Академический Документы
Профессиональный Документы
Культура Документы
Chemist Mohd Irwan Idris
Загружено:
Eonegte IdrisАвторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
Chemist Mohd Irwan Idris
Загружено:
Eonegte IdrisАвторское право:
Доступные форматы
1/M.
IRWAN IDRIS
_________________________________________________________________________ SBCH 3203 ORGANOMETALLIC CHEMISTRY /KIMIA ORGANOLOGAM SEPTEMBER 2011 _________________________________________________________________________
NAMA : MOHD IRWAN BIN IDRIS MATRIK NO :810320145167001 NO. TEL :0166665247 EMAIL : eoneg e!"#$oo.%o& '( : S$#$ A)#&
2/M.IRWAN IDRIS
*+ESTION 1
a) In the Electron - Sea model, the valence electrons of each atom are attracted to positively charged nuclei of several close neighbours. As the result , the valence electrons become free from attractives force of its o n nucleus. !he electrostatic force of attraction bet een the positive ions and the sea of valence electrons is the metallic bond. Sodium has the electronic structure "s##s##p$%s". &hen sodium atoms come together, the electron in the %s atomic orbital of one sodium atom shares space ith the corresponding electron on a neighbouring atom to form a molecular orbital . !he electrons can move freely ithin these molecular orbitals, and so each electron becomes detached from its parent atom. !he electrons are said to be delocalised. !his cause the distance bet een the central metal cations ith the electron cloud farther, so electrostatic attraction bet een the metal cations ith ea'er electrons cloud.
3/M.IRWAN IDRIS
(agnesium, end up ith stronger bonds and so a higher melting point. (agnesium has the outer electronic structure %s#. )oth of these electrons become delocalised, so the *sea* has t ice the electron density as it does in sodium. !he remaining *ions* also have t ice the charge and so there ill be more attraction bet een *ions* and *sea*.
(ore realistically, each magnesium atom has one more proton in the nucleus than a sodium atom has, and so not only ill there be a greater number of delocalised electrons, but there ill also be a greater attraction for them. (agnesium atoms have a slightly smaller radius than sodium atoms, and so the delocalised electrons are closer to the nuclei. Each magnesium atom also has t elve near neighbours rather than sodium+s eight. )oth of these factors increase the strength of the bond still further. !his ill e,plain hy the increasing boiling point from -a to (g to Al across the ro in a periodic table
4/M.IRWAN IDRIS
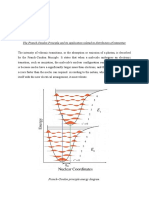
b) .ifferent ligands have different effects on the energies of the d orbitals of the central ion. Some ligands have strong field ligand hich cause a large energy gap hen the d orbitals split into t o groups. !he si/e of gap determines the avelength of lights is going to get absorbed. !he list belo sho s some common ligands ith the splitting
Cl F OH H2O NH3 CN largest splitting smallest splitting
0-1 is a strong field
ith the largest splitting need more energy to promote an
electron from the lo er group of orbitals to the higher ones. In terms of the colour of the light absorbed, greater energy corresponds to shorter avelength, higher fre2uency. In order to promote an electron, )lue light has a higher energy complementary yello coloured. !hose containing 3#4 is a ea' field ligand ill create a comple, ith a smaller 5 , hich ill absorb light of longer 6 and thus lo er fre2uency, v. !he comple, ith 3#4 ligands ill absorbed red light, in order to give the complementary green colour. ill be absorbed, to give the
5/M.IRWAN IDRIS
c) A covalent bond is formed by t o atoms sharing a pair of electrons. !he atoms are held together because the electron pair is attracted by both of the nuclei. In the formation of a simple covalent bond, each atom supplies one electron to the bond - but that doesn+t have to be the case. A coordinate bond 7also called a dative covalent bond) is a covalent bond 7a shared pair of electrons) in hich both electrons come from the same atom. Le,-. A%-/: a species that accepts an electron pair and ill have vacant orbitals E,amples8 )9%, 3:, 0u#:, 0r%: Le,-. B#.e8 a species that donates an electron pair and ill have lone-pair electrons E,amples8 -3%, 43-, 3#4 !he reaction of a ;e is acid and a ;e is base ill produce a coordinate covalent bond, as sho n in figure " belo . A coordinate covalent bond is <ust a type of covalent bond in hich one reactant gives it electron pair to another reactant.
6/M.IRWAN IDRIS
A coordination comple, is the product of a ;e is acid-base reaction in hich neutral molecules 7ligands) bond to a central metal atom by coordinate covalent bonds. 0ompounds that contain a coordination comple, are called coordination compound. E,ample8 T$e 0e#% -on 1e ,een #&&on-# #n/ $"/0ogen %$)o0-/e
If these colourless gases are allo ed to mi,, a thic' hite smo'e of solid ammonium chloride is formed.
Ammonium ions, -3=:, are formed by the transfer of a hydrogen ion from the hydrogen chloride to the lone pair of electrons on the ammonia molecule.
&hen the ammonium ion, -3=:, is formed, the fourth hydrogen is attached by a dative covalent bond, because only the hydrogen+s nucleus is transferred from the chlorine to the nitrogen. !he hydrogen+s electron is left behind on the chlorine to form a negative chloride ion.
7/M.IRWAN IDRIS
d) 3e,aammine 0obalt 7III) chloride, > 0o7-3%)$?0l% in its octahedral geometry ith $ coordination number. !he central ion is 0o@: and three 0lAion as ionic bonds
.iagram sho 8
BBBBBBBBBB ..
ionic bonds 0ovalent bond
Вам также может понравиться
- Jsu5yakin SainsДокумент41 страницаJsu5yakin SainsEonegte IdrisОценок пока нет
- The Yellow House: A Memoir (2019 National Book Award Winner)От EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Рейтинг: 4 из 5 звезд4/5 (98)
- Best Answer From The Answers Marked A, B, C and D.: Paper 1Документ11 страницBest Answer From The Answers Marked A, B, C and D.: Paper 1Eonegte IdrisОценок пока нет
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeОт EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeРейтинг: 4 из 5 звезд4/5 (5794)
- Match The Phrases To The Pictures and Write Correctly: Eonegte/1/biy2sksДокумент6 страницMatch The Phrases To The Pictures and Write Correctly: Eonegte/1/biy2sksEonegte IdrisОценок пока нет
- RPH Bi Y2Документ23 страницыRPH Bi Y2Eonegte IdrisОценок пока нет
- The Little Book of Hygge: Danish Secrets to Happy LivingОт EverandThe Little Book of Hygge: Danish Secrets to Happy LivingРейтинг: 3.5 из 5 звезд3.5/5 (400)
- Question 1 To 5. Read The Passage and Circle The Correct AnswerДокумент5 страницQuestion 1 To 5. Read The Passage and Circle The Correct AnswerEonegte IdrisОценок пока нет
- Year 2 Phonemic Awareness Textbook Page 29Документ12 страницYear 2 Phonemic Awareness Textbook Page 29Eonegte IdrisОценок пока нет
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureОт EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureРейтинг: 4.5 из 5 звезд4.5/5 (474)
- MathДокумент5 страницMathEonegte Idris100% (1)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryОт EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryРейтинг: 3.5 из 5 звезд3.5/5 (231)
- SBPH4203 Light and Modern Physics/Cahaya Dan Fizik Moden September 2011Документ5 страницSBPH4203 Light and Modern Physics/Cahaya Dan Fizik Moden September 2011Eonegte IdrisОценок пока нет
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceОт EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceРейтинг: 4 из 5 звезд4/5 (895)
- Introduction To Spectroscopy Pavia 4th Solutions ManualДокумент5 страницIntroduction To Spectroscopy Pavia 4th Solutions Manualسیاہ پوشОценок пока нет
- Team of Rivals: The Political Genius of Abraham LincolnОт EverandTeam of Rivals: The Political Genius of Abraham LincolnРейтинг: 4.5 из 5 звезд4.5/5 (234)
- Chemical Bonding (Basic) : Inorganic ChemistryДокумент37 страницChemical Bonding (Basic) : Inorganic ChemistrySATYAM GAMINGОценок пока нет
- Never Split the Difference: Negotiating As If Your Life Depended On ItОт EverandNever Split the Difference: Negotiating As If Your Life Depended On ItРейтинг: 4.5 из 5 звезд4.5/5 (838)
- UV VIS Phenol LabДокумент6 страницUV VIS Phenol LabJoão Paulo FioriОценок пока нет
- The Emperor of All Maladies: A Biography of CancerОт EverandThe Emperor of All Maladies: A Biography of CancerРейтинг: 4.5 из 5 звезд4.5/5 (271)
- Class 11 Chemistry Chapter 3 Theories of Covalent BondingДокумент46 страницClass 11 Chemistry Chapter 3 Theories of Covalent BondingSafa Farhad- 2912149Оценок пока нет
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaОт EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaРейтинг: 4.5 из 5 звезд4.5/5 (266)
- Chemical Bonding PDFДокумент80 страницChemical Bonding PDFQuỳnh PhạmОценок пока нет
- IB Chem2 5 Assess T2Документ2 страницыIB Chem2 5 Assess T2Trúc Hồ100% (2)
- Thermo Spectronic Genesys 6 Manual: Read/DownloadДокумент3 страницыThermo Spectronic Genesys 6 Manual: Read/DownloadaseelОценок пока нет
- The Unwinding: An Inner History of the New AmericaОт EverandThe Unwinding: An Inner History of the New AmericaРейтинг: 4 из 5 звезд4/5 (45)
- Nuclear Magnetic ResonanceДокумент34 страницыNuclear Magnetic ResonanceD.N.S DE ZOYSAОценок пока нет
- "Principle of Operation" Page 1-2Документ2 страницы"Principle of Operation" Page 1-2Nur RahMiОценок пока нет
- CHM3105 Short PaperДокумент4 страницыCHM3105 Short PaperTrimal AccraОценок пока нет
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersОт EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersРейтинг: 4.5 из 5 звезд4.5/5 (345)
- 5.1 Mass Spectrometry FactfileДокумент5 страниц5.1 Mass Spectrometry FactfileFasiha RazaОценок пока нет
- Auger Electron SpectrosДокумент1 страницаAuger Electron SpectrossamerОценок пока нет
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyОт EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyРейтинг: 3.5 из 5 звезд3.5/5 (2259)
- Topic 7 Unit 07 Lp01ps - Van Der Waal Forces OkkkkaaaaayyyyyyyyДокумент26 страницTopic 7 Unit 07 Lp01ps - Van Der Waal Forces OkkkkaaaaayyyyyyyyRodjhen Anne P. BarquillaОценок пока нет
- Magnetoresistance Experiment and SEM Analysis & EDX of FeCoДокумент32 страницыMagnetoresistance Experiment and SEM Analysis & EDX of FeCoLakshay BhardwajОценок пока нет
- Introduction To Intermolecular ForcesДокумент8 страницIntroduction To Intermolecular ForcesLouie DelavegaОценок пока нет
- This Study Resource Was: Activity: Strong vs. Weak AcidsДокумент2 страницыThis Study Resource Was: Activity: Strong vs. Weak AcidsJelaena Dean NavalОценок пока нет
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreОт EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreРейтинг: 4 из 5 звезд4/5 (1090)
- Phosphorescence Excitation Spectrum of Benzophenone at Liq.N TemperatureДокумент5 страницPhosphorescence Excitation Spectrum of Benzophenone at Liq.N TemperatureNisar Ali Mphil-Chem AОценок пока нет
- 07 Chem 105 LComputation ResultsДокумент10 страниц07 Chem 105 LComputation ResultsBandiyah Sri AprilliaОценок пока нет
- Summative Assessment 1 2nd QuarterДокумент2 страницыSummative Assessment 1 2nd QuarterJudith DurensОценок пока нет
- Energy Levels MS PDFДокумент3 страницыEnergy Levels MS PDFLoh Jun XianОценок пока нет
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)От EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Рейтинг: 4.5 из 5 звезд4.5/5 (121)
- 20 Chapter Physics TestДокумент1 страница20 Chapter Physics TesthasnainОценок пока нет
- Molymod EnglishДокумент20 страницMolymod EnglishVina Zavira Nizar100% (1)
- Physical Science: Submitted By: Criestefiel Ann S. Lolo Grade 12 - GatesДокумент13 страницPhysical Science: Submitted By: Criestefiel Ann S. Lolo Grade 12 - GatesCriestefiel LoloОценок пока нет
- Class XII Physics - Chapt 12 AtomДокумент31 страницаClass XII Physics - Chapt 12 AtomTABENDRA MОценок пока нет
- Atomic ND Molecular PhysДокумент21 страницаAtomic ND Molecular PhysBinita SedhaiОценок пока нет
- Given The Electron Configuration For C and H, Imagine How Their Atomic Orbitals Might OverlapДокумент38 страницGiven The Electron Configuration For C and H, Imagine How Their Atomic Orbitals Might OverlapMarwan JBОценок пока нет
- De Novo Sequencing by Tandem Mass Spectrometry (MS-MS)Документ10 страницDe Novo Sequencing by Tandem Mass Spectrometry (MS-MS)api-3696530Оценок пока нет
- First Page PDFДокумент1 страницаFirst Page PDFUnidos Con PuerresОценок пока нет
- Biophysics Handout Bits PilaniДокумент3 страницыBiophysics Handout Bits PilaniAryaman MandhanaОценок пока нет
- Raman of Catalyst)Документ30 страницRaman of Catalyst)lotannaОценок пока нет