primarily by the collagen fibrils of the choroidal stroma. After midterm, the elastic component forms a nearly continuous fenestrated sheet. The collagenous layers thicken apparently without the direct intervention of fibroblasts, which now lie within the choroid. Incomplete basal lamina formation around the proliferating choriocapillary endothelia is the last component of Bruch's membrane to appear.156,188 Back to Top OPTIC NERVE AND DISC OPTIC STALK The optic stalk forms a connecting channel between the vesicular cavities and that of the forebrain. (2 to 4 mm at 24 days; see Fig. 4E).27,189 Its involution commences simultaneously with the collapse of the vesicles into the optic cup stage at about day 29 (5 to 7 mm). A shallow groove is formed in the stalk (see Fig. 9F), extending from the optic (choroid) fissure almost to the forebrain. In the 5 mm embryo, the hyaloid artery is within this depression. Some drainage from the sinusoids surrounding the optic cup occurs through a tributary of the primitive maxillary vein. This channel, also located within the optic stalk groove, is probably the precursor of the future central retinal vein. Direct continuity exists between the inner layers of the cup and stalk (Fig. 33A); the region of the disc is outlined by the neuroepithelial tissue of the primitive papilla.
Fig. 33. Schematic drawing of the optic stalk and early optic nerve formation; their relation to the periocular vasculature. A. Developing optic vesicle and stalk seen from below. Embryonic fissure of the cup is closed except for a notch at the tip. It remains open in the stalk. The hyaloid artery and a terminal branch of the dorsal ophthalmic artery from the internal carotid artery are trapped within the fissure, as is a small twig from the maxillary vein. The other branch of the dorsal ophthalmic artery, which continues outside the cup, is the temporal long ciliary artery (hollow arrow). Ventral ophthalmic artery has a transitory anastomosing branch with the dorsal ophthalmic. The nasal ciliary artery came off this connection, which then disappeared (not shown), so that the dorsal ophthalmic artery eventually remains the only branch from the internal
carotid to the eye (see B). Upper and lower venous plexuses draining the blood channels in the mesenchyme next to the pigment epithelium form the primitive superior and inferior vortex veins that connect with the cavernous sinus. B. By 6 weeks, the proximal portion of the fetal fissure is closed up to the small opening for the hyaloid vessels. The interior of the eye is drained by terminal branches of the maxillary vein, which accompany the hyaloid artery and eventually empty into the cavernous sinus. (Redrawn from the film Embryology of the Eye. By permission of the American Academy of Ophthalmology.) C. Relation of the growing optic nerve to the vessels supplying the intraocular structures. The optic nerve has grown to 7 to 8 mm in length and 1.2 mm in width and its orbital portion is being vascularized from the septa (not drawn). Hyaloid artery is marked by curved arrow. (Drawing partly from a cleared specimen at 3 months.) Closure of the optic fissure commences during the fourth week, between 7 and 9 mm, with fusion of the central part of the optic cup. Its inner and outer margins fuse subsequently; closure of the cup is complete at 5 weeks (Fig. 34; see Fig. 33A). The lips of the optic stalk begin to close over the hyaloid artery at 12 to 17 mm, starting from the region near the forebrain and gradually extending distally (see Fig. 33A and B). Thus, fusion of the margins of the stalk lags behind that of the optic cup. The opening within the stalk through which the hyaloid artery enters is closed by 19 to 20 mm. Fig. 34. Almost completely fused embryonic (or optic) fissure has discarded cells around the contacting margins of the optic cup (arrow). Arrowhead indicates pigment epithelium (PE) without pigment granules around the region of fusion. Hy, arborization of hyaloid artery; Le, lens; NEp, neural epithelium. (Phase contrast, 450; courtesy of Professor Vrabec.) The margins of the optic fissure are covered by basal lamina. Breakdown of the basal lamina, inversion of the outer layers of the cup and stalk, degeneration of the superfluous cells, and eventual reconstitution of the basal lamina are essential events in normal closure.190 It is suggested that cell death helps to control the growth rate of optic cup and fissure.191 Given that cell degeneration precedes invagination, it may serve to retard or inhibit it locally, or to integrate the series of infoldings in the dorsal optic cup and optic fissure.192,193 MIGRATION OF NERVE FIBERS INTO THE INNER STALK LAYER
Some cells in the inner wall of the optic stalk vacuolate and receive axons from the ganglion cells of the retina. The fibers force their way through these spaces; by 19 mm, the fused optic stalk is almost completely filled by nerve fibers that surround the hyaloid artery. The primitive epithelial papilla with the hyaloid artery in its center is isolated by the confluent axons that course toward the brain. There is a potential space between the basal lamina around the hyaloid, which is covered by glia, and the basal lamina of the retinal surface, where the hyaloid artery enters from the papilla. This space between the artery and the glial sheath becomes accentuated with the atrophy of the hyaloid system. The segregated cells are converted to glia, which become the constituents of the primitive optic disc. Some of these glia are destined to form a conical formation around the hyaloid artery, called Bergmeister's papilla (Fig. 35). The cells of this last structure proliferate; by 4.5 months, there is a mantle around the regressing artery. The extent of the degeneration of these cells late in gestation defines the limit of the excavation at the disc.194196 Fig. 35. Section through the optic nervehead of a 65-mm fetus at 3 months. Bergmeister's papilla (arrowheads) represent the neuroepithelial cells that were displaced toward the center of the optic stalk around the hyaloid artery's entrance at the time (15 mm) when the axons of the ganglion cells made their right angle turn to pass through the stalk. Glial cells of the papilla also extend around the hyaloid artery as its sheath (hollow arrow). Optic nerve fiber bundles surrounded by rudimentary glial septa make a nearly right angle turn toward the scleral foramen (double arrows). Scleral condensation (Sc) merges into that of the developing dura mater (arrow). The inner nuclear and ganglion cell layers end sooner than the outermost cells of the outer nuclear layer of the retina at the exit of the optic nerve fibers. NF, nerve fiber layer; R, retina; Vi, secondary vitreous ( 160). LAMINA CRIBROSA The collagenous fibers of the sclera progress from the perilimbal region posteriorly, where they encircle the developing optic nerve, thus forming the scleral foramen. In the fourth month, connective tissue fibers penetrate the optic nerve, running between groups of glia-covered axons to reach the hyaloid vessel. Thereafter, a network of collagenous and elastic fibers forming a sievelike scaffolding, the lamina cribrosa, bridges the scleral foramen. The latter attains its mature structure during the seventh month. It should be emphasized that the openings filled with axons are present first, and the sieve subsequently invades around them. A faint suggestion of the
optic nerve sheath commences in the seventh week (before 20 mm), but it is precisely defined only in the fifth month (see Fig. 35). The sheaths are derived from the cranial neural crest mesenchyme. By the end of the third month, the optic nerve is 1.2 mm in diameter and 7 to 8 mm long (see Fig. 33C).197 MYELINATION Myelination starts in the fetus near the chiasm about the seventh month, progresses distally and stops at the lamina cribrosa about 1 month postpartum.198202 In the newborn, the myelin is extremely thin and seems to contain more cholesterol in the portion near the brain. During childhood, the number of myelin layers around the axons increases. Back to Top VITREOUS AND HYALOID SYSTEM Development of the vitreous includes both the embryonic blood vessels and the cells of the vitreous cortex, the hyalocytes. Many cytologic and biochemical investigations have been made on the developing hyaloid system and its regression.203215 PRIMARY VITREOUS Hyaloid Vasculature The primary vitreous mostly consists of the hyaloid vasculature with some minimal associated matrix and cellular (neural crest) components. Vascular endothelia are of mesodermal origin. The matrix may be a combined secretion of the vascular and neural crest tissue. The primitive dorsal ophthalmic artery gives off the hyaloid artery, which passes through the optic fissure during the fifth week of gestation (see Fig. 33) and branches within the cavity of the primary optic vesicle (Fig. 36A; see Fig. 9A). Arborization of the hyaloid artery produces terminal branches around the posterior lens capsule (the tunica vasculosa lentis). Other branches surround the lens equator and anastomose with the annular vessel around the outer edge of the optic cup (see Fig. 36B). The annular vessel sends loops forward and centrally, which compose the anterior tunica vasculosa lentis. Near the margins of the cup, anastomoses occur between the annular vessel and terminal branches of the hyaloid artery.
Fig. 36. Schema of the main features in vitreous development and regression of the hyaloid system shown in drawings of sagittal sections. A. At 5 weeks, the hyaloid vessels and their branches, the vasa hyaloidea propria, occupy much of the space between the lens and the neural ectoderm. One capsulopupillary branch (left) approaches the annular vessel. A capillary net joins the capsula perilenticularis fibrosa (curved arrow), which is composed of some ectodermal fibrils associated with vasoformative mesenchyme from the periphery. The ground substance of this primary vitreous is finely fibrillar. B. By 2 months, the vascular primary vitreous reaches its greatest extent. Arborization of the vasa hyaloidea propria (curved arrow) fills most of the retrolental area. It is embedded in collagen fibrils. An avascular secondary vitreous or more finely fibrillar composition forms a narrow zone between the peripheral (outer) branches of the vasa hyaloidea propria and the retina. Thick arrow indicates the posterior vascular capsule of the lens; in front of it, the channels with a palisadelike arrangement are the capsulopupillary vessels. They connect with the annular vessel. Hooked arrow points to the vessel of the pupillary membrane. Drawing is a composite of embryos at 15 to 30 mm. C. During the fourth month, the hyaloid vessels and the vasa hyaloidea propria, together with the tunica vasculosa lentis atrophies progressively, with the smaller peripheral channels regressing first. Large curved arrow points to remnants of involuted vessels of the superficial portion of the vasa hyaloidea propria in the secondary vitreous. The small curved arrow indicates the pupillary membrane (not sketched). The straight arrow points to the remnants of the atrophied capsulopupillary vessels. Zonular fibers (tertiary vitreous) begin to stretch from the growing ciliary region toward the lens capsule. Vessels through the center of the optic nerve connect with the hyaloid artery and vein and send small loops into the retina (open hollow arrow). The drawing is a composite of fetuses at 75 to 110 mm. The hyaloid vasculature reaches its greatest development at about 9 weeks (33 to 40 mm; see Fig. 36B). Venous drainage from the vessels of the tunica vasculosa lentis and the pupillary membrane is accomplished through vessels that assemble into a net in the region where the ciliary body will subsequently arise. This plexus eventually communicates with venules of the choroid. No hyaloid vein is present. SECONDARY (DEFINITIVE) VITREOUS Development of secondary vitreous occurs during the seventh to eighth weeks, after closure of the optic fissure. A finer, more compact fibrillar
network of monocytes and a small amount of hyaluronic acid characterize it.203208,213,215,216 This newer vitreous also contains cells, the primitive hyalocytes, which most likely originate from the vascular primary vitreous (mesoderm) and the neural crest mesenchyme, rather than the retina. Collagen fibrils are produced by the hyalocytes and result in expansion of the secondary vitreous volume. Vessel walls of the hyaloid system consist of endothelial cells with a discontinuous mural cell covering.210,211 The nonfenestrated endothelium is underlined by a continuous basal lamina. Expansion of the vitreous is associated with an overall increase in the volume of the globe through active production of aqueous humor.54 Failure of closure of the optic fissure prevents this normal establishment of intraocular pressure necessary for globe expansion. This is one mechanism of (colobomatous) microphthalmia. REGRESSION OF HYALOID VASCULATURE Beginning first with the atrophy of the distal branches of the hyaloid, followed by that of the capillaries of the tunica vasculosa lentis, and finally by that of the hyaloid artery itself (at end of the fourth month), the primary vitreous retracts with the atrophying vessels (see Fig. 36C). By 160 mm (in the fifth month), atrophy of the vasculature posterior to the lens creates the funnel-like expansion of Cloquet's canal. Before the capillaries disappear, they are occluded by macrophages.217 Persistence of the primary vitreous and failure of the posterior tunica vasculosa lentis to regress results in persistent hyperplastic primary vitreous (PHPV).214,218,219 TERTIARY VITREOUS AND ZONULAR FIBERS The secondary vitreous in the anterior peripheral region at the rim of the cup contains thicker, presumably aggregated collagen fibers. Some of these fibers abut the proliferating mesenchyme near and between the lens and the rim of the cup by the end of the third month (65 mm). These fibers form the marginal bundle of Drualt (Fig. 37; see Figs. 21, 22, 24, and 36C). Here, the vitreous is firmly attached to the internal limiting membrane of the retina. This constitutes the embryonic aspect of the vitreous base. Fig. 37. Commencement of tertiary vitreous, or zonular fibers in an 85-mm fetus (fourth month) ( 160). Newly formed zonula fibers (zf) span the space between the inner, nonpigmented ciliary epithelium (Ci) and the lens capsule (hollow arrow). The zonular fibers have crossed the faisceau isthmique (or marginal bundle of Drualt) at right angles (see Figs. 21, 22, and 24). The faisceau isthmique has retreated posteriorly with the
forward growth of the anterior portion of the optic cup and its anterior anchorage at this age is in the posterior portion of the developing pars plicata (arrow). This attachment will be displaced further posteriorly with growth of the eyeball. It is the fetal origin of the vitreous base. PC, posterior chamber; Vi, vitreous; Le, lens. Regression of the peripheral branches of the hyaloid vasculature precedes zonular fiber appearance. During the sixth and seventh months of development (200 to 300 mm), the stainable vitreous regresses to its base on the pars plana and to its attachment to the lens (capsulohyaloidal ligament). Late in gestation, the fibers of the suspensory ligament (zonules) appear to originate from precursors in the valleys between the ciliary processes just anterior to the ora serrata. Early zonular fibers seem to be a continuation of the internal limiting membrane, which thickens over the nonpigmented epithelial layer covering the ciliary muscle.220,221 Back to Top ADNEXA EYELIDS The eyelid territory is determined by the optic vesicle during the fourth week of gestation. Thus, microphthalmia that originates at the optic vesicle stage is associated with a small palpebral fissure whereas colobomatous microphthalmia may be associated with a more normally sized palpebral fissure. The upper and lower eyelids develop from mesenchymal condensations referred to as the frontonasal (paranasal) and maxillary (visceral) processes. The ectoderm of the skin proliferates in the region of the future upper lid at the outer canthus at 11 to 14 mm (6 to 7 weeks). In the second month (approximately 20 mm), both eyelid prominences are visible (Fig. 38A and B; see Fig. 26). Mesenchyme beneath the epithelium proliferates and is provided with blood vessels and accompanying macrophages. During the next few days, the basal lamina beneath the epithelium thickens. Invading nerve fibers and newly deposited collagenous fibrils are seen.224
Fig. 38. Eyelid closur e. A. At 6 weeks ' gestati on in the huma n, lid folds are appar ent (arrows). Note the relationship to the lateral nasal prominence (LNP), maxillary prominence (Mx), external ear (E), and forelimb (F) ( 40). B. At 8 weeks' gestation, the facial prominences have fused and the eyelids are beginning to close ( 20). C. At 9 weeks' gestation, the eyelids begin to fuse ( 300). D. Light micrograph illustrating the marginal epithelium (M) undergoing simultaneous proliferation and cell death in preparation for lid fusion ( 929). E and F. At 10 weeks' gestation, fusion is complete, and the epithelial proliferation associated with formation of the seam can be seen (E, 58; F, 280). (A-C, E, and F, courtesy of Kathy Sulik.) Lid Adhesion The lid folds not only move together by differential growth above and below the developing eye but also elongate laterally. The lid margins contact each other during the third month of gestation (approximately 35 to 40 mm; see Fig. 38C and D). Muscle cells originating from the mesoderm of the second visceral arch migrate to the face and eventually reach the area around the eye. After lid fusion at 10 weeks (45 mm; see Fig. 38E and F), rudiments of the orbicularis palpebrae muscle are present in the mesenchyme between the dermal and palpebral surfaces. Lid Disjunction According to observations made by electron microscopy, the two lids are temporarily joined by desmosomes during their adhesion, thus isolating the eye from the amniotic fluid.223 The period of lid disjunction varies but usually occurs through breaking of the connecting epithelial bridges (desmosomes) during the fifth month (150 to 170 mm) anteriorly, and at about 180 to 200 mm near the posterior surface (in the sixth month). The
main causes of this process are attributed to keratinization and the appearance of keratohyalin granules, which, in turn, are preceded by lipid manifestation in the Meibomian anlage. The adherence of the lids probably prevents the corneal and conjunctival epithelium from keratinizing. LACRIMAL GLAND About 25 mm, the developing lacrimal gland is seen in the form of epithelial buds arising from the basal cells of the conjunctiva covering the temporal portion of the upper fornix. The resultant solid cords are the core around which the surrounding mesenchyme condenses and proliferates. At around 3 months (approximately 60 mm), the central cells of the cords start to vacuolate and lumina appear. The growing tendon of the levator palpebrae divides the gland during the fifth month. Full development is reached by 3 to 4 years postnatally. EYELASHES The first cilia appear at the lid junction at about 40 mm. As the surface epithelial cells proliferate, they protrude, together with their basal laminae, into the underlying mesenchyme. Hair follicles of the cilia arise on both lid margins in an anteroposterior direction. The first row on the lower lid is completed before the second row on the upper lid.222 The glycogen and acid phosphatase content of most epithelial cells is high at this time. CONJUNCTIVAL AND LID GLANDS Mucus-secreting goblet cells in the conjunctival sac are visible at 10 weeks (52 mm). The Meibomian (sebaceous, holocrine) gland precursors are seen at 80 mm as epithelial buds and down-growths from the basal cells of the inner edge of the adhering lid margins (Fig. 39). The apocrine glands of Moll have their onset early during the fourth month (approximately 80 mm); their ducts arise from the walls of the ciliary hair sacs (see Fig. 39). The sebaceous glands of Zeiss appear at about 90 to 100 mm as lateral outgrowths on the epithelial invaginations constituting the first row of cilia. Soon after lipid production begins in these cells, a lipid-filled canal empties through the prospective hair shaft to the lid junction surface at 4 months (100 mm). The canals of the cilia keratinize at 110 to 120 mm. Hairs (cilia) are formed from the overlying epidermis and penetrate downward through the lipid-filled spaces. Keratinization of the walls of the Meibomian glands takes place at 5.5 months (170 mm). During the development of the cilia and Meibomian glands, their thickened basal lamina and the collagen fibers related to the orbicularis muscle fibers represent the future tarsus.
Fig. 39. Fetus at 4.5 months ( 45). The two lids are still sealed by epithelial cells, and the orbicularis muscle (large hollow arrow) is well formed. Lash follicles can be seen (arrows), the one on the right with a developing gland of Moll. Primitive meibomian glands and their ducts (arrowheads) are present. Stubby arrow indicates incipient keratinization. C, cornea. BONY ORBIT The lesser wing of the sphenoid is initially cartilaginous, derived from the base of the skull, while the greater wing of the sphenoid and the rest of the bones are membranous processes that ossify between the sixth and seventh months. Osseous structures of the orbit are mostly derived from the cranial neural crest cells, which migrate to surround the developing eye and additionally form the frontonasal and maxillary processes. The maxillary process contributes to the floor and lateral wall of the orbit, and the nasal process provides the lacrimal and ethmoid bones. The air sinuses develop mainly postnasally. The ethmoid is the first to take shape, between the sixth and eighth weeks. A fibrous trochlea is seen at 37 to 40 mm. During the third month, orbital walls are differentiated and later become incompletely ossified. The angle between the orbital axis starts at nearly 180 degrees, diminishes to about 105 degrees at 3 months, and reaches 71 degrees at birth. The adult condition is 68 degrees. This decrease results from growth of the tissue behind and lateral to the eyes. At term, the morphology of the orbital structures approaches that of the adult. The orbit fits closely to the eye at first and is nearly hemispherical. It grows as the orbital contents increase. If the eye does not grow, the orbit remains small, about 90% of normal size. The eye reaches its adult size by the age of 3, but the adult dimensions of the orbit are attained subsequently, sometimes as late as 16 years. EXTRAOCULAR MUSCLES The orbital contents consist of fat, connective tissue septa, and eye muscles. The former two are of neural crest origin. The human skeletal muscles of the trunk and limbs arise segmentally from each somite, as do the nerves.33,40,42 50,225,226 The mesoderm of the head region is in the form of somitomeres. In avian models, somitomeres 1 and 2 give rise to the dorsal, medial, and ventral recti, and to the ventral oblique muscles. Somitomere 3 forms the dorsal oblique and the lateral rectus arises from somitomere 5.51 There is a prechordal mass (first head somite) that gives rise to the premandibular
condensation, from which the four oculomotor muscles develop (third cranial nerve).225 The other two muscle primordia arise in the maxillomandibular mesoderm and give origin to the lateral rectus (from the third head somite) (sixth cranial nerve) and the superior oblique (from the second head somite, fourth cranial nerve). Mesenchyme within the orbit is the source of in situ differentiation.226,227 The cranial nerves grow from the brain into their respective mesodermal condensations in the following sequence: oculomotor, abducens, trochlear. In addition to these primordia, there are four other condensations around the outer rim of the optic vesicle. These are the future insertion sites of the rectus muscles, and they also participate in scleral morphogenesis. Condensation of the future fascia bulbi (Tenon's) is present at 45 mm (10 weeks, approximately). Near the end of the third month (60 mm), the tendons of the rectus muscles fuse with the sclera in the vicinity of the equator of the bulb. The levator muscle forms from the dorso medial aspect of the superior rectus muscle at about 22 to 30 mm and grows laterally and over the superior rectus toward the upper eyelid. It is complete and in its permanent position during the fourth month. NASOLACRIMAL DRAINAGE APPARATUS At about 32 days (8 to 9 mm), the maxillary processes, which contact the paraxial mesoderm surrounding the eye, extend forward to the nasal pit and processes. Their surface ectoderm covering is thicker over the grooved interface separating these processes. As the maxillary mesenchyme grows forward and upward over the thickened ectodermal strip and fuses with the lateral nasal processes and the paraxial mesoderm, it buries the ectodermal cells that line this rudimentary fissure. The origin of the lacrimal tract is attributed to this irregular ectodermal cord of cells, which separates from the surface ectoderm and extends caudal and cephalic branches into the mesenchyme beneath it.228 These cells come to lie between the future medial canthus and nasal cavity during the sixth week. The lacrimal sac anlage is derived from the adjacent upper portion of this nasolacrimal cord when it begins to thicken and bulge. Partial canalization is observed during the fourth month. The lacrimal puncta open after separation of the eyelids. The inferior extremity of the nasolacrimal canal fuses with a superiorly directed outgrowth of cells that originate from the nasal fossa during the sixth month or later. Back to Top CONCLUSIONS Much morphologic development of the eye occurs early in gestation with all major tissue elements present by 2 months of gestation. However, the
differentiation of epithelial or endothelial structures that control metabolic exchanges and intraocular fluid transport occurs later in gestation. These are expressed by the appearance of membranous infoldings in the plasmalemma of the ciliary epithelium, its vesiculation, and ciliary channels between the two apposed epithelia; similarly, vacuoles appear in Schlemm's canal when aqueous outflow is demonstrable. Morphologic indications of transport in the cytoplasm of the pigment epithelium, such as vesiculation and plication of the plasma membrane, also appear after midterm. Even without direct experimental data in the human or primate, the development of the functional specialization of the pigment epithelium can still be inferred from the presence and configuration of its other cytoplasmic organelles. Synthetic and secretory activity is usually assigned to the tubular smooth endoplasmic reticulum, which becomes abundant during the last trimester. It is associated, in other species, with fatty acid esterification of vitamin A. The contacts made by portions of the smooth-surfaced endoplasmic reticulum with mitochondria, as seen in premelanosome formation in the pigment epithelium, may indicate a morphologic mechanism for the enzymatic requirements to carry out pigment formation. Back to Top ACKNOWLEDGMENTS The specimens illustrated in Figures 4, 9, 11, 12, 38 were prepared in the laboratory of Kathy Sulik, University of North Carolina. Sulik's advice and guidance are gratefully acknowledged. Material from embryos and fetuses of known developmental stages were obtained through the courtesy of the late George K. Smelser. The source of some of the fetuses was the Population Council, Rockefeller University, New York. Some nonhuman embryos and fetuses were received from the Bionetics Research Laboratories of Litton Bionetics, Kensington, Maryland. In all experimental animal tissues, timed matings were used to control gestational age precisely. Photomicrographs from the collection of Ft. Vrabec, Chairman, First Eye Clinic, Charles University, Prague, the Czech Republic, are gratefully acknowledged. The technical assistance of Mary Rayborn and Deborah Dehart is acknowledged. Back to Top REFERENCES 1. Mann I: Development of the Human Eye. Philadelphia: Grune & Stratton, 1964 2. Barber AN: Embryology of the Human Eye. St Louis: CV Mosby, 1955 3. Dejean C, Leplat G, Hervout F: L'Embryologie de l'Oeil et sa Teratologie, p 288. Paris: Masson et Cie, 1958 4. Duke-Elder S, Cook C: In Duke-Elder S (ed): System of Ophthalmology.
St Louis: CV Mosby, 1963 5. Bartelmez GW: The formation of neural crest from the primary optic vesicle in man. Contrib Embryol Carnegie Inst 35:603, 1954 6. Berson D: The development of the choroid and sclera in the eye of the foetal rat with particular reference to their developmental interrelationship. Exp Eye Res 4:102, 1965 7. Clavert A: Role de la coupule optique et du cristallin dans la morphogense oculair. Arguments fournis par la teratogense. Arch Ophthalmol Rev Gen Ophthalmol 33:289, 1973 8. Coulombre AJ: Regulation of ocular morphogenesis. Invest Ophthalmol 8:25, 1969 9. Coulombre AJ, Coulombre JL: Lens development I. Role of lens in eye growth. J Exp Zool 156:39, 1964 10. Coulombre AJ, Coulombre JL: Mechanisms of ocular development. Int Ophthalmol Clin 15:7, 1975 11. Genis-Galvez JM: Role of the lens in the morphogenesis of the iris and cornea. Nature 210:209, 1966 12. Hay ED: Origin and role of collagen in the embryo. Am Zool 13:1085, 1973 13. Meier S, Hay ED: Control of corneal differentiation by extracellular materials. Collagen as a promoter and stabilizer of epithelial stroma production.Dev Biol 38:249, 1974 14. Lopashov GV: Developmental Mechanisms of Vertebrate Eye Rudiments. New York: Pergamon Press, 1963 15. Grainger R, Henry J, Henderson R: Reinvestigation of the role of the optic vesicle in embryonic lens induction. Development 102:517, 1988 16. Karkinen-Jaaskelainen M: Permissive and directive interactions in lens induction. J Embryol Exp Morphol 44:167, 1978 17. Kuno N, Kadomatsu K, Muramatsu T: Determination of the optimal time and dosage of all-trans retinoic acid for induction of murine exencephaly. Teratology 60:63, 1999 18. McKeehan M: Cytological aspects of embryonic lens induction in the
chick. J Exp Zool 31, 1951 19. Selleck MA, Bronner-Fraser M: The genesis of avian neural crest cells: A classic embryonic induction. Proc Natl Acad Sci USA 93:9352, 1996 20. Streit A, Stern CD: Neural induction: A bird's eye view. Trends Genet 15:20, 1999 21. McKeehan MS: The relative ribonucleic acid content of lens and retina during lens induction in the chick. Am J Anat 99:131, 1956 22. Cook CS, Nowotny AZ, Sulik KK: Fetal alcohol syndrome. Eye malformations in a mouse model. Arch Ophthalmol 105:1576, 1987 23. Cook C, Sulik K: Keratolenticular dysgenesis (Peters' anomaly) as a result of acute embryonic insult during gastrulation. J Pediatr Ophthalmol Strabismus 25:60, 1988 24. Cook C: Experimental models of anterior segment dysgenesis. Ophthalm Paediatr Genet 10:33, 1989 25. Stromland K, Miller M, Cook C: Ocular teratology. Surv Ophthalmol 35:429, 1991 26. Cook C: Embryogenesis of congenital eye malformations. Vet Comp Ophthalmol 5:109, 1995 27. O'Rahilly R: The early development of the eye in staged human embryos. Contrib Embryol Carnegie Inst 38:1, 1966 28. Le Lievre C, Le Douarin N: Mesenchymal derivatives in the neural crest: Analysis of chimaeric quail and chick embryos. J Embryol Exp Morphol 34:125, 1975 29. Jakobiec FA, Tannenbaum M: Embryological perspectives on the fine structure of orbital tumors. Int Ophthalmol Clin 15:85, 1975 30. Johnston M, Noden D, Hazelton R et al: Origins of avian ocular and periocular tissues. Exp Eye Res 29:27, 1979 31. O'Rahilly R. In Rohen J (ed): Eye Structure II Symposium p 557. Stuttgart: Schattauer Verlag, 1965 32. Torczynski E, Jakobiec FA, Johnston MC et al: Synophthalmia and cyclopia: A histopathologic, radiographic and organogenetic analysis. Doc
Ophthalmol 44:311, 1977 33. Meier S: The distribution of cranial neural crest cells during ocular morphogenesis. Prog Clin Biol Res 82:1, 1982 34. Blankenship TN, Peterson PE, Hendrickx AG: Emigration of neural crest cells from macaque optic vesicles is correlated with discontinuities in its basement membrane. J Anat 188:473, 1996 35. Dencker L, d'Argy R, Danielsson B et al: Saturable accumulation of retinoic acid in neural and neural crest derived cells in early embryonic development. Dev Pharmacol Ther 10:212, 1987 36. Greenberg J, Seppa S, Hewitt A: Role of collagen and fibronectin in neural crest cell adhesion and migration. Dev Biol 87:259, 1981 37. Nataf V, Lecoin L, Eichmann A et al: Endothelin-B receptor is expressed by neural crest cells in the avian embryo. Proc Natl Acad Sci USA 93:9645, 1996 38. Noden D. In Tasman W, Jaeger E (eds): Duane's Foundations of Clinical Ophthalmology, p 1. Philadelphia: JB Lippincott, 1993 39. Nye JS, McLone DG, Charrow J et al: Neural crest anomaly syndromes in children with spina bifida. Teratology 60: 179, 1999 40. Trainor PA, Tam PP: Cranial paraxial mesoderm and neural crest cells of the mouse embryo: Co-distribution in the craniofacial mesenchyme but distinct segregation in branchial arches. Development 121:2569, 1995 41. Meier S, Packard DS Jr: Morphogenesis of the cranial segments and distribution of neural crest in the embryos of the snapping turtle, Chelydra serpentina. Dev Biol 102:309, 1984 42. Jacobson AG: Somitomeres: Mesodermal segments of vertebrate embryos. Development 104:209, 1988 43. Tam PPL, Meier S: The establishment of a somitomeric pattern in the mesoderm of the gastrulating mouse embryo. Am J Anat 164:209, 1982 44. Meier S, Tam PPL: Metameric pattern development in the embryonic axis of the mouse. I: Differentiation of the cranial segments. Differentiation 21:95, 1982 45. Meier SP: The development of segmentation in the cranial region of
vertebrate embryos. Scan Electron Microsc 3:1269, 1982 46. Packard DS Jr, Meier S: An experimental study of the somitomeric organization of the avian segmental plate. Dev Biol 97:191, 1983 47. Packard DS Jr, Meier S: Morphological and experimental studies of the somitomeric organization of the segmental plate in snapping turtle embryos. J Embryol Exp Morphol 84:35, 1984 48. Tam PP: A study of the pattern of prospective somites in the presomitic mesoderm of mouse embryos. J Embryol Exp Morphol 92:269, 1986 49. Tam PP, Meier S, Jacobson AG: Differentiation of the metameric pattern in the embryonic axis of the mouse. II: Somitomeric organization of the presomitic mesoderm. Differentiation 21:109, 1982 50. Tam PP, Trainor PA: Specification and segmentation of the paraxial mesoderm. Anat Embryol (Berl) 189:275, 1994 51. Noden DM: The embryologic origins of avian craniofacial muscles and associated connective tissues. Am J Anat 168:257, 1983 52. Wrenn JT, Wessels NK: An ultrastructural study of lens invagination in the mouse. J Exp Zool 171:359, 1969 53. Schook P: A review of data on cell actions and cell interactions during the morphogenesis of the embryonic eye. Acta Morphol Neerl Scand 16:267, 1978 54. Beebe D, Latker C, Jebens J et al: Transport and steady-state concentration of plasma proteins in the vitreous humor of the chicken embryo: Implications for the mechanism of the eye growth during early development. Dev Biol 114:361, 1986 55. Woerdeman MW: The differentiation of the crystallin lens. J Embryol Exp Morphol 301, 1953 56. Coulombre JL, Coulombre AJ: Lens development IV. Size, shape and orientation. Invest Ophthalmol 8:251, 1969 57. Hendrix RW, Zwaan J: Changes in the glycoprotein concentration of the extracellular matrix between lens and optic vesicle associated with early lens differentiation. Differentiation 2:357, 1974 58. Zwaan J, Hendrix RW: Changes in cell and organ shape during early
development of the ocular lens. Am J Zool 13:1039, 1973 59. Beebe DC: Ocular Growth and Differentiation Factors. New York: John Wiley & Sons, 1985 60. Silver PHS, Wakely J: The initial stage in the development of the lens capsule in chick and mouse embryo. Exp Eye Res 19:73, 1974 61. Beebe D, Compart P, Johnson M et al: The mechanism of cell elongation during lens fiber cell differentiation. Dev Biol 92:54, 1982 62. Grainger RM, Henry JJ, Saha MS et al: Recent progress on the mechanisms of embryonic lens formation. Eye 6:117, 1992 63. Graw J: Genetic aspects of embryonic eye development in vertebrates. Dev Genet 18:181, 1996 64. Muggleton-Harris AL, Higbee N: Factors modulating mouse lens epithelial cell morphology with differentiation and development of a lentoid structure in vitro. Development 99:25, 1987 65. Menko AS, Philip NJ: Beta 1 integrins in epithelial tissues: a unique distribution in the lens. Exp Cell Res 218:516, 1995 66. Kuwabara T: The maturation of the lens cell: A morphologic study. Exp Eye Res 20:427, 1975 67. Wulle KG, Lerche W: Zur Feinstruktur der embryonalen menschlichen Linsenblase. Graefes Arch Klin Exp Ophthalmol 173:141, 1967 68. Wulle KG, Lerche W: Elektronenmikroskopischer Beitrag zur Embryonalentwicklung der menschlichen Linse. Graefes Arch Klin Exp Ophthalmol 176:126, 1968 69. Zwaan J, Hendrix RW, Johnson RA: Lens invagination: A theory and a mathematical model. Ophthalmic Res 3:22, 1972 70. Zwann J: Fine structure of the developing lens. Int Ophthalmol Clin 15:39, 1975 71. Yamada T. In Hagen E, Wechsler W, Zilliken P et al (eds): Experimental Biology and Medicine I, p 77. Basel: Karger, 1967 72. Lerche W, Wulle KG: Electron microscopic studies on the development of the human lens. Ophthalmologica 158: 296, 1969
73. Garcia-Porrero JA, Colvee E, Ojeda JL: The mechanisms of cell death and phagocytosis in the early chick lens morphogenesis: A scanning electron microscopy and cytochemical approach. Anat Rec 208:123, 1984 74. Garcia-Porrero JA, Collado JA, Ojeda JL: Cell death during detachment of the lens rudiment from ectoderm in the chick embryo. Anat Rec 193:791, 1979 75. Smelser GK: Embryology and morphology of the lens. Invest Ophthalmol 4:398, 1965 76. Brini A, Porte A, Stoeckel ME: Developpement de la corne chez l'embryon de poulet: Etude en microscope electronique. Doc Ophthalmol 20:309, 1966 77. Coulombre AJ. In Abercrombie M, Brachet J (eds): Advances in Morphogenesis, p 81. San Diego: Academic Press, 1965 78. Dublin I: Vergleichend-embryologische Untersuchungen ber die Fruhentwicklung der Hornhaut und der Pupillarmembran bei Reptilien, V geln and Saugern. Acta Anat 76:381, 1970 79. Ewer MS: Zur Fruhentwicklung des Stroma corneae und der Pupillarmembran beim Menschen. Acta Anat 75:37, 1970 80. Hay ED, Revel JP. In Wolsky A, Chen P (eds): Monographs in Developmental Biology. Basal: A Karger, 1969 81. Kaye GI. In Rohen J (ed): Die Struktur des Auges, II Symposium, p 441. Stuttgart: Schattauer, 1965 82. Messeni ML: A study of the prenatal developmental of corneal mucopolysaccharides in man. Boll Soc Ital Biol Sper 49:1166, 1973 83. Miyashita S: Electron microscopic studies on the cornea of fetuses. III. The human embryo cornea. Nippon Ganka Gakkai Zasshi 68:526, 1964 84. Pei YF, Rhodin JAG: The prenatal development of the mouse eye. Anat Rec 168:105, 1970 85. Pei YF, Rhodin JAG: Electron microscopic study of the development of the mouse corneal epithelium. Invest Ophthalmol 10:811, 1971 86. Praus R, Brettschneider I: Glycosaminoglycans in embryonic and
postnatal human cornea. Ophthalmic Res 7:452, 1975 87. Smelser GK: In Duke-Elder S (ed): The transparency of the cornea: A Symposium, p 23. Springfield, IL: Charles C Thomas, 1960 88. Schwarz W: Elektronenmikroskopische Untersuchungen fiber die Differenzierung der Cornea and Sklera Fibrillen des Menschen. Z Zellforsch 38:78, 1953 89. Schwartz W. In Smelser G (ed): The Structure of the Eye, p 393. San Diego: Academic Press, 1961 90. Zinn KM, Mockel-Pohl S: Fine structure of the developing cornea. Int Ophthalmol Clin 15:19, 1975 91. Wulle KG, Lerche W: Electron microscopic observations of the early development of the human corneal endothelium and Descemet's membrane. Ophthalmologica 157:451, 1969 92. Wulle KG: Electron microscopy of the fetal development of the corneal endothelium and Descemet's membrane of the human eye. Invest Ophthalmol 11:897, 1972 93. Wulle KG, Ruprecht KW, Windrath LC: Electron microscopy of the development of the cell junctions in the embryonic and fetal human corneal endothelium. Invest Ophthalmol 13:923, 1974 94. Smelser GK, Ozanics V (eds): Symposium on the Cornea: Transactions of the New Orleans Academy of Ophthalmology, p 20. St Louis: CV Mosby, 1972 95. Hay E: Development of the vertebrate cornea. Int Rev Cytol 63:263, 1980 96. Bahn CF, Glassman RM, MacCallum et al: Postnatal development of corneal endothelium. Invest Ophthalmol Vis Sci 27:44, 1986 97. Bee JA, Hay RA, Lamb EM et al: Positional specificity of corneal nerves during development. Invest Ophthalmol Vis Sci 27:38, 1986 98. Clarke ND, Bee JA: Innervation of the chick cornea analyzed in vitro. Invest Ophthalmol Vis Sci 37:1761, 1996 99. De Vries A, Gorgels TGMF, Berg RJW et al: Ultraviolet-B induced hyperplasia and squamous cell carcinomas in the cornea of XPA-deficient
mice. Exp Eye Res 67:53, 1998 100. Fitch JM, Gordon MK, Gibney EP: Analysis of transcriptional isoforms of collagen types IX, II, and I in the developing avian cornea by competitive polymerase chain reaction. Dev Dyn 202:42, 1995 101. Toole B, Trelstad R: Hyaluronate production and removal during corneal development in the chick. Dev Biol 26:28, 1971 102. You LT, Kruse FE, Pohl J et al: Bone morphogenetic proteins and growth and differentiation factors in the human cornea. Invest Ophthalmol Visual Sci 40:296, 1999 103. Hirsch M, Noske W, Prenant G et al: Fine structure of the developing avian corneal stroma as revealed by quick-freeze, deep-etch electron microscopy. Exp Eye Res 69:267, 1999 104. Anseth A: Glycosaminoglycans in the developing corneal stroma. Exp Eye Res 1:116, 1961 105. Kitano S: An embryological study on the human corneal nerves. Jpn J Ophthalmol 1:48, 1957 106. Ozanics V, Rayborn M, Sagun D: Observations on the morphology of the developing primate cornea epithelium, its innervation and anterior stroma. J Morphol 153:263, 1977 107. Whitear M: An electron microscopic study of nerves in the corneal epithelium. Experientia 13:287, 1957 108. Pouliquen Y, Faure JP, Bisson J et al: La zone fibrillaire accellulaire sous epitheliale de la corne de l'embryon de poulet: Ses rapports avec la formation de la membrane basale de l'pithelium et de la membrane de Bowman. Arch Ophthalmol Paris 26:59, 1966 109. Ozanics V, Rayborn M, Sagun D: Some aspects of corneal and scleral differentiation in the primate. Exp Eye Res 22:305, 1976 110. Herrmann H: In Smelser G (ed): Structure of the Eye, p 421. San Diego: Academic Press, 1961 111. Smelser GK, Ozanics V: The development of the trabecular meshwork in primate eyes. Am J Ophthalmol 71:366, 1971 112. Reme C, D'Epinay SL: Periods of development of the normal human
chamber angle. Doc Ophthalmol 51:241, 1981 113. Hansson HA, Jerndal T: Scanning electron microscopic studies on the development of the iridocorneal angle in human eyes. Invest Ophthalmol Vis Sci 10:252, 1971 114. Burian HM, Braley AE, Allen L: A new concept of the development of the angle of the anterior chamber of the human eye. Arch Ophthalmol 55:439, 1956 115. Wulle KG: The development of the productive and draining system of the aqueous humor in the human eye. Adv Ophthalmol 26:269, 1972 116. Anderson DR: The development of the trabecular meshwork and its abnormality in primary infantile glaucoma. Trans Am Ophthalmol Soc 79:458, 1981 117. Kupfer C, Ross K: The development of outflow facility in human eyes. Invest Ophthalmol 10:513, 1971 118. Pandolfi M, Astedt B: Outflow resistance in the fetal eye. Acta Ophthalmol Copenh 49:344, 1971 119. Adamis AP, Molnar ML, Tripathi BJ et al: Neuronal-specific enolase in human corneal endothelium and posterior keratocytes. Exp Eye Res 41:665, 1985 120. Wulle KG: Electron microscopic observations of the development of Schlemm's canal in the human eye. Trans Am Acad Ophthalmol Otolaryngol 72:765, 1968 121. Cook C, Sulik K, Wright K: In Wright KW (ed): Pediatric Ophthalmology and Strabismus, p 3. St Louis: Mosby, 1995 122. Imaizumi M, Kuwabara T: Development of the rat iris. Invest Ophthalmol 10:733, 1971 123. Matsuo N, Smelser GK: Electron microscopic studies on the pupillary membrane: The fine structure of the white strands of the disappearing stage of this membrane. Invest Ophthalmol 10:108, 1971 124. Ito M, Yoshioka M: Regression of the hyaloid vessels and pupillary membrane of the mouse. Anat Embryol 200: 403, 1999 125. Yamashita T, Sohal G: Development of smooth and skeletal muscle cells in the iris of the domestic duck, chick and quail. Cell Tissue Res
244:121, 1986 126. Lai YL: The development of the sphincter muscle in the iris of the albino rat. Exp Eye Res 14:196, 1972 127. Tamura T, Smelser GK: Development of the sphincter and dilator muscles of the iris. Arch Ophthalmol 89:332, 1973 128. Lai YL: The development of the dilator muscle in the iris of the albino rat. Exp Eye Res 14:203, 1972 129. Hu F, Endo H, Alexander NJ: Morphological variations of pigment granules in eyes of the rhesus monkey. Am J Anat 136:167, 1973 130. Mund ML, Rodrigues MM, Fine BS: Light and electron microscopic observations on the pigmented layers of the developing human eye. Am J Ophthalmol 73:167, 1972 131. Wulle KG: Frhentwicklung der Ciliarfortsatze im menschlichen Auge. Phasenkontrast und electronenmikroskopische Untersuchungen. Z Zellforsch 71:545, 1966 132. Bard J, Ross A: The morphogenesis of the ciliary body of the avian eye. Dev Biol 92:87, 1982 133. Wulle KG: Elektronenmikroskopie der Kapillarentwick-lung in menschlichen Ziliarfortsatzen. Anat Anz 120(Suppl.):465, 1967 134. Takei Y: Development of the ciliary non-pigmented epithelium in monkey fetusesA light and electron microscopic study. Jpn J Ophthalmol 22:259, 1978 135. Ozanics V, Rayborn ME, Sagun D: Observations on the ultrastructure of the developing primate choroid coat. Exp Eye Res 26:25, 1978 136. Brihaye-Van Geertruyden M: Contribution l'tude des tumeurs mlaniques de l'uve et de leur origine. Doc Ophthalmol 17:163, 1963 137. Feeney L, Grieshaber JA, Hogan MJ. In Rohen J (ed): Eye Structure II: Symposium, p 535. Stuttgart: Schattauer Verlag, 1965 138. Heimann K: The development of the choroid in man: Choroidal vascular system. Ophthalmol Res 3:257, 1972 139. Spira AW, Hollenberg MJ: Human retinal development: Ultrastructure
of the inner retinal layers. Dev Biol 31:1, 1973 140. Smelser GK, Ozanics V, Rayborn M et al: Retinal synaptogenesis in the primate. Invest Ophthalmol 13:340, 1974 141. Hollenberg MJ, Spira AW: Human retinal development: Ultrastructure of the outer retina. Am J Anat 137:357, 1973 142. Yamada E, Ishikawa T: Some Observations on the Submicroscopic Morphogenesis of the Human Retina, pp 15. Stuttgart: Schattauer Verlag, 1965 143. Vrabec F: The temporal raphe of the human retina. Am J Ophthalmol 62:926, 1966 144. Blechschmidt E: Developmental movements of the human retina at the time of development of the iris: Development of the optic ganglion as an instance of the development of the cytoarchitecture amenable to ultramicroscopic study. Ophthalmologica 154:531, 1967 145. Fisher SK, Linberg KA: Intercellular junctions in the early human embryonic retina. J Ultrastruct Res 51:69, 1975 146. Hollenberg MJ, Spira AW: Early development of the human retina. Can J Ophthalmol 7:472, 1972 147. Breathnach AS, Wyllie LM: Ultrastructure of retinal pigment epithelium of the human fetus. J Ultrastruct Res 16:584, 1966 148. Streeten BW: Development of the human retinal pigment epithelium and the posterior segment. Arch Ophthalmol 81:383, 1969 149. Zhou G, Williams RW: Eye1 and eye2: Gene loci that modulate eye size, lens weight, and retinal area in the mouse. Invest Ophthalmol Visual Sci 40:817, 1999 150. Bhattacharjee J, Sanyal S: Developmental origin and early differentiation of retinal Muller cells in mice. J Anat 120:367, 1975 151. Graymore C: Metabolism of the developing retina. Exp Eye Res 3:5, 1964 152. Keefe JR, Ordy JM, Samorajski T: Prenatal development of the retina in a diurnal primate (Macaca Mulatta). Anat Rec 154:759, 1966 153. Kuwabara T, Weidman TA: Development of prenatal rat retina. Invest
Ophthalmol 13:725, 1974 154. Olney JW: An electron microscopic study of synapse formation, receptor outer segment development and other aspects of developing mouse retina. Invest Ophthalmol 7:250, 1968 155. Smelser GK, Ozanics V, Rayborn M et al: The fine structure of the retinal transient layer of Chiewitz. Invest Ophthalmol 12:504, 1973 156. Tsukahara I, Uehara M, Okuma H: Development of the retinal inner limiting membrane and Bruch's membrane. Nippon Ganka Gakkai Zasshi 76:783, 1972 157. Uga S, Smelser GK: Electron microscopic study of the development of retinal Mullerian cells. Invest Ophthalmol 12:295, 1973 158. Weidman TA: Fine structure of the developing retina. Int Ophthalmol Clin 15:65, 1975 159. Lopashov GV, Stroeva OG: In Ambercrombie M, Brachet J (eds): Advances in Morphogenesis, p 331. San Diego: Academic Press, 1961 160. De Robertis E: Some observations on the ultrastructure and morphogenesis of photoreceptors. J Gen Physiol 43(Suppl 43):1, 1960 161. Hendrickson A, Kupfer C: The histogenesis of the fovea in the macaque monkey. Invest Ophthalmol 15:746, 1976 162. Wilmer HA, Scammon RE: Growth of the components of the human eyeball. Arch Ophthalmol 43:599, 1950 163. Ashton N: Studies on developing retinal vessels. VIII: Effect of oxygen on the retinal vessels of the ratling. Br J Ophthalmol 45:321, 1961 164. Ashton N, Pedler C: Studies on developing vessels. IX: Reaction of endothelial cells to oxygen. Br J Ophthalmol 46:257, 1962 165. Ashton N: Oxygen and the growth and development of retinal vessels: In vivo and in vitro studies. Am J Ophthalmol 62:412, 1966 166. Ashton N, Tripathi B, Knight G: Effect of oxygen on the developing retinal vessels of the rabbit. I: Anatomy and development of the retinal vessels of the rabbit. Exp Eye Res 14:214, 1972 167. Ashton N, Tripathi B, Knight G: Effect of oxygen on the developing retinal vessels of othe rabbit. II. In vivo experiments. Exp Eye Res 14:221,
1972 168. Braekevelt CR, Hollenberg MJ: Comparative electron microscopic study of development of hyaloid and retinal capillaries in albino rats. Am J Ophthalmol 69:1032, 1970 169. Engerman RL, Meyer RK: Development of retinal vasculature in rats. Am J Ophthalmol 60:628, 1965 170. Oshima K, Ishikawa T: Effects of oxygen on developing vessels in the kitten retina. Nippon Ganka Gakkai Zasshi 79:813, 1975 171. Shakib M, De Oliveira F: Studies on developing retinal vessels. X: Formation of the basement membrane and differentiation of intramural pericytes.Br J Ophthalmol 50:124, 1966 172. Shakib M, De Oliveira F, Henkind P: Development of retinal vessels. II: Earliest stages of vessel formation. Invest Ophthalmol 7:689, 1968 173. Tripathi B, Knight R, Ashton N: Effect of oxygen on the developing retinal vessels of the rabbit. IV. Exp Eye Res 19:449, 1974 174. Mutlu F, Leopold IH: The structure of the retinal vascular system of the human fetus eye. Arch Ophthalmol 71:531, 1964 175. Cogan DG: Development and senescence of the human retinal vasculature. Trans Ophthalmol Soc UK 83:465, 1963 176. Engerman RL: Development of macular circulation. Invest Ophthalmol 15:835, 1976 177. Henkind P, Bellhorn RW, Murphy ME et al: Development of macular vessels in monkey and cat. Br J Ophthalmol 59:703, 1975 178. Wise GN, Dollery CT, Henkind P. The Retinal Circulation. New York: Harper & Row, 1971 179. Ashton N: Retinal angiogenesis in the human embryo. Br Med Bull 26:103, 1970 180. Michaelson IC: The mode of development of the vascular system of the retina with some observations on its significance for certain retinal diseases. Trans Ophthalmol Soc UK 68:137, 1948 181. Ashton N (ed): The William MacKenzie Centenary Symposium on the
Ocular Circulation in Health and Disease, p 7. St Louis: CV Mosby, 1969 182. Cook CS, Generoso WM, Hester D et al: RPE dysplasia with retinal duplication in a mutant mouse strain. Exp Eye Res 52:409, 1991 183. Moyer F (ed): The Retina: Morphology, Function and Clinical Characteristics, p 10. Berkeley: University of California Press, 1969 184. Tombran-Tink J, Shivaram SM, Chader GJ et al: Expression, secretion, and age-related downregulation of pigment epithelium-derived factor, a serpin with neurotrophic activity. J Neurosci 15:4992, 1995 185. Kong Y, Nagata T: Nucleic acid synthesis in mouse retina and retinal pigment epithelium by radioautography. Cell Molec Biol 41:171, 1995 186. Zhao S, Thornquist SC, Barnstable CJ: In vitro transdifferentiation of embryonic rat retinal pigment epithelium. 1995 187. Robb RM: Regional changes in RPE cell density during ocular development. Invest Ophthalmol Vis Sci 26:614, 1985 188. Takei Y, Ozanics V: Origin and development of Bruch's membrane in monkey fetuses: An electron microscopic study. Invest Ophthalmol 14:903, 1975 189. O'Rahilly R: The prenatal development of the human eye. Exp Eye Res 21:93, 1975 190. Geeraets R: An electron microscopic study of the closure of the optic fissure in the golden hamster. Am J Anat 145:411, 1976 191. Spira AW, Price PG. In Yamada E, Mishima S (eds): Structure of the Eye, p 229. Tokyo: 1976 192. Silver J, Hughes AFW: The role of cell death during morphogenesis of the mammalian eye. J Morphol 140:159, 1973 193. Berman M, Pierro L: Lens detachment and choroid fissure closure in the embryonic mouse eye. Am Zool 9:365, 1969 194. Kuwabara T: Development of the optic nerve of the rat. Invest Ophthalmol 14:732, 1975 195. Sturrock RR: A light and electron microscopic study of proliferation and maturation of fibrous astrocytes in the optic nerve of the human embryo.
J Anat 119:223, 1975 196. Vaughn JE, Peters A: Electron microscopy of early postnatal development of fibrous astrocytes. Am J Anat 121:131, 1967 197. Hogan MJ, Alvarado JA, Weddell JE: Histology of the Human Eye. An Atlas and Textbook. Philadelphia: WB Saunders, 1971 198. Blunt MG, Baldwin F, Wendell-Smith CP: Glyogenesis and myelination in kitten optic nerve. Z Zellsforsch 124:293, 1972 199. Vaughn J: An electron microscopic analysis of gliogenesis in rat optic nerves. Z Zellforsch 94:293, 1969 200. Rawlines FA: A quantitative electron microscopic analysis of myelination in the optic nerve of suckling rats treated with an inhibitor of cholesterol biosynthesis. Z Zellforsch 140:9, 1973 201. Kanazawa S: Electron microscopic study of the human foetal optic nerve. Nippon Ganka Gakkai Zasshi 73:1330, 1969 202. Friede RL, Hu KH: Proximo-distal differences in myelin development in human optic fibers. Z Zellforsch 79:259, 1967 203. Balazs EA, Laurent TC, Laurent UBG: Studies on the structure of the vitreous body. VI: Biochemical changes during development. Biol Chem 234:422, 1959 204. Balazs EA, Toth LZ, Jutheden G et al: Cytological and biochemical studies of the developing chicken vitreous. Exp Eye Res 4:237, 1965 205. Balazs EA: Fine structure of developing vitreous. Int Ophthalmol Clin 15:53, 1975 206. Balazs EA, Toth LZ, Ozanics V: Cytological studies on the developing vitreous as related to the hyaloid vessel system. Graefes Arch Klin Exp Ophthalmol 213:71, 1980 207. Balazs EA, Bloom GD, Ozanics V: The fine structure of the hyaloid arteriole in bovine vitreous. Exp Eye Res 31:129, 1980 208. Falbe-Hansen I, Ehlers N, Degn JK: Development of the human foetal vitreous body. 1. Biochemical changes. Acta Ophthalmol 47:39, 1969 209. Jack RL: Ultrastructural aspects of hyaloid vessel development. Arch
Ophthalmol 87:427, 1972 210. Jack RL: Ultrastructure of the hyaloid vascular system. Arch Ophthalmol 87:555, 1972 211. Hamming NA, Apple DJ, Gieser DK et al: Ultrastructure of the hyaloid vasculature in primates. Invest Ophthalmol Vis Sci 16:408, 1977 212. Mutlu F, Leopold IH: The structure of the fetal hyaloid system and tunica vasculosa lentis. Arch Ophthalmol 71:102, 1964 213. Porte A, Brini A, Stoeckel ME: In Rohen J (ed): The Structure of the Eye II, Symposium, p 515. Stuttgart: Schattauer Verlag, 1965 214. Ozeki H, Shirai S, Ikeda K et al: Critical period for retinoic acidinduced developmental abnormalities of the vitreous in mouse fetuses. Exp Eye Res 68:223, 1999 215. Bremer FM, Rasquin F: Histochemical localization of hyaluronic acid in vitreous during embryonic development. Invest Ophthalmol Visual Sci 39:2466, 1998 216. Sang DN: Embryology of the vitreous. Congenital and developmental abnormalities. Bull Soc Belge Ophtalmol 223:11, 1987 217. Jack RL: Regression of the hyaloid vascular system: An ultrastructural analysis. Am J Ophthalmol 74:261, 1972 218. Stades F: Persistent hyperplastic tunica vasculosa lentis and persistent hyperplastic primary vitreous (PHTVL/PHPV) in 90 closely related Doberman Pinschers: Clinical aspects. J Am Anim Hosp Assoc 16:739, 1980 219. van der Linde-Sipman J, Stades F, de Wolff-Rouendaal D: Persistent hyperplastic tunica vasculosa lentis and persistent hyperplastic primary vitreous in the Doberman Pinscher: Pathological aspects. J Am Anim Hosp Assoc 19:791, 1983 220. Takei Y: Electron microscopic studies on zonule. Jpn J Ophthalmol 19:376, 1975 221. Okita M: Embryological and structural studies on the chicken zonule (1): Embryonic development. Nippon Ganka Gakkai Zasshi 75:432, 1971 222. Andersen H, Ehlers N, Matthiessen ME: Histochemistry and development of the human eyelids. Acta Ophthalmol 43:642, 1965
223. Andersen H, Ehlers N, Matthiessen ME et al: Histochemistry and development of the human eyelids. II. Acta Ophthalmol 45:288, 1967 224. Makino K: On the distribution and growth of nerves in the eyelid of human embryo. Igaku Kenkyu 30:124, 1960 225. Gilbert PW (ed): The origin and development of the human extrinsic ocular muscles. Carnegie Inst Publication, 246:59, 1957 226. Sevel D: Reappraisal of the origin of human extraocular muscles. Ophthalmology 88:1330, 1981 227. Sevel D: The origins and insertions of the extraocular muscles: Development, histologic features, and clinical significance. Trans Am Ophthalmol Soc 84:488, 1986 228. Cassady JV. In Veirs E (ed): The Lacrimal System Clinical Application, p 20. Philadelphia: Grune & Stratton, 1955
Вам также может понравиться
- Uveit FosterДокумент954 страницыUveit FosterEdmond IsufajОценок пока нет
- Embryology of The EyeДокумент29 страницEmbryology of The EyeBimo Juliansyah100% (1)
- Alzheimers Disease PaperДокумент6 страницAlzheimers Disease Paperapi-339853259Оценок пока нет
- Acute PericarditisДокумент8 страницAcute Pericarditisalina abu rumiОценок пока нет
- Imagine You Being Loved! 11 - 28 - 20 PDFДокумент166 страницImagine You Being Loved! 11 - 28 - 20 PDFGlenis AcostaОценок пока нет
- Development of The Vertebrate EyeДокумент5 страницDevelopment of The Vertebrate Eyezari_pak2010Оценок пока нет
- The Iris Understanding The EssentialsДокумент257 страницThe Iris Understanding The EssentialsgabbbbbbbbyОценок пока нет
- Ant. Segment DysgenesisДокумент19 страницAnt. Segment DysgenesisSwati TyagiОценок пока нет
- Tissue Da 1Документ10 страницTissue Da 1Mansi SinghОценок пока нет
- The Histology and Biology of The LensДокумент8 страницThe Histology and Biology of The LensIdham BaharudinОценок пока нет
- Anatomy and Pathology of The Eye: Role of MR Imaging and CTДокумент25 страницAnatomy and Pathology of The Eye: Role of MR Imaging and CTCucută Alexandru-DanielОценок пока нет
- Articulo PaparpadoДокумент8 страницArticulo PaparpadoJONATHAN CEDEÑOОценок пока нет
- Adv Bio Morphogenesis NotesДокумент13 страницAdv Bio Morphogenesis NotesHappy HookОценок пока нет
- Congenital Eye Anomalies: More Mosaic Than Thought?Документ71 страницаCongenital Eye Anomalies: More Mosaic Than Thought?FelipeA.DuránRodriguezОценок пока нет
- Embryology of The Orbit: Raymond I. Cho Alon KahanaДокумент5 страницEmbryology of The Orbit: Raymond I. Cho Alon Kahanaleandro.silvaОценок пока нет
- Nervul OpticДокумент10 страницNervul OpticIulia BuzdugăОценок пока нет
- Suprachoroidal Space InterventionsОт EverandSuprachoroidal Space InterventionsShohista SaidkasimovaОценок пока нет
- Bing MentahanДокумент15 страницBing Mentahansecondfery2021Оценок пока нет
- Unit Organogenesis of Eye And: StructureДокумент40 страницUnit Organogenesis of Eye And: Structurekaladhar reddyОценок пока нет
- Guercio2007Документ28 страницGuercio2007Priscilla GeraldineОценок пока нет
- BIO109 Neurulation-M6Документ44 страницыBIO109 Neurulation-M6Sherly MAKIJIОценок пока нет
- Molecular Basis PART 1 / Orthodontic Courses by Indian Dental AcademyДокумент188 страницMolecular Basis PART 1 / Orthodontic Courses by Indian Dental Academyindian dental academyОценок пока нет
- Lec 41 - Dav - EmbeyeДокумент8 страницLec 41 - Dav - EmbeyeAymen MageedОценок пока нет
- Dev't of MuskuloskeletalДокумент70 страницDev't of MuskuloskeletalMilkoo sabaОценок пока нет
- Malformed Vertebrae: A Clinical and Imaging Review: EmbryologyДокумент13 страницMalformed Vertebrae: A Clinical and Imaging Review: EmbryologyYovanka Naryai ManuhutuОценок пока нет
- Embryonic InductionДокумент2 страницыEmbryonic InductionRaza Butt100% (1)
- #5 Morfogénesis de Los Músculos Cráneo-FacialesДокумент25 страниц#5 Morfogénesis de Los Músculos Cráneo-FacialesValentina MoralesОценок пока нет
- Dental Distress Syndrome QuantifiedДокумент29 страницDental Distress Syndrome QuantifiedmichalОценок пока нет
- Krigestin Et Al 2006, Neural Stem Cell PrgenitorДокумент8 страницKrigestin Et Al 2006, Neural Stem Cell PrgenitorSalma Moustafa MahmoudОценок пока нет
- Coloboma 2Документ1 страницаColoboma 2maria astridОценок пока нет
- (Perspectives in Vision Research) Elaine R. Berman (Auth.) - Biochemistry of The Eye (1991, Springer US) PDFДокумент489 страниц(Perspectives in Vision Research) Elaine R. Berman (Auth.) - Biochemistry of The Eye (1991, Springer US) PDFBoris HildebrandtОценок пока нет
- 1991 Bookmatter BiochemistryOfTheEyeДокумент14 страниц1991 Bookmatter BiochemistryOfTheEyeAndre C100% (1)
- MeningesДокумент2 страницыMeningesDurga MadhuriОценок пока нет
- Prenatal Growth of Head and Face: Presented by Amritha. Vasudevan First Year PG Department of OrthodonticsДокумент50 страницPrenatal Growth of Head and Face: Presented by Amritha. Vasudevan First Year PG Department of OrthodonticsReenaChauhanОценок пока нет
- Sci2015 601731Документ7 страницSci2015 601731angelos96papОценок пока нет
- Theories of GrowthДокумент67 страницTheories of GrowthsoujanyaОценок пока нет
- Embryology, Anatomy, and Physiology of The Afferent Visual PathwayДокумент80 страницEmbryology, Anatomy, and Physiology of The Afferent Visual PathwayTea Abramia AbramiaОценок пока нет
- Chapter 01Документ30 страницChapter 01sarahОценок пока нет
- HDTD-E - 6 - Embryonic PeriodДокумент33 страницыHDTD-E - 6 - Embryonic PeriodMariam QaisОценок пока нет
- 1 s2.0 S2352396421001535 MainДокумент13 страниц1 s2.0 S2352396421001535 MainAmy Lalringhluani ChhakchhuakОценок пока нет
- The Multifunctional ChoroidДокумент38 страницThe Multifunctional ChoroidyinvilllОценок пока нет
- Head and Neck. Embryology. Topographic RegionsДокумент56 страницHead and Neck. Embryology. Topographic RegionsXerox medОценок пока нет
- Embryology of Inner Ear Recent TrendsДокумент22 страницыEmbryology of Inner Ear Recent TrendsDr. T. BalasubramanianОценок пока нет
- Materials ApplicationДокумент16 страницMaterials ApplicationMurali RameshОценок пока нет
- Activity 1: Frog's Egg, Cleavage, and Blastulation: Late-Yolk-Plug-4x-Ss - JPGДокумент7 страницActivity 1: Frog's Egg, Cleavage, and Blastulation: Late-Yolk-Plug-4x-Ss - JPGIvanОценок пока нет
- The Ocular Lens: A Classic Model For Development, Physiology and DiseaseДокумент3 страницыThe Ocular Lens: A Classic Model For Development, Physiology and DiseaseWahyudi Permana DarlisОценок пока нет
- Human Malformations - 08 - Facial BonesДокумент30 страницHuman Malformations - 08 - Facial BonesAhmed H. Ali ElbestaweyОценок пока нет
- Eye & Ear Embryology ObjectivesДокумент3 страницыEye & Ear Embryology ObjectivesDr. DОценок пока нет
- Why Lordotic in Cervical and LumbalДокумент13 страницWhy Lordotic in Cervical and LumbalKunni MardhiyahОценок пока нет
- Procrsmed00410 0051 PDFДокумент10 страницProcrsmed00410 0051 PDFMilton David Rios SerratoОценок пока нет
- Embryology and Anatomy: Spine/Spinal CordДокумент5 страницEmbryology and Anatomy: Spine/Spinal CordFernandoo CunhaОценок пока нет
- Anatomy, Development, and Physiology of The Visual System PDFДокумент23 страницыAnatomy, Development, and Physiology of The Visual System PDFNivedha AvoodaiappanОценок пока нет
- Sonic HedgehogДокумент11 страницSonic HedgehogPaola Rivera ÁvilaОценок пока нет
- Bies 20137Документ15 страницBies 20137Jerahmeel Sombilon GenillaОценок пока нет
- Cerebellar Ataxia Pathophysiology and RehabilitationДокумент23 страницыCerebellar Ataxia Pathophysiology and RehabilitationMikail AtiyehОценок пока нет
- 02 - CNS DevelopmentДокумент3 страницы02 - CNS DevelopmentCemre KuzeyОценок пока нет
- Principles of Development. Cap 1Документ32 страницыPrinciples of Development. Cap 1Gre Vega ÁlvarezОценок пока нет
- Fate Maps & Fate of Germ LayersДокумент5 страницFate Maps & Fate of Germ LayersAmar Kant JhaОценок пока нет
- Simple Anatomy of The RetinaДокумент24 страницыSimple Anatomy of The RetinaLateepheart YakubОценок пока нет
- Cell Differentiation and Organogenesis 2Документ34 страницыCell Differentiation and Organogenesis 2Dahal Babin100% (1)
- TelomereДокумент32 страницыTelomeremumunooОценок пока нет
- Brjopthal00028 0026 PDFДокумент5 страницBrjopthal00028 0026 PDFmumunooОценок пока нет
- Guidelines For Pediatric Fluid ResuscitationДокумент6 страницGuidelines For Pediatric Fluid Resuscitationbalab2311Оценок пока нет
- DermatologyДокумент44 страницыDermatologymumunooОценок пока нет
- Otitis ExternaДокумент14 страницOtitis ExternamumunooОценок пока нет
- Yale Swallow Protocol PDFДокумент2 страницыYale Swallow Protocol PDFmumunooОценок пока нет
- Atopic DermatititsДокумент7 страницAtopic DermatititsRaissa Alfaathir HeriОценок пока нет
- Burns Management PDFДокумент7 страницBurns Management PDFRoh Bungaria N Garingging100% (1)
- Pediatric Population: Anisometropia Treatment OptionsДокумент5 страницPediatric Population: Anisometropia Treatment OptionsmumunooОценок пока нет
- Alagille SyndromeДокумент8 страницAlagille SyndromemumunooОценок пока нет
- Studies On The Mechanism of Action of Timolol and On The Effects of Suppression and Redirection of Aqueous Flow On Outflow FacilityДокумент2 страницыStudies On The Mechanism of Action of Timolol and On The Effects of Suppression and Redirection of Aqueous Flow On Outflow FacilitymumunooОценок пока нет
- AnisometropДокумент6 страницAnisometropmumunooОценок пока нет
- Downregulation and UpregulationДокумент2 страницыDownregulation and UpregulationmumunooОценок пока нет
- Step by Step Laser in Ophthalmology - B. Bhattacharyya (Jaypee, 2009) BBSДокумент247 страницStep by Step Laser in Ophthalmology - B. Bhattacharyya (Jaypee, 2009) BBSmumunoo100% (3)
- Kjo 27 7Документ5 страницKjo 27 7mumunooОценок пока нет
- GlaucomaДокумент29 страницGlaucomaRaja Alfian IrawanОценок пока нет
- Jurnal 3 rthrthhhr6h Rthfwefw Wefwf Wefew RetinoДокумент6 страницJurnal 3 rthrthhhr6h Rthfwefw Wefwf Wefew RetinomumunooОценок пока нет
- In Infants and Children Undergoing Lens Implantation IndoДокумент3 страницыIn Infants and Children Undergoing Lens Implantation IndomumunooОценок пока нет
- Article 1Документ8 страницArticle 1mumunooОценок пока нет
- Chapter 2 BiochemistryДокумент7 страницChapter 2 BiochemistrymumunooОценок пока нет
- Comparison of Handheld Rebound Tonometry With Goldmann ApplanationДокумент4 страницыComparison of Handheld Rebound Tonometry With Goldmann ApplanationmumunooОценок пока нет
- Bag PlacementДокумент2 страницыBag PlacementmumunooОценок пока нет
- Kjo 27 7Документ5 страницKjo 27 7mumunooОценок пока нет
- Bag PlacementДокумент2 страницыBag PlacementmumunooОценок пока нет
- Diabetes Mellitus: Overview and TreatmentsДокумент36 страницDiabetes Mellitus: Overview and TreatmentsGlenn Nadine N. Masicampo100% (1)
- Selecting Intraocular Lens Power in ChildrenДокумент3 страницыSelecting Intraocular Lens Power in ChildrenmumunooОценок пока нет
- Sun Screen:: The Burning FactsДокумент6 страницSun Screen:: The Burning FactsCourier JournalОценок пока нет
- RR 58 e 324Документ209 страницRR 58 e 324mumunooОценок пока нет
- Severe Odontogenic InfectionsДокумент8 страницSevere Odontogenic InfectionsmumunooОценок пока нет
- Plantilla Powerpoint Del Sistema MuscularДокумент48 страницPlantilla Powerpoint Del Sistema MuscularLimberg ArhuataОценок пока нет
- Cross Section Drawing of The Eye - (Side View) With Major Parts LabeledДокумент7 страницCross Section Drawing of The Eye - (Side View) With Major Parts LabeledCamille ReneeОценок пока нет
- Panca InderaДокумент26 страницPanca Inderakema_hiperkes100% (1)
- Uveal TractДокумент56 страницUveal Tractmaf manОценок пока нет
- Ocular Blood FlowДокумент451 страницаOcular Blood FlowDwi Atikah SariОценок пока нет
- Structure of The EyeДокумент2 страницыStructure of The EyeminaОценок пока нет
- Ophtho BookДокумент181 страницаOphtho BookSymss MathewОценок пока нет
- Anatomy of The Left EyeДокумент3 страницыAnatomy of The Left EyeJaessa FelicianoОценок пока нет
- Notes On Eye of RabbitДокумент3 страницыNotes On Eye of RabbitShailesh NiroulaОценок пока нет
- Kuliah Histologi MataДокумент47 страницKuliah Histologi MataLuna Litami100% (1)
- OphthalmologyДокумент102 страницыOphthalmologyPatty Reyes100% (1)
- Basic Course Lectures in Ophthalmology 2013: Date Time Topic LecturerДокумент2 страницыBasic Course Lectures in Ophthalmology 2013: Date Time Topic LecturerSteffi ClaroОценок пока нет
- Anatomy-Physiology of The Eye DNB Ophthalmology Theory DnbCentral - inДокумент66 страницAnatomy-Physiology of The Eye DNB Ophthalmology Theory DnbCentral - inporuОценок пока нет
- (Ophthalmology) Clinical Retina (2002)Документ493 страницы(Ophthalmology) Clinical Retina (2002)Marianna Ivanova100% (7)
- Zhu2012 - Eye AnatomyДокумент9 страницZhu2012 - Eye Anatomytobing704Оценок пока нет
- Subject Name: Shalakya Tantra Unit No: Unit Name: Eye Anatomy (Font: Source Sans Pro, Size: 32)Документ15 страницSubject Name: Shalakya Tantra Unit No: Unit Name: Eye Anatomy (Font: Source Sans Pro, Size: 32)Sambamurthi Punninnair NarayanОценок пока нет
- PPTДокумент8 страницPPTAndo yuОценок пока нет
- Anatomy and Physiology of The EyeДокумент6 страницAnatomy and Physiology of The Eyesen_subhasis_58Оценок пока нет
- The Human EyeДокумент41 страницаThe Human EyeJasmine SebastianОценок пока нет
- Eye Pathology An Atlas and TextДокумент697 страницEye Pathology An Atlas and TextEric Wong100% (3)
- 2.biophysics of VisionДокумент109 страниц2.biophysics of Visionkrueg3rОценок пока нет
- Introduction To Optometry: The Normal EyeДокумент21 страницаIntroduction To Optometry: The Normal EyeMwanja MosesОценок пока нет
- Anatomy of Human EyeДокумент41 страницаAnatomy of Human EyeCarly MelachioОценок пока нет
- Examination of Eye PT IIДокумент77 страницExamination of Eye PT IIMihaela TomaОценок пока нет
- Anatomy of The Eye PresentationДокумент12 страницAnatomy of The Eye Presentationfathul rahman100% (2)
- Sheep Eye Dissection.190121501 PDFДокумент8 страницSheep Eye Dissection.190121501 PDFcarofrancoeОценок пока нет
- Eye StructureДокумент15 страницEye StructurekathyОценок пока нет
- 3800lecture 1 - Eye AnatomyДокумент17 страниц3800lecture 1 - Eye AnatomyAkshay Dhawan100% (1)
- Retinopathy of Prematurity (Rop)Документ21 страницаRetinopathy of Prematurity (Rop)ArinYuniastikaEkaPutriОценок пока нет
- EYE Notes IgcseДокумент3 страницыEYE Notes IgcseTay HermyОценок пока нет