Академический Документы
Профессиональный Документы
Культура Документы
High Temperature Materials
Загружено:
Veeramani ShankarОригинальное название
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
High Temperature Materials
Загружено:
Veeramani ShankarАвторское право:
Доступные форматы
w
w
w
.
t
o
p
p
e
r
s
a
r
e
n
a
.
c
o
m
HIGH TEMPERATRE MATERIALS
Short Questions and Answe
1 Write five important factors affecting the mechanical properties of metal and
thereby its life.
Ans:- (a) Alloy content such as tungsten(W), Cr and Ni which improves hardness
and strength.
(b) Fine grain size materials exhibit higher strength and vice versa.
(c) Crystal imperfections such as dislocations which cause deformation within the
allowable stress. More dislocations means higher strain and lesser strength.
(d) Excessive cold working or strain hardening results in interaction of dislocations
and hence dislocation mobility vanishes and strength of material increases.
(e) Oxidation and corrosion property.
2 Write few important mechanical properties that enhances materials functional
life.
Ans:- Elasticity(E), Toughness, Tensile and yield strength, impact strength, ductility
and malleability, brittleness vs hardness, fatigue strength and creep resistance are the
important mechanical properties that enhances materials functional life.
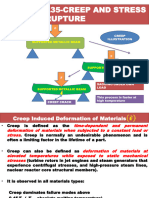
3 Define Creep.
Ans:- It is defined as the property of material by virtue of which it deforms slowly,
continuously under constant stress and high temperature
Draw Fig 1.1 of page -4 of Module-I.
4 Draw a typical creep curve and mark various stages. How does the ratio r/h, rate
of softening to hardening vary for each stage?
Ans:- Draw fig 1.2 of page-5 of Module-I.
At the beginning of the first stage of creep, rate of thermal softening(r) is much faster
than the rate of strain hardening(h). In stage-I, r/h ratio continuously decrease and
become constant in stage-II. Here the rate of softening is fast enough and rate of
hardening is slow that a balance is reached between these competing factors. In stage-
III, r/h ratio continuously increases up to rupture.
For More B.Tech Notes & Projects Visit www.toppersarena.com
For More B.Tech Notes & Projects Visit www.toppersarena.com
w
w
w
.
t
o
p
p
e
r
s
a
r
e
n
a
.
c
o
m
5 Draw the variation of strain() with time and strain rate( ) with time for two sets
of stress and temperature.
Ans:- Draw fig 1.3(a) & (b) of page-6 of Module-I
6 Define rupture life of a material. Draw the variation of rupture life with applied
stress for different temperatures.
Ans:- Draw fig 1.5 of page-8 of module-I and explain.
7 Write how total creep mechanism can be studied by super-position of various
strain-time curves.
Ans:- Draw fig 1.6 of page-8 of module-I and explain.
8 What are the well known relations connecting failure time(t
f
) and temperature
compensated time()? Write their parametric relations.
Ans:- Sherby-Dorn parameter(P
SD
) and Larsen-Miller parameter(P
LM
) are the
relations.
log log log
SD f
Q
P t e
RT
= = .
log (log log )
LM f
Q
P e T t
R
= = .
9 What are the material aspects for creep design?
Ans:- (a) Creep resistance is improved if diffusion rates are reduced.
(b) An increased modulus (E) improves resistance to dislocation creep like coarse
grain improves resistance to diffusion creep.
(c) Inter granularly positioned II phase particles reduce grain-boundary sliding.
10 What are the chief creep deformation mechanisms and write the stress ranges(/G)
in which they occurs.
Ans:- (i)Dislocation Glide which occurs at high stress(/G >10
-2
)
(ii) Dislocation creep which occurs for 10
-4
< /G < 10
-2
.
(iii) Diffusion creep occurs for /G < 10
-4
.
(iv) Grain-boundary sliding.
11With a graph show the relation between total creep rate and stress.
Ans:- Draw fig 2.3 of page 5 of module-II note.
Total creep rate (
T GC DFF
= + )
12 What is hardening? Write different methods of hardening/strengthening.
Ans:- Hardening is the process of strengthening of metals and plastics by addition of
alloys/high strength fibers. Extrinsic load on the matrix(base metal) is transferred to
the high strength fibers and hence intrinsic resistance to deform in the matrix is not
changed. Major strengthening mechanisms are (a) strain hardening or dislocation-
dislocation interactions (b) Grain boundaries solute atoms(solid solution
strengthening) (c) Precipitation hardening (d) Dispersion strengthening and (e) Grain
boundary strengthening.
For More B.Tech Notes & Projects Visit www.toppersarena.com
For More B.Tech Notes & Projects Visit www.toppersarena.com
w
w
w
.
t
o
p
p
e
r
s
a
r
e
n
a
.
c
o
m
13 Explain strain hardening?
Ans:- Strain Hardening or Work hardening is a phenomenon which results in
increase of hardness & strength of metals subjected to plastic deformation at low
temperature(below recrystallization range). Strain hardening results from a dramatic
increase in the no: of dislocation-dislocation interactions and which reduces
dislocation mobility.
14 Explain the effect of dislocation density on the strength of metals.
Ans:- Draw fig 2.4 of page 6 of module-II note.
The strength of the metal approaches extremely high levels when there are either no
dislocations present or when the no: of dislocations are extremely high( >
10 2
10 / cm ).
15 What do you mean by Stress-Rupture test?
Ans:- Stress-rupture tests are basically similar to creep tests, except the tests are
carried out upto failure of material and done at high stress and temperature. These
tests are done for shorter periods(<1000 hrs) with total strain around 50%. Stress-
rupture tests are suited to determine the relatively high temperature strength of new
alloys used for jet engine applications.
16 efine Monkman & Grant relationship.
Ans:- Regarding the rupture life(t
R
) and steady state creep rate(
ss
), Monkman &
Grant identified an empirical relation,
log log
R ss
t m B + = .
Where
R
t = rupture life,
m, B are constants
To a first approximation,
R
t was found to be inversely proportional to
ss
.
17 Explain Twinning in crystalline materials.
Ans:- Studies show that crystalline order in the solid is not lost during plastic
deformation even though more imperfections are introduced. The atom movements
are such that the crystal structure remains same after plastic deformation. Twinning
changes the orientation of the twinned parts. The atoms above the twinning plane
move in a coordinated manner so as to produce the shape changes and produce a
mirror image of the crystal across the twin plane. Twinning occurs in low temperature
plastic deformation of a no: of HCP and BCC transition metals.
18. Give salient differences between slip and twinning.
Ans:-
Slip Twinning
All atoms are in one block Atoms in each successive block move in
different directions.
Under microscope, slip appears as thin
line
Twinning appears as broad lines or bands.
There is very little change in lattice
orientation of slipped region.
There is marked difference in lattice
orientation in the twinned region
For More B.Tech Notes & Projects Visit www.toppersarena.com
For More B.Tech Notes & Projects Visit www.toppersarena.com
w
w
w
.
t
o
p
p
e
r
s
a
r
e
n
a
.
c
o
m
Twinning usually requires a higher shear stress than slip.
Slip systems are considerably limited in H.C.P.
In metals, twinning becomes an important mode of deformation.
19. What is relaxation process?
Ans:- The time-dependent phenomena in material in response to applied load are
called relaxation processes.
Examples of relaxation processes are:- (a) Jumping of atoms by diffusion.
(b) Atomic vibrations.
(c) Flow of grain boundaries
(d) Bolt holding two rigid parts in tight contact relaxes after prolonged hours.
20. What is jog dislocation?
Ans:- Dislocation of Burgers vector b lying in a plane other than the slip plane or
glide plane are called jog under certain condition. Condition is Burger vector of
dislocation is equal to Burger vector lying in the slip plane. These dislocations can
move and/or jump from one plane to another. Jog may be treated as a short
dislocation.
21. What are the differences between crystalline and non-crystalline solids?
Ans:-
Crystalline solid Non-crystalline solid
Long range periodicity Entangled chain without periodicity
Higher density due to closely packed
atoms
Lower density due to zig zag packing of
atoms.
Sharp diffraction pattern Diffraction pattern is not sharp
Melting temperature-pin pointed. Melts over range of temperatures.
Well defined crystal structure and
geometrics
Varying structure and geometrics.
22. What do you mean by dislocations?
Ans:- In materials science, a dislocation is a crystallographic defect or irregularity,
within a crystal structure. The presence of dislocations strongly influences many of
the properties of materials. Some types of dislocations can be visualized as being
caused by the termination of a plane of atoms in the middle of a crystal. In such a
case, the surrounding planes are not straight, but instead bend around the edge of the
terminating plane so that the crystal structure is perfectly ordered on either side. Two
dislocations of opposite orientation, when brought together, can cancel each other, but
a single dislocation typically cannot disappear on its own.
23. Explain edge dislocations.
Ans:- An edge dislocation is a defect where an extra half-plane of atoms is introduced
mid way through the crystal, distorting nearby planes of atoms. When enough force is
applied from one side of the crystal structure, this extra plane passes through planes
of atoms breaking and joining bonds with them until it reaches the grain boundary.
The dislocation has two properties, a line direction, which is the direction running
For More B.Tech Notes & Projects Visit www.toppersarena.com
For More B.Tech Notes & Projects Visit www.toppersarena.com
w
w
w
.
t
o
p
p
e
r
s
a
r
e
n
a
.
c
o
m
along the bottom of the extra half plane, and the Burgers vector which describes the
magnitude and direction of distortion to the lattice. In an edge dislocation, the
Burgers vector is perpendicular to the line direction.
24. Explain screw dislocations.
Ans:- Imagine cutting a crystal along a plane and slipping one half across the other by
a lattice vector, the halves will fit back together without leaving a defect. If the cut
only goes part way through the crystal, and then slipped, the boundary of the cut is a
screw dislocation. It comprises a structure in which a helical path is traced around the
dislocation line by the atomic planes in the crystal lattice. In pure screw dislocations,
the Burgers vector is parallel to the line direction.
25. What is dislocation climb?
Ans:- The driving force for dislocation climb is the movement of vacancies through a
crystal lattice. If a vacancy moves next to the boundary of the extra half plane of
atoms that form an edge dislocation, the atom in the half plane closest to the vacancy
can "jump" and fill the vacancy. This atom shift "moves" the vacancy in line with the
half plane of atoms, causing a shift, or positive climb, of the dislocation. The process
of a vacancy being absorbed at the boundary of a half plane of atoms, rather than
created, is known as negative climb. Since dislocation climb results from individual
atoms "jumping" into vacancies, climb occurs in single atom diameter increments.
26.What is the difference between slip and dislocation climb?
Ans:- Difference between dislocation slip and climb is the temperature dependence.
Climb occurs much more rapidly at high temperatures than low temperatures due to
an increase in vacancy motion. Slip, on the other hand, has only a small dependence
on temperature.
27. Explain the mechanism of dislocation creep?
Ans:- Dislocation creep is a mechanism for deformation in crystalline materials.
Dislocation creep involves the movement of dislocations through the crystal lattice of
the material. It causes plastic deformation of the individual crystals and in the end the
material itself. Dislocation creep is highly sensitive to the differential stress on the
material. At relatively low temperatures it is the dominant deformation mechanism in
most crystalline materials
28. Define Burgers vector.
Ans:- The length of the displacement in the crystal caused by the movement of the
dislocation is called the Burgers vector. It equals the distance between two atoms or
ions in the crystal lattice. Therefore each material has its own characteristic Burgers
vectors for each glide plane.
29. Give difference between isotropic and anisotropic materials.
Ans:- When the properties of a material vary with different crystallographic
orientations, the material is said to be anisotropic. When a material is formed, the
grains are usually distorted and elongated in one or more directions which make the
For More B.Tech Notes & Projects Visit www.toppersarena.com
For More B.Tech Notes & Projects Visit www.toppersarena.com
w
w
w
.
t
o
p
p
e
r
s
a
r
e
n
a
.
c
o
m
material anisotropic. When the properties of a material are the same in all directions,
the material is said to be isotropic. For many polycrystalline materials the grain
orientations are random before any working (deformation) of the material is done.
Therefore, even if the individual grains are anisotropic, the property differences tend
to average out and, overall, the material is isotropic.
30. Explain dislocation recovery.
Ans:- Dislocations are imperfections in a crystal lattice, that from a thermodynamic
point of view reduce the amount of free energy in the system. Therefore, parts of a
crystal that have more dislocations will be relatively unstable. By recrystallisation the
crystal can heal itself. Recovery of the crystal structure can also take place when two
dislocations with opposite displacement meet each other.
31. What is dislocation glide?
Ans:- Dislocation motion along a crystallographic direction is called glide or slip.
There must be a local shear stress in an appropriate direction on the dislocation for
glide to occur. Dislocation glide allows plastic deformation to occur at a much lower
stress than would be required to move a whole plane of atoms past another.
32. Explain diffusion creep.
Ans:- Diffusion creep is more sensitive to temperature than other deformation
mechanisms. It usually takes place at high homologous temperatures (i.e. within
about a tenth of its absolute melting temperature). Diffusion creep is caused by the
migration of crystallographic defects through the lattice of a crystal such that when a
crystal is subjected to a greater degree of compression in one direction relative to
another, defects migrate to the crystal faces along the direction of compression,
causing a net mass transfer that shortens the crystal in the direction of maximum
compression.
33. What are the different types of diffusion creep?
Ans:- Diffusion of vacancies through a crystal can happen in a number of ways.
When vacancies move through the crystal, this is called Nabarro-Herring creep.
Another way in which vacancies can move is along the grain boundaries, is called
Coble creep.
34. Explain coble creep.
Coble creep, a form of diffusion creep, is a mechanism for deformation of crystalline
solids. Coble creep occurs through the diffusion of atoms in a material along the grain
boundaries, which produces a net flow of material and a sliding of the grain
boundaries. The strain rate in a material experiencing Coble creep is given by:
/
3
coble
Q RT
gb
d
D e
dt d
=
Where
is the applied stress
d is the average grain boundary diameter
D
gb
is the diffusion coefficient in the grain boundary
Q
Coble
is the activation energy for Coble creep
For More B.Tech Notes & Projects Visit www.toppersarena.com
For More B.Tech Notes & Projects Visit www.toppersarena.com
w
w
w
.
t
o
p
p
e
r
s
a
r
e
n
a
.
c
o
m
R is the molar gas constant
T is the temperature in Kelvin
35. Define various types of fracture
Ans: Type of fracture are (a) Low temperature tensile fracture caused by separation
of atomic bonds under static loading (b) High temperature tensile fracture (creep
fracture ) caused by separation of atomic bonds under static loading, aided by
diffusion flow. (c) Fatigue fracture-caused by cyclic stress and strain . It happens at
stress levels much less than the stress for tensile fracture. (d) Embrittlement and static
fracture caused by the stress along with hostile environment. Adverse environment
creates material embrittlement.
36. Give briefly the processes of fracture and classes of tensile fracture.
Ans: The process of fracture consists of two components; (a) Crack initiation
(b) Crack propagation. Tensile fracture can be classified into (a) ductile fracture (b)
brittle fracture.
37. Define the various types of cleavage failures.
Ans: In brittle or cleavage fracture , the crack propagates rapidly with minimum
elongation or energy absorption. The propagation of crack involves very little plastic
deformation of the metal adjacent to the crack. The process of cleavage fracture is
made up of three steps, (a) plastic deformation to produce dislocation pile-up (b)
crack initiation (c) crack propagation. Second-phase particles (alloys) crack during
deformation.
38. Explain the brittle to ductile transition from low temperature to high temperature.
Ans: Draw fig- 3.3 given in page 4 of M3 Note. Temperature variation of charpy-
impact energy is show in the above fig. for several types of metals. Low strength
ductile metals do not show any change in fracture mode (impact energy) with
temperature. But for low strength BCC transition metals show a typical ductile to
brittle transition. High strength materials other than steels are fairly insensitive to
temperature.
39. What are the important causes controlling the brittle to ductile transition?
Ans: This transition is related by (
i
D
0.5
+K) K = G
s
where
i
is the lattice
resistance to dislocation motion, K` is a parameter related to release of dislocations
from a pile-up,
s
is the effective surface energy and = shear stress/normal stress.
Causes controlling the brittle to ductile transition are ; (a) High frictional resistance
leading to brittle fracture (b)
i
D
0.5
term in the above equation shows fine grain
For More B.Tech Notes & Projects Visit www.toppersarena.com
For More B.Tech Notes & Projects Visit www.toppersarena.com
w
w
w
.
t
o
p
p
e
r
s
a
r
e
n
a
.
c
o
m
metals can withstand high i (low temperature) before becoming brittle (c) If s is
large at a given temperature , B.F is suppressed (d) Environment factors like
corrosion or H-embrittlement lowers the
s.
40. What are the brittle fracture modes?
Ans: Brittle fracture occurs in 3 modes. In ModeI fracture occurs without plastic
deformation. In Mode-II , microscopic plastic deformation preceeds which nucleates
cracks that may propagate by cleavage or brittle inter-granular fracture (BIF). In
Mode-III, generalized plastic deformation preceeds brittle fracture. A limited
reduction in cross section is found in tensile test for Mode-III.
41. What is Griffith`s theory of brittle fracture ?
Ans: Griffith proposed that a brittle material contains large No. of fine cracks which
attains
max
at crack tips even at low nominal stress. When crack propagates, it needs
surface energy to produce new surfaces which is obtained through the release of
strain energy (PE). According to him ``A crack will propagate when the decrease in
strain energy is at least equal to the energy required to create new crack surfaces ``.
42. At what condition Griffiths equation fails to predict the fracture.
Ans: Griffith equation fails to predict the fracture stress especially when there is
plastic deformation before fracture (Mode-III). Plastic deformation at the root of the
crack makes the tip blunt, there by increasing radius of the crack tip and hence
increasing fracture stress (
f
). Hence Griffith equation can be made applicable for
Mode-III brittle fracture by including a term
p
(plastic work to extend crack).
Hence
f
= j
0.5
2 ( ) E s p c +
0.5
2
p
E
c
(
~
(
( Since s is 1to2J/m2 and
p
is 100 to 1000 J/m2)
43. What are the important characteristics of ductile fracture (D.F)?
Ans: Important characteristics of D.F is it occurs by a slow tearing with the
absorption of considerable energy. At high temperature (but T< 0.4 Tm) tensile
fracture of most of metal is ductile. D.F is always preceded by localized deformation
called necking. Fracture of very ductile metals with 100% R.A is called rupture.
44. Explain the mechanism of micro void coalescans (MVC).
Ans: Study shows that most of the fracture energy associated with MVC is consumed
during the growth of micro-voids. Two growth mechanisms have been identified.
(1) Plastic flow of matrix that surrounds the M-V nucleation site (2) Plastic flow
enhanced by the de-cohesion of small particles in the matrix. Final step of MVC that
leads to final failure involves the coalescence of countless micro-voids into large
crack.
45. What are the 3 stress states of MVC morphology?
For More B.Tech Notes & Projects Visit www.toppersarena.com
For More B.Tech Notes & Projects Visit www.toppersarena.com
w
w
w
.
t
o
p
p
e
r
s
a
r
e
n
a
.
c
o
m
Ans: Three stress stats on MVC morphology are (a) Tensile stress produce equiaxed
micro-voids (b) Pure shear stress generate micro-voids elongated in the shearing
direction. Here the voids point in the opposite direction on the two fracture surfaces.
(c)Tearing associated with non-uniform stresses which produce elongated dimples on
both fracture surfaces that points back to crack origin.
46. What is diffusional void growth ?
Ans: Rapid surface diffusion, i.e. mass transport is required within the void to
maintain the shape of the void. If it is suppressed, the void becomes elongated. The
shape of the growing void is determined by the rate of surface diffusion and boundary
diffusion. If surface diffusion rate is higher than the boundary diffusion rate, the void
will grow like a spherical shape.
47. How the shape of the diffusion void growth is controlled?
Ans: The shape of the growing void is determined by the rate of surface diffusion and
boundary diffusion. If surface diffusion rate is higher than the boundary diffusion
rate, the void will grow like a spherical shape. If surface diffusion rate is lesser than
the boundary diffusion rate, the void will grow like a penny- shaped crack.
48. What are the fracture regimes of FCC metals?
Ans: Fracture regimes of FCC metal are ; (a)Dynamic fracture, occurs at highest
stress levels, which are not achievable. (b) High temperature creep fracture (ICF)
occurs at low stress levels and relatively from low temperature to high temperature.
(c) Low temperature fracture at medium stress levels is the ductile fracture, i.e.TCF.
49. What are the fracture regimes of BCC metals?
Ans: Fracture regimes of BCC metal are the 3 brittle fracture Modes and ICF at low
stress and ductile fracture (TCF) at medium stress levels. Dynamic fracture regime is
at the highest stress levels which are difficult to achieve. Mode-I brittle fracture (BF)
occurs at relatively lower stress levels and Mode-II & III fracture regimes are seen at
medium stress at different temperature ranges.
50. Draw the fracture map of HCP Mg metal.
Ans: Draw fig. 3.11 given in page 19 of M-3 Notes.
51. Draw the fracture maps of alkali halides (NaCl).
Ans: Draw fig. 3.12 given in page 19 of M-3 Notes.
52. Draw the fracture maps of refractory oxides
Ans: Draw fig. 3.13 given in page 20 of M-3 Notes.
53. Define oxidation and give some examples.
Ans: Oxidation generally refers to oxygen-producing reaction . This is also used to
designate the reaction between metal and air/oxygen in the absence of water. Some
of the oxidation processes are ; scaling ,tarnishing and dry corrosion.
54. What are the preventive measures against oxidation & corrosion?
For More B.Tech Notes & Projects Visit www.toppersarena.com
For More B.Tech Notes & Projects Visit www.toppersarena.com
w
w
w
.
t
o
p
p
e
r
s
a
r
e
n
a
.
c
o
m
Ans: Preventive measures against oxidation & corrosion are :
(a) Use of corrosion/oxidation resistant materials like, Cu, Bronze & Brass.
(b) Use of protective coatings; e.g. Paints, grease, enamels etc.
(c) Galvanic protection; e.g. iron with Zn coating, iron with Al.
(d) Use of inhibitors , (e) Avoid formation of galvanic cell by proper design . Avoid
physical contact b/w two metals.
55. What are the two important reactions necessary for completing the oxidation?
Ans: Oxidation by gaseous O2 in presence of water (H
+
) is an electrochemical
process. It is not simply chemical combination of metal (M) and O2, but it consists of
two partial, spatially separated processes.
M M
2+
+ 2e- (metal oxidation at metal scale interface)
O2 + 2e- O
2-
(oxidant reduction at scale- gas interface)
Hence it is essential to have both anode and cathode reactions to complete the
corrosion/oxidation of metal.
56. Define Pitting- Bed worth ratio and give its impact on type of corrosion.
Ans: Pilling & Bed worth proposed that oxidation resistance is related to the volume
ratio of oxide and metal per gram atom of meal.
Mole. Wt. of oxide/ density of oxide Molar vol. of oxide
i.e. P-B ratio = ------------------------------------------- = --------------------------
Mole. Wt. of metal/density of metal Molar vol. of metal
If P-B ratio is less than 1, the scale is not protective, B/w 1& 2.3 it gives a
protective scale and more than 2.3 it is again non-protective.
57. Comments on the various kinetic laws of oxidation.
Ans: Examination of various kinetic laws of oxidation indicate that a linear oxidation
rate is least desirable , since W(weight gain per unit area due to oxide) increases at
constant rate with time(t). Parabolic and logarithmic oxidation rates are more
desirable for high temperature alloys. Al oxidies according to this log rate law and
hence it stops after a few days exposure.
58. Draw different oxidation rate law curves.
Ans: Draw fig. 4.1 of page-4 of M-4 Note.
59. What are N-type and P-type oxides?
Ans: Some oxides have excess and others a deficiency of metallic ions, or
equivalently, deficiency or excess of oxide ions. For e.g: ZrO2 contains excess
electrons which are carriers of current and termed as N-type oxides . similarly NiO is
a metal deficient oxide. For each Ni ion (Ni
2+
) vacancy, there are two trivalent
(Ni
3+
) Nickel ions in normal lattice positions. Ni
3+
can be considered as divalent
ions (Ni
2+
) and an electron hole. Hence this oxide is termed as P-type oxide.
For More B.Tech Notes & Projects Visit www.toppersarena.com
For More B.Tech Notes & Projects Visit www.toppersarena.com
w
w
w
.
t
o
p
p
e
r
s
a
r
e
n
a
.
c
o
m
60. How oxidation is controlled in N-type oxides?
Ans: ZrO2 contains excess electrons and termed as n-type oxides. Addition of
cations of lower valence (Ca
2+
) than Zr
4+
in ZrO2 lattice, increases anion vacancy
(+) and decreases the concentration of excess electrons in N-type oxides. Hence
oxidation is decreased. But addition of higher valence cations (Ta
5+
) into ZrO2
lattice decreases anion vacancy (+) and increases the concentration of excess
electrons and hence increases the oxidation of N-type oxides.
61. How oxidation is controlled in P-type oxides?
Ans: In P-type oxides like NiO with excess electron holes, addition of lower valence
cations (Li+) decreases the no. of electron holes and hence decreases the oxidation
rate. On the other hand addition of higher valance cations (Cr
3+
) increases the no. of
electron holes and hence increases the oxidation rate.
62. How oxidation resistance/ protection is achieved?
Ans: There are numerous properties an oxide layer must posses to be protective.
When such properties exist in the oxide layer formed, a high degree of oxidation
resistance /protection is achieved. Ni, Co & Fe exhibit a moderate degree of
oxidation resistance. But alloy addition like , Cr, Al allow formation of relatively
good protective oxide phases (Cr
2
O
3
, Al
2
O
3
) . If Cr & Al alloys are too less in the
metal, the internally produced oxides will not be effective as a protective oxide layer.
63. Define basic and acidic fluxing in hot corrosion.
Ans: Fluxing (dissolving) can be caused either by the combination of oxides (Al
2
O
3
)
with O
2-
to form anions and it is basic fluxing.
i.e. Al
2
O
3
+ O
2-
2Al O
2-
Acid fluxing takes by decomposition of oxides into corresponding cations and O
2-
.
i.e. Al
2
O
3
2Al
2+
+ 3 O
2-
Acid fluxing occurs when O
2-
activity in the molten salt is much lowered. Acid
fluxing causes much more severe oxidation than basic fluxing.
64. What are the major areas at which hot corrosion occurs?
Ans: Hot corrosion is the accelerated corrosion of materials in the presence of salt
contaminants like Na Cl, Na
2
SO
4
& V
2
O
5
, which damages the protective oxide layer.
Hot corrosion occurs in areas like petrochemical industries for coal gasification and
thermoelectric power stations where residual oil is used as the energy source. Na & S
present in fuel oil and coal react to form Na
2
SO
4
in the combustion system and act as
hot corrosion agent in the entire system.
65. What are the mechanisms of hot corrosion?
Ans: Hot corrosion can occur when the salt contaminants are either in liquid or solid
form. In the temperature range of 850-950
0C
high temperature hot corrosion occurs
For More B.Tech Notes & Projects Visit www.toppersarena.com
For More B.Tech Notes & Projects Visit www.toppersarena.com
w
w
w
.
t
o
p
p
e
r
s
a
r
e
n
a
.
c
o
m
with the condensation of fused alkali metal salt (Na Cl , Na
2
SO
4
) on the surface of
components and attack the protective oxide film. In temperature range of 630-806
0C
,
salt contaminants like Ca
2
SO
4
& Na
2
SO
4
cause typical pitting on the oxide layer and
it is called low temperature hot corrosion.
66. What are salt fluxing mechanisms?
Ans: Salt fluxing mechanism was originally proposed by Goebel and Pettit. As per
this , protection efficiency of surface oxide layer is lost as a result of fluxing of this
layer in molten salt. It can take place by combination of oxide (Al
2
O
3
) with O
2-
(basic fluxing) or by decomposition of oxide into cations and O
2-
(acid fluxing).
Al
2
O
3
+ O
2-
2Al O
2-
(basic fluxing)
Al
2
O
3
2Al
2+
+ 3 O
2
(acid fluxing).
Acid fluxing is much more corrosive and self sustaining than basic fluxing.
67. How hot corrosion resistance is improved by adding alloy elements?
Ans: Hot corrosion resistance of super alloys are improved because of its complex
microstructure which contains several phases like carbides ,borides and TCP
(topologically closed packed ) intermetallic compounds such as , (Ni
3
Al) in Ni-
base alloys with a FCC solid solution matrix. The optimization of all desired
properties including hot corrosion resistance involves a balance of kind and
concentration of alloying elements.
68. Define and list various TCP phases and explain whether they are beneficial or
detrimental for high temperature properties.
Ans: TCP (topologically closed packed ) intermetallic compounds are of the type ,
(Ni
3
Al) in Ni-base alloys with a FCC solid solution matrix. For e.g: Cr is a good hot
corrosion resistant element. However, it promotes formation of TCP phases that are
harmful to high temperature ductility and strength if added in excess.
69. What are the two simple methods to combat hot corrosion?
Ans: Superalloy Superni 75 (Ni-base) subjected to hot corrosion test with plasma
sprayed Ni Cr Al Y and Ni 20Cr metallic coating. The performance of uncoated as
well as coated super alloy was evaluated and found that uncoated superalloy suffered
accelerated corrosion. Ni Cr Al Y coated specimen showed marginal spalling towards
the end of exposure of 50 cycles. The Ni 20Cr coated super alloy did not suffer from
spalling of its oxide scale. Ni 20Cr coated showed maximum weight gain, while
Ni Cr Al Y coated showed the least. Both coatings were found sufficient to combat
hot corrosion.
70. How modified hot corrosion is achieved?
Ans: Addition of active elements in H.T.M may improve corrosion resistance
extensively. For this Yttrium (Y), which is an oxygen active element was ion-plated
before or after pack aluminizing IN713C alloy to maximize the corrosion resistance.
From various coating combinations, best corrosion resistance was obtained from
H/A+Y (high activity aluminizing + Y-ion plating) type multi-layer coatings. High
For More B.Tech Notes & Projects Visit www.toppersarena.com
For More B.Tech Notes & Projects Visit www.toppersarena.com
w
w
w
.
t
o
p
p
e
r
s
a
r
e
n
a
.
c
o
m
activity aluminide coating gave better uniformity of Y deposition than the low
activity aluminide coating. High corrosion resistance for H/A+Y coating was
obtained because Y present between Al
2
O
3
columns improves the Al
2
O
3
scale
adherence and prohibit Al depletion.
71. How high temperature capability like strength & ductility of Fe-alloy can be
enhanced by adding nickel?
Ans: Nickel- base alloys are either solid solution strengthened or precipitation
strengthened. First is for moderate strength and temperature (matrix in FCC phase)
And latter (`) is for high temperature strength and ductility.
72. What are the different species (compounds) that improve the high temperature
properties of Ni-base alloys?
Ans: In Ni-base alloys, gamma() formers (group 5,6,7 elements) tent to keep the
base lattice in FCC by solid solution strengthening . The - prime formers are
precipitates (Ni
3
Al or Ti) which increases the high temperature strength. They are
group 3,4.5 elements. Third species is the carbide formers mainly of Cr, Mo, W, Nb,
Ta etc. The primary grain boundary elements use B, C, & Zr whose atomic dia. is 21-
27% different from Ni atoms.
73. Briefly explain solid solution strengthening.
Ans: Elastic interaction between stress fields of solute atoms and dislocations in the
base lattice produces solid solution strengthening. Edge dislocations have both shear
and hydrostatic stress fields around them. Screw dislocations have only shear stress
field . Again the type of interaction depends on the type of misfit (symmetrical or
non-symmetrical) which depends on the size difference between solute and solvent
atoms. When stress field of solute atom is symmetrical, it interact with edge
dislocation only and solid solution strengthening is limited (d/dc = G/100 toG/10).
When stress field of solute atom is non-symmetrical, solute atom interacts strongly
with both edge and screw dislocations. In this case solid solution strengthening is
much greater (d/dc = 2G to 9G).
74. What are the various types of precipitation hardening and define them?
Ans: Micro structural precipitates with second phase () particles formation is one
type of strengthening. The strengthening depends on aging temperature which
decides the amount of precipitates (). Dislocation- particle interaction is another
method of precipitation strengthening.
75. What is grain boundary strengthening and briefly explain?
For More B.Tech Notes & Projects Visit www.toppersarena.com
For More B.Tech Notes & Projects Visit www.toppersarena.com
w
w
w
.
t
o
p
p
e
r
s
a
r
e
n
a
.
c
o
m
Ans: The presence of grain boundaries act as barriers to the movement of glide
dislocations and hence act as strengthening agent. The yield stress (
ys
) is contributed
by three components which are decided by their stress fields. They are (a) the short
range order peierls stress effect (<10 A
0
), (b) Long range order dislocation stress
field effect (100 to !000 A
0
) (c) Very long-range structural size effects (> 10
4
A
0
).
76. Define and list various TCP phases and explain whether they are beneficial or
detrimental for high temperature properties.
Ans: TCP compounds are important in cobalt alloys and contain , , Laves and
phases. phase is an electron compound with 30 atoms per unit cell, crystallizes in
Body-centered tetragonal (BCT) form. contains 13 atoms per unit cell,
crystallizes in a rhombohedral form . Laves are non-electron compounds of the form
A
2
B- (Co2 Ta). ` contains semi-carbide of Co, Ni, Cr,& W. These phases mostly
reduces high temperature strength and ductility and hence they are detrimental to high
properties.
77. What is embrittlement and define various types?
Ans: The term embrittlement is applied to variety of mechanical performance
degradation of a stressed material exposed to hostile environment. It happens when
the material is exposed to LME (liquid metal embrittler), an aqueous solution (results
in stress corrosion cracking- SCC) and IAE (impurity atom embrittlement). LME
occurs when alloy contains a low melting element (e.g: steel with lead). SCC happens
due to anodic dissociation at crack tip.
78. What are intermetallics and give the different types of materials formed with
intermetallics?
Ans: Intermetallics are compounds formed by two or more metals in well defined
integral proportion and have crystal structure up to its melting point. They have high
melting point and high resistance to deformation. Some of intermetallics Nickel
aluminide (Ni3 Al, Ni Al), Titanium aluminide (Ti
3
Al, Ti Al) and Iron aluminide
(Fe
3
Al).
79. What are ceramics and briefly explain with examples?
Ans: Ceramics are non-organic, non-metallic materials including oxides, borides,
carbides, nitrides and silicides. Major engineering ceramics are Alumina (Al
2
O
3
),
Zirconia (Zr O
2
), Silicon carbide (Si C) and silicon nitride (Si
3
N
4
). Ceramics are used
as insulating materials and high temperature filtering elements in addition to use as
structural components. High temperature capability of Si
3
N
4
over Ni-super alloy
with lower density is of much importance for G.T application.
80. Explain why the single crystal turbine blades perform better than directionally
solidified and coarse grained cast products.
For More B.Tech Notes & Projects Visit www.toppersarena.com
For More B.Tech Notes & Projects Visit www.toppersarena.com
w
w
w
.
t
o
p
p
e
r
s
a
r
e
n
a
.
c
o
m
Ans: Single crystal materials have no grain boundaries and hence leads to improved
mechanical properties. It also reduce susceptibility to creep and cracking along grain
boundaries. Hence single crystal turbine blades perform better than directionally
solidified and coarse grained cast products.
81. What is gamma in Nickel-base superalloy ?
Ans: The continuous matrix (called gamma ) is an FCC nickel-based austenitic
phase that usually contains a high percentage of solid-solution elements such
as Co, Cr, Mo and W.
82. What is role of gamma prime () in nickel-based alloy?
Ans: The primary strengthening phase in nickel-based superalloys is Ni
3
(Al,Ti), and
is called gamma prime. It is a coherently precipitating phase with ordered FCC
crystal structure. The close match in matrix/precipitate lattice parameter (~0-1%)
combined with the chemical compatability allows the to precipitate
homogeneously throughout the matrix and have long-time stability. Interestingly,
the flow strees of the increases with increasing temperature up to about 650
0
C.
In addition, is quite ductile and thus imparts strength to the matrix without
lowering the fracture toughness of the alloy. Ai & Ti are the major constituents
and are added in amounts and mutual proportions to precipitate a high volume
fraction in the matrix. In some modern alloys the volume fraction of the is
around 70%. There are many factors that contribute to the hardening imparted by
the and include faulty energy, strength, coherency strains, volume
fraction of and particle size.
83. What are carbides in Nickel-based superalloys and their roe?
Ans: Carbon, added at levels of 0.05-0.2%, combine with reactive and refractory
elements such as Ti, Ta, and hafnium to form carbides (eg., TiC, TaC, or HfC).
During heat treatment and service, these begin to decompose and form lower
carbides such as M23C6 and M6C, which lend to form on the grain boundaries.
These common carbides all have an FCC crystal structure. Results vary on
whether carbides are detrimental or advantageous to superalloy properties. The
general opinion is that in superalloys with grain boundaries, carbides are
beneficial by increasing rupture strength at high temperature.
84. How directionally solidified (DS) materials are produced?
Ans: In DS processing, columnar grains are formed parallel to the growth axis. In
Nickel-based alloys, the natural growth direction is along the <100>
crystallographic direction. This morphology is accomplished by poring liquid
metal into a mold that contains a water-cooled bottom plate. Solidification first
For More B.Tech Notes & Projects Visit www.toppersarena.com
For More B.Tech Notes & Projects Visit www.toppersarena.com
w
w
w
.
t
o
p
p
e
r
s
a
r
e
n
a
.
c
o
m
occurs at the bottom plate, after which the mold is slowly withdrawn from the
furnace, allowing the metal inside to directionally solidify from bottom to top .
85. How single crystal (SC) alloy parts are produced?
Ans: SC casting are produced in a similar fashion to DS by selecting a single
grain,via a grain selector. During solidification, this single grain grows to
encompass the entire part. Single crystals obtain their outstanding strength
through the elimination of grain boundaries that are present in both equiaxed and
directionally solidified materials. In addition, the elimination of grain boundary
strengtheners such as C,B, Si, and Zr raises the single crystals melting point. By
increasing the alloys melting point, the homogenization heat-treatment
temperature can be increased without fear of incipient melting, thus allowing for
more complete solutioning of the and thereby increasing alloy strength and
maximum use temperature.
86. What are advantages of DS & SC alloys?
Ans: 1. The alignment or elimination of any weak grain boundaries oriented
transverse to the eventual loading direction.
2. The low modulus associated with <100> directions enhances thermal
mechanical fatigue resistance in areas of constrained thermal expansion
Particularly turbine vanes. In general, the lack of transverse grain boundaries
coupled with the lower modulus can result in 3-5 times improvement in
rupture life.
87. What are properties of superalloys and their crystal structure ?
Ans: A superalloy, or high- performance alloy , is an alloy that exhibits excellent
mechanical strength and creep resistance at high temperatures, good surface
stability , and corrosion and oxidation resistance. Superalloys typically have
an austenitic FCC crystal structure. A superalloys base alloying element is
usually Ni, Co, or Ni-Fe. Superalloy development has relied heavily on both
chemical and process innovations.
88. What is dentrite ?
Ans: In metals, the crystals that form in the liquid during freezing generally follow
a pattern consisting of a main branch with many appendages. A crystal with
this morphology slightly resembles a pine tree and is called a dentrite, which
means branching. The formation of dentrites occurs because crystals grow in
defined planes due to the crystal lattice they create.
For More B.Tech Notes & Projects Visit www.toppersarena.com
For More B.Tech Notes & Projects Visit www.toppersarena.com
w
w
w
.
t
o
p
p
e
r
s
a
r
e
n
a
.
c
o
m
89. What are shrinkage problems during casting ?
Ans: Shrinkage can sometimes cause cracking to occur in component as it
solidifies. Since the coolest area of a volume of liquid is where it contacts a
mold or Die, solidification usually begins first at this surface. As the crystals
grow inward, the material continues to shrink. If the solid surface is too rigid
and will not deform to accommodate the internal shrinkage, the stresses can
become high enough to exceed the tensile strength of the material and cause a
crack to form. Shrinkage cavitation sometimes occurs because as a material
solidifies inward, shrinkage occurred to such an extent that there is not enough
atoms present to fill the available space and a void is left.
90. How grain size is controlled ?
Ans: The size of the grains within a material also has an effect on the strength of the
material. The boundary between grains acts as a barrier to dislocation
movement and the resulting slip because adjacent grains have different
orientations. Since the atoms alignment is different and slip planes are
discontinuous between grains. The smaller the grains, the shorter the distance
atoms can move along a particular slip plane. Therefore, smaller grains
improve the strength of a material. The size and number of grains within a
material is controlled by the rate of solidification from the liquid phase.
For More B.Tech Notes & Projects Visit www.toppersarena.com
For More B.Tech Notes & Projects Visit www.toppersarena.com
Вам также может понравиться
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryОт EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryРейтинг: 3.5 из 5 звезд3.5/5 (231)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)От EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Рейтинг: 4.5 из 5 звезд4.5/5 (119)
- Never Split the Difference: Negotiating As If Your Life Depended On ItОт EverandNever Split the Difference: Negotiating As If Your Life Depended On ItРейтинг: 4.5 из 5 звезд4.5/5 (838)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaОт EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaРейтинг: 4.5 из 5 звезд4.5/5 (265)
- The Little Book of Hygge: Danish Secrets to Happy LivingОт EverandThe Little Book of Hygge: Danish Secrets to Happy LivingРейтинг: 3.5 из 5 звезд3.5/5 (399)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyОт EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyРейтинг: 3.5 из 5 звезд3.5/5 (2219)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeОт EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeРейтинг: 4 из 5 звезд4/5 (5794)
- Team of Rivals: The Political Genius of Abraham LincolnОт EverandTeam of Rivals: The Political Genius of Abraham LincolnРейтинг: 4.5 из 5 звезд4.5/5 (234)
- The Emperor of All Maladies: A Biography of CancerОт EverandThe Emperor of All Maladies: A Biography of CancerРейтинг: 4.5 из 5 звезд4.5/5 (271)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreОт EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreРейтинг: 4 из 5 звезд4/5 (1090)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersОт EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersРейтинг: 4.5 из 5 звезд4.5/5 (344)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceОт EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceРейтинг: 4 из 5 звезд4/5 (890)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureОт EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureРейтинг: 4.5 из 5 звезд4.5/5 (474)
- The Unwinding: An Inner History of the New AmericaОт EverandThe Unwinding: An Inner History of the New AmericaРейтинг: 4 из 5 звезд4/5 (45)
- The Yellow House: A Memoir (2019 National Book Award Winner)От EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Рейтинг: 4 из 5 звезд4/5 (98)
- Aluminum Silicon Casting AlloysДокумент126 страницAluminum Silicon Casting AlloysEdson Araga67% (3)
- Fractography of Ceramic and Metal FailureДокумент415 страницFractography of Ceramic and Metal FailureKhaled BOUALIОценок пока нет
- Low and High Cycle FatigueДокумент85 страницLow and High Cycle FatigueKevin TsuiОценок пока нет
- ASTM Grain Size Measurement GuideДокумент27 страницASTM Grain Size Measurement GuidenareshОценок пока нет
- Prediction of residual stress and distortion in heat treated aluminum partsДокумент60 страницPrediction of residual stress and distortion in heat treated aluminum parts임학진Оценок пока нет
- 4-Lecture Notes Aerodynamics Introductory LectureДокумент30 страниц4-Lecture Notes Aerodynamics Introductory Lecturerogerfisica100% (2)
- Mechanical Properties of MetalsДокумент258 страницMechanical Properties of MetalsIsza Marie N. SocorinОценок пока нет
- ForgingДокумент8 страницForgingVeeramani ShankarОценок пока нет
- Flight Dynamics Two MarkДокумент43 страницыFlight Dynamics Two MarkVeeramani ShankarОценок пока нет
- Avionics - QBДокумент5 страницAvionics - QBVeeramani ShankarОценок пока нет
- Aero Sybl R 2013Документ89 страницAero Sybl R 2013Maniyarasu OppilamaniОценок пока нет
- Casting DefectДокумент5 страницCasting DefectVeeramani ShankarОценок пока нет
- EE CONTROL ENGINEERINGДокумент17 страницEE CONTROL ENGINEERINGSaberish Kumar AОценок пока нет
- Radiator: Radiators AreДокумент5 страницRadiator: Radiators AreVeeramani ShankarОценок пока нет
- Gun TunnelsДокумент4 страницыGun TunnelsVeeramani ShankarОценок пока нет
- Engines and Repair LabДокумент19 страницEngines and Repair LabkarthipriyaОценок пока нет
- Aircraft Structure 1Документ21 страницаAircraft Structure 1pasaattttОценок пока нет
- Numerical MethodsДокумент37 страницNumerical MethodsVeeramani ShankarОценок пока нет
- Aircraft Systems Lab Course PlanДокумент2 страницыAircraft Systems Lab Course PlanVeeramani ShankarОценок пока нет
- High Temperature MaterialsДокумент101 страницаHigh Temperature MaterialsVeeramani Shankar100% (1)
- Int3 Ad Question PaperДокумент2 страницыInt3 Ad Question PaperVeeramani ShankarОценок пока нет
- Flow Visualization inДокумент2 страницыFlow Visualization inVeeramani ShankarОценок пока нет
- Ad-II Revision Test - IДокумент2 страницыAd-II Revision Test - IVeeramani ShankarОценок пока нет
- Anna University B.E. Degree ExaminationДокумент2 страницыAnna University B.E. Degree ExaminationVeeramani ShankarОценок пока нет
- Ad-Ii SylДокумент1 страницаAd-Ii SylVeeramani ShankarОценок пока нет
- High Temperature MaterialsДокумент101 страницаHigh Temperature MaterialsVeeramani Shankar100% (1)
- MIT16 50S12 Lec8Документ7 страницMIT16 50S12 Lec8Veeramani ShankarОценок пока нет
- Rajalakshmi Engineering College: Ae 2305 Aircraft Structures Lab-IiДокумент31 страницаRajalakshmi Engineering College: Ae 2305 Aircraft Structures Lab-IiVeeramani ShankarОценок пока нет
- Introduction To CFD ModuleДокумент44 страницыIntroduction To CFD ModuleVeeramani ShankarОценок пока нет
- M.E Aeronautical Engg Syllabus Reg: 2013Документ27 страницM.E Aeronautical Engg Syllabus Reg: 2013Veeramani ShankarОценок пока нет
- 34-Creep Deformation-31-10-2023Документ22 страницы34-Creep Deformation-31-10-2023NandiniОценок пока нет
- Ferromagnetic Domain Theory: A Survey of the Physical Principles and Crucial ExperimentsДокумент51 страницаFerromagnetic Domain Theory: A Survey of the Physical Principles and Crucial ExperimentsEdward Raja KumarОценок пока нет
- Frank Girgsdies Phase Analysis and Structure Refinement 131129Документ91 страницаFrank Girgsdies Phase Analysis and Structure Refinement 131129MegaTypers100% (1)
- Revealing Hidden Interfaces in Organic SemiconductorsДокумент7 страницRevealing Hidden Interfaces in Organic SemiconductorsalkanderiОценок пока нет
- Studies of Welded Joints: Archives of Foundry EngineeringДокумент6 страницStudies of Welded Joints: Archives of Foundry EngineeringnkchandruОценок пока нет
- Rolls-Royce Corporation, Indianapolis, Indiana Howmet Research Corporation, Whitehall, Michigan Cannon-Muskegon Corporation, Muskegon, Michigan ++NASA Glenn, Cleveland, OhioДокумент9 страницRolls-Royce Corporation, Indianapolis, Indiana Howmet Research Corporation, Whitehall, Michigan Cannon-Muskegon Corporation, Muskegon, Michigan ++NASA Glenn, Cleveland, OhioCaio Fazzioli TavaresОценок пока нет
- The Effect of Constrained Groove Pressing On Grain Size, Dislocation Density and Electrical Resistivity of Low Carbon SteelДокумент7 страницThe Effect of Constrained Groove Pressing On Grain Size, Dislocation Density and Electrical Resistivity of Low Carbon SteelMoin AОценок пока нет
- Dislocation Theory For Engineers: Worked ExamplesДокумент80 страницDislocation Theory For Engineers: Worked Examplesa khosraviОценок пока нет
- Quian Lippold 2003 Liquation Phenomena in The SimulatedДокумент7 страницQuian Lippold 2003 Liquation Phenomena in The Simulatedvladimirsoler01Оценок пока нет
- Notes 01 Spring2014Документ47 страницNotes 01 Spring2014annerivervalleyОценок пока нет
- Materia Engineering Cha 1Документ236 страницMateria Engineering Cha 1amanuelfitsum589Оценок пока нет
- 7th Congr Mat TestДокумент6 страниц7th Congr Mat TestAlireza KhodabandehОценок пока нет
- Viewdocdsp - Asp Doc 32964&lib CENTRODOCTEC&version 32923&mimetype Application/pdf&file PDFДокумент430 страницViewdocdsp - Asp Doc 32964&lib CENTRODOCTEC&version 32923&mimetype Application/pdf&file PDFRosa San MartinОценок пока нет
- Quiz Show FileДокумент18 страницQuiz Show FileLeaniel SilvaОценок пока нет
- Defects in Crystals-Che222Документ18 страницDefects in Crystals-Che222mgfmhcvjhj;Оценок пока нет
- Strengthening MechanismsДокумент19 страницStrengthening MechanismsAnas Anwar ArainОценок пока нет
- Restlife AssessmentДокумент12 страницRestlife AssessmentsachzenОценок пока нет
- Lecture-2 - MLL100 (Lattice, Motif)Документ21 страницаLecture-2 - MLL100 (Lattice, Motif)Sibasish RoutОценок пока нет
- Lecture TEMДокумент20 страницLecture TEMKevin McNallyОценок пока нет
- Jfap 0501 P 041 WДокумент14 страницJfap 0501 P 041 WJulio C. Sierra PalominoОценок пока нет
- Chapter 3c X Ray DiffractionДокумент40 страницChapter 3c X Ray DiffractiondhandametОценок пока нет
- Crystal Structure PDFДокумент51 страницаCrystal Structure PDFKUSHAGRA MANIKОценок пока нет
- Pre-U PhysicsДокумент182 страницыPre-U PhysicsBobОценок пока нет
- IISC Bangalore - Materials Engineering Courses-2013-14Документ8 страницIISC Bangalore - Materials Engineering Courses-2013-14Sri PuduОценок пока нет