Академический Документы
Профессиональный Документы
Культура Документы
Corrosion
Загружено:
ranjankathuriaОригинальное название
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
Corrosion
Загружено:
ranjankathuriaАвторское право:
Доступные форматы
Volume : 2 | Issue : 2 | Feb 2013 ISSN No 2277 - 8160
Research Paper
Chemistry
Study on the Corrosion Inhibition of Mild Steel by Phosphono and Benzotriazole Derivative in Ground Water Media Dr. V. Manivannan ABSTRACT
Professor & Head, Department of Chemistry, Paavai College of Engineering, Pachal, Namakkal, Tamilnadu, India
Corrosion inhibition by benzotriazole derivative 1-(2- pyrrolecarbonyl)benzotriazole (PCBT) and phosphono derivative 2-Phosphonoacetic acid (2-PAA) on mild steel in ground water media has been investigated by weight loss method, potentiodynamic polarization, electrochemical impedance spectroscopy (EIS) and atomic absorption spectroscopy(AAS) method. The experimental results obtained reveal that among the two inhibitors PCBT is the best eective inhibitor than 2-PAA. The variation in inhibitive eciency mainly depends on the type and nature of the substituent present in the inhibitor molecule. Inhibition eciency (IE %) values obtained from various methods used are in good agreement.
KEYWORDS: Mild steel, Benzotriazole, Phosphonic acid, Corrosion, Potentiodynamic polarization, Inhibition.
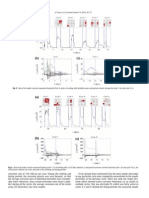
1. INTRODUCTION Corrosion control of metals is an important activity of technical, economical, environmental and aesthetical importance. Thus, the search for new and ecient corrosion inhibitors has become a necessity to secure metallic materials against corrosion. Over the years, considerable eorts have been deployed to nd suitable compounds of organic origin to be used as corrosion inhibitors in various corrosive media, to either stop or delay to the maximum the attack of a metal [1-10]. The adsorption of corrosion inhibitor depends mainly on physico-chemical properties of the molecule such as functional groups, steric factor, molecular size, molecular weight, molecular structure, aromaticity, electron density of the donor atoms and p- orbital character of donating electrons [11-12] and also on the electronic structure of the molecules. The aim of the present study is to determine the inhibition eciency of benzotriazole with Zn2+ and 2-phosphonoacetic acid. 2. EXPERIMENTAL Mild steel samples with the composition C-0.13%, P-0.032%, Si-0.014%, S-0.025%, Mn-0.48% and balance Fe were used and the standard electrode preparation was followed. For weight loss measurements metal specimens of 4.0 cm x 2.0 cm x 0.2 cm dimension were used. For each electrochemical study, specimens of size 1.0 cm 9 1.0 cm 9 0.3 cm were cut, embedded in epoxy resin and mechanically polished with silicon carbide papers. All the electrochemical measurements were performed using an Electrochemical Workstation (Model No: CHI 760, CH Instruments, USA). The potentiodynamic polarization studies were carried out from -1200 to 0 mV at a scan rate of 0.1 mV s-1. Electrochemical impedance studies were carried out with the same setup used for potentiodynamic polarization studies. The applied ac perturbation signal was about 10 mV within the frequency range 100 kHz to 1Hz. The amount of iron leached-out to the solution after carrying out potentiodynamic polarization measurement of mild steel in ground water in the absence and presence of inhibitors was estimated by AAS technique. 3. RESULTS AND DISCUSSION 3.1 Weight loss measurements The results of weight loss measurements for mild steel in ground water in the absence and presence of optimum concentrations of benzotriazole, phosphono derivatives are listed in Table 1. According to these results, among the benzotriazole derivatives PCBT had shown the maximum inhibition eciency of 74.4 % at the optimum concentration of 12 ppm and the phosphono derivative 2-PAA had shown the maximum inhibition eciency of 61.96 % at the optimum concentration of 12 ppm. 3.2 Electrochemical Studies The potentiodynamic polarization curves of mild steel in ground water without and with the presence of various concentrations of PCBT and 2-PAA are shown in Fig. 1 & 2. It is evident from the gure that the anodic and cathodic curves for mild steel with benzotriazole derivative was shifted towards negative potential region compared to the blank and at the same time in the presence of phosphono derivative, the anodic and cathodic curves for mild steel were shifted towards positive potential region compared to the blank. The values of the electrochemical parameters like Ecorr and its corresponding corrosion current density are obtained from the polarization curves and are summarized in Table 2.With the aid of Tafel extrapolation the corrosion inhibition eciency of PCBT was found to be 75.5% for optimum concentrations of 12 ppm. In the case of phosphono derivative, the corrosion inhibition eciency of 2-PAA is found to be 62.6%. Impedance measurements of the mild steel electrode at its open circuit potential after 1 hr of immersion in ground water alone and in the presence of various optimum inhibitor combinations were performed over the frequency range from 100 kHz to 1 Hz. represents the capacitance of the inhibitor lm on the metal surface due to adsorption. Typical Nyquist plots obtained in the absence and presence of optimum concentration of all the studied inhibitors are shown in Fig. 3. The impedance diagrams obtained are not perfect semicircles and this dierence has been attributed to frequency dispersion. The values of the dierent elements present in the equivalent circuit were evaluated using a tting procedure and the parameters obtained are shown in Table 3. From the impedance plots of the individual inhibitors, it is evident that the values of charge transfer resistance of PCBT found to be 0.5728 104 and 2-PAA found to be 0.3168 104 All the ac measurements show the same trend as those observed from dc polarization.
Fig. 1. Potentiodynamic polarization curves of mild steel in ground water in the absence and presence of various concentrations of PCBT at 30 C.
Fig. 2. Potentiodynamic polarization curves of mild steel in ground water in the absence and presence of various concentrations of 2-PAA at 30 C
GRA - GLOBAL RESEARCH ANALYSIS X 14
Volume : 2 | Issue : 2 | Feb 2013 ISSN No 2277 - 8160
WWTRTable 2. Potentiodynamic polarization parameters of mild steel in ground water in the absence and presence of various concentrations of PCBT and 2-PAA at 30 C Ecorr icorr Corrosion Inhibition Inhibitor Inhibitor conc. (ppm) (mV) (A cm-2) rate (mpy) eciency (%) Blank 6 8 10 12 14 16 18 6 8 10 12 14 16 -640 12.59 -715 -785 -715 -720 -748 -770 -774 -669 -744 -681 -627 -714 -713 7.08 6.30 5.82 3.09 4.16 5.71 6.96 7.23 6.38 5.15 4.71 6.07 6.42 5.75 3.23 2.87 2.66 1.41 1.90 2.61 3.18 3.30 2.91 2.35 2.15 2.77 2.93 43.8 50.0 53.8 75.5 66.9 54.6 44.7 42.5 49.3 59.0 62.6 57.7 49.0
PCBT
Fig. 3. Nyquist plots of mild steel in ground water without and with the presence of optimum concentration of triazole, phosphono derivatives at 30 C
2-PAA 18 -684 7.50 3.42 40.4
Table 3. Electrochemical impedance parameters for mild steel in ground water in the absence and presence of optimum concentration of benzotriazole and phosphono derivative at 30 C Fig.4. Estimation of leached-out iron present in the ground water in the absence and presence of optimum concentration of 2-PAA and PCBT by AAS method. Table 1. Weight loss measurements of mild steel in ground water in the absence and presence of various concentration of benzotriazole and Phosphono derivative at 30 C. Inhibitor Blank Inhibitor conc. (ppm) 6 8 10 PCBT 12 14 16 18 6 8 10 2-PAA 12 14 16 18 Corrosion rate (mpy) 6.6 3.71 3.39 3.10 1.70 2.19 3.02 3.68 3.85 3.43 2.75 2.51 2.90 3.37 4.01 Inhibition eciency (%) 43.78 48.64 53.00 74.40 66.80 54.24 44.24 42.27 48.03 58.33 61.96 56.06 48.93 39.24 Inhibitor Blank PCBT 2-PAA Inhibitor conc. (ppm) 12 12 Rct x 104 (W) 0.0130 0.5728 0.3168 Cdl (mF) 15.03 6.49 7.83
3.3. AAS Studies Figure 4 depicts the estimation of leached-out iron present in the ground water in the absence and presence of optimum concentration of individual inhibitors. In the absence of inhibitor, the leachedout iron present in the solution was found to be 15.1 ppm. While, in the presence of inhibitor the leaching of iron was signicantly decreased, it may be due to the adsorption of inhibitor molecules on the surface of mild steel and it prevents the dissolution of iron in the electrolyte. Among the studied individual inhibitor systems, PCBT was found to be very low dissolution of iron in the electrolyte (3.8 ppm). 4. CONCLUSIONS 1. All the examined benzotriazole derivative, phosphono derivative were found to be good corrosion inhibitors for mild steel in ground water medium. 2. Studied phosphono derivative were acting as mixed-type inhibitors with anodic predominance, benzotriazole derivative act as mixed-type inhibitors with cathodic predominance. 3. PCBT act as better inhibitor than 2-PAA and a good agreement was observed between weight loss measurements coupled with electrochemical and AAS measurements. A., Boulkamh, & Djebbar, K. (2007). Investigations of the inhibition of copper corrosion in nitric acid solutions by ketene dithioacetal derivatives: Appl. Surf. Sci. 253, 9347. doi.org/10.1016/j.apsusc.2007.05.066.
GRA - GLOBAL RESEARCH ANALYSIS X 15
Volume : 2 | Issue : 2 | Feb 2013 ISSN No 2277 - 8160
REFERENCES
1. Bentiss, F., Traisnel. M & Lagrene`e. (2000). The substituted 1,3,4-oxadiazoles: a new class of corrosion inhibitors of mild steel in acidic media: Corros. Sci. 42, 127.doi.org/10.1016/S0010-938x(99)00049-9. | 2. Bouklah, M. Hammouti, B., Benkaddour M.,& Benhadda. (2005). Thiophene derivatives as effective inhibitors for the corrosion of steel in 0.5 M H2SO4: J. Appl. Electrochem. 35, 1095. doi:10.1007/S10800-005-9004-z. | 3. Elkad, L., Mernari B., Traisnel, M., Bentiss, M., & Lagrene`e, M. (2000). The inhibition action of 3,6-bis(2-methoxyphenyl)-1,2-dihydro1,2,4,5-tetrazine on the corrosion of mild steel in acidic media: Corros. Sci. 42, 703.doi ;org/10.1016/soo10-938x(99)00101-8. | 4. Wang, H.L., Liu, R.B., & Xin, J. (2004). Inhibiting effects of some mercapto-triazole derivatives on the corrosion of mild steel in 1.0 M HCl medium: Corros. Sci. 46, 2455.doi.org/10.1016/j.corsci.2004.01.023. | 5. Mahmoud, S.S. (2007). Corrosion inhibition of iron by amphoteric surfactants in hydrochloric acid solutions: J. Mater. Sci. 42, 989. doi: 10.1007/s10853-006-1389-5. | 6. Fouda, A.S., Mostafa, H.A., Ghazy, S.E., & El-Farah, S.A. (2007). Use of Hydrazone Derivates as Inhibitors for the Corrosion of Nickel in Hydrochloric Acid Solution): Int. J. Electrochem. Sci. 2, 182. | 7. Villamizar, W., Casales, M., & Gonzalez-Rodriguez , J.G. (2007). CO2 corrosion inhibition by hydroxyethyl, aminoethyl, and amidoethyl imidazolines in wateroil mixtures: J. Solid State Electrochem. 11, 619. Doi: 10.1007/s10008-006-0208-x. | 8. Fiala, A., Chibani, A., Darchen, A., Boulkamh, & Djebbar, K. (2007). Investigations | of the inhibition of copper corrosion in nitric acid solutions by ketene dithioacetal derivatives: Appl. Surf. Sci. 253, 9347. doi.org/10.1016/j. apsusc.2007.05.066. | 9. Fouda, A.S., Abd El-Aal, A., & Kandil, A.B. (2006). The effect of some phthalimide derivatives on corrosion behavior of copper in nitric acid: Desalination 201, 216. | 10. Bockris, JOM & Yang, B. (1991). The Mechanism of Corrosion Inhibition of Iron in Acid Solution by Acetylenic Alcohols: J. Electrochem. Soc. 138, 2237. doi: 0.1149/1.2085956. | 11. Khamis, E., (1990). The Effect of Temperature on the Acidic Dissolution of Steel in the Presence of Inhibitors: Corrosion. 46, 476 doi.org/10.5006/1.358535. | 12. Stupnisek Lisac, E.,& Podbrscek, S. (1994). Non-toxic organic zinc corrosion inhibitors in hydrochloric acid: J. Appl. Electrochem. 24, 779.
GRA - GLOBAL RESEARCH ANALYSIS X 16
Вам также может понравиться
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeОт EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeРейтинг: 4 из 5 звезд4/5 (5794)
- Rapid Prototyping 15209024Документ9 страницRapid Prototyping 15209024ranjankathuriaОценок пока нет
- The Little Book of Hygge: Danish Secrets to Happy LivingОт EverandThe Little Book of Hygge: Danish Secrets to Happy LivingРейтинг: 3.5 из 5 звезд3.5/5 (400)
- How To Detect Casting DefectsДокумент1 страницаHow To Detect Casting DefectsranjankathuriaОценок пока нет
- Derek A. Paice-Power Electronics Converter Harmonics Multipulse Methodsfor Clean Power-Ieee (1995)Документ113 страницDerek A. Paice-Power Electronics Converter Harmonics Multipulse Methodsfor Clean Power-Ieee (1995)HemaSinghОценок пока нет
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceОт EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceРейтинг: 4 из 5 звезд4/5 (895)
- 8 WaterconflictДокумент22 страницы8 WaterconflictGCVishnuKumarОценок пока нет
- The Yellow House: A Memoir (2019 National Book Award Winner)От EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Рейтинг: 4 из 5 звезд4/5 (98)
- Metal Casting PDFДокумент1 страницаMetal Casting PDFranjankathuriaОценок пока нет
- The Emperor of All Maladies: A Biography of CancerОт EverandThe Emperor of All Maladies: A Biography of CancerРейтинг: 4.5 из 5 звезд4.5/5 (271)
- 1 s2.0 S0010938X13002175 Main - 009Документ1 страница1 s2.0 S0010938X13002175 Main - 009ranjankathuriaОценок пока нет
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryОт EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryРейтинг: 3.5 из 5 звезд3.5/5 (231)
- Casting StuffДокумент1 страницаCasting StuffranjankathuriaОценок пока нет
- Never Split the Difference: Negotiating As If Your Life Depended On ItОт EverandNever Split the Difference: Negotiating As If Your Life Depended On ItРейтинг: 4.5 из 5 звезд4.5/5 (838)
- 1 s2.0 S0010938X13002175 Main - 007Документ1 страница1 s2.0 S0010938X13002175 Main - 007ranjankathuriaОценок пока нет
- 1 s2.0 S0010938X13002175 Main - 001Документ1 страница1 s2.0 S0010938X13002175 Main - 001ranjankathuriaОценок пока нет
- 1 s2.0 S0010938X13002175 Main - 006Документ1 страница1 s2.0 S0010938X13002175 Main - 006ranjankathuriaОценок пока нет
- 1 s2.0 S0010938X13002175 Main - 004Документ1 страница1 s2.0 S0010938X13002175 Main - 004ranjankathuriaОценок пока нет
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureОт EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureРейтинг: 4.5 из 5 звезд4.5/5 (474)
- How To Do Breakeven AnalysisДокумент3 страницыHow To Do Breakeven AnalysisranjankathuriaОценок пока нет
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaОт EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaРейтинг: 4.5 из 5 звезд4.5/5 (266)
- Rajender PachaudiДокумент2 страницыRajender PachaudiranjankathuriaОценок пока нет
- The Unwinding: An Inner History of the New AmericaОт EverandThe Unwinding: An Inner History of the New AmericaРейтинг: 4 из 5 звезд4/5 (45)
- QuadricsДокумент5 страницQuadricsDebo AkandeОценок пока нет
- Mechanical Metallurgy 'G.e. Dieter' - 001Документ1 страницаMechanical Metallurgy 'G.e. Dieter' - 001ranjankathuriaОценок пока нет
- Team of Rivals: The Political Genius of Abraham LincolnОт EverandTeam of Rivals: The Political Genius of Abraham LincolnРейтинг: 4.5 из 5 звезд4.5/5 (234)
- Mett.4th SemДокумент10 страницMett.4th SemranjankathuriaОценок пока нет
- 4.1.3 Properties of Coals PDFДокумент7 страниц4.1.3 Properties of Coals PDFJaco KotzeОценок пока нет
- Section13 6Документ4 страницыSection13 6ranjankathuriaОценок пока нет
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyОт EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyРейтинг: 3.5 из 5 звезд3.5/5 (2259)
- TT Metadepartment EvenДокумент3 страницыTT Metadepartment EvenranjankathuriaОценок пока нет
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreОт EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreРейтинг: 4 из 5 звезд4/5 (1090)
- Mett.4th SemДокумент10 страницMett.4th SemranjankathuriaОценок пока нет
- Section13 6Документ4 страницыSection13 6ranjankathuriaОценок пока нет
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersОт EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersРейтинг: 4.5 из 5 звезд4.5/5 (344)
- Spectrofluorimetry: Minia University Faculty of Pharmacy Department of Analytical ChemistryДокумент35 страницSpectrofluorimetry: Minia University Faculty of Pharmacy Department of Analytical ChemistryAhmed Zaghloul100% (2)
- Objective: Experiment 2: Determination of Ash ContentДокумент2 страницыObjective: Experiment 2: Determination of Ash ContentRaj Kumar Purkayastha100% (2)
- Some Basic Concepts of Chemistry Shobhit NirwanДокумент15 страницSome Basic Concepts of Chemistry Shobhit NirwanBhavya Goyal XI Non med100% (1)
- Areas clasificadas-IECДокумент1 страницаAreas clasificadas-IECChristian ChdОценок пока нет
- 001 - Shell Turbo Oil CC 32Документ2 страницы001 - Shell Turbo Oil CC 32Irvan NandaОценок пока нет
- Plate Hydraulic Design Procedure111Документ17 страницPlate Hydraulic Design Procedure111Gebrekiros ArayaОценок пока нет
- Biomolec ConceptMaps CHO, Lipid, ProtДокумент4 страницыBiomolec ConceptMaps CHO, Lipid, ProtLorence ArnedoОценок пока нет
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)От EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Рейтинг: 4.5 из 5 звезд4.5/5 (121)
- Hydrocarbon and Alkyl Halide-1Документ10 страницHydrocarbon and Alkyl Halide-1Aarya Vardhan ShandilyaОценок пока нет
- Chemistry Primer ANSWERSДокумент81 страницаChemistry Primer ANSWERSYazdan KelawalaОценок пока нет
- Corrosion of MartensiticДокумент40 страницCorrosion of MartensiticDinta PratiwiОценок пока нет
- Vendor Directory Amendment No - 5Документ10 страницVendor Directory Amendment No - 5annayya.chandrashekar Civil EngineerОценок пока нет
- Hanwha Total PP HJ700Документ2 страницыHanwha Total PP HJ700Nguyễn Hồng NamОценок пока нет
- Gusto Ko Garlic RiceДокумент28 страницGusto Ko Garlic RiceAngelica Mae Dela FuenteОценок пока нет
- Welding For Design EngineersДокумент518 страницWelding For Design EngineersHumberto Magno FukeОценок пока нет
- CV - Anil K Shukla (Nih, Usa)Документ6 страницCV - Anil K Shukla (Nih, Usa)Mehedi HossainОценок пока нет
- 100-028-544 - ALLIANT SPORT PISTOL POWDER, 1LB - 69B - DefaultДокумент13 страниц100-028-544 - ALLIANT SPORT PISTOL POWDER, 1LB - 69B - DefaultDavid WilliamsОценок пока нет
- Cavitation and Pipe DetailsДокумент34 страницыCavitation and Pipe DetailsSANDIP ROYОценок пока нет
- Electrochimica Acta: Abrar Khan, Raja Arumugam Senthil, Junqing Pan, Sedahmed Osman, Yanzhi Sun, Xin ShuДокумент10 страницElectrochimica Acta: Abrar Khan, Raja Arumugam Senthil, Junqing Pan, Sedahmed Osman, Yanzhi Sun, Xin Shusalsa bilaОценок пока нет
- Bioanalytical Method Validation - ICHДокумент3 страницыBioanalytical Method Validation - ICHfdfsdfdssfsfsОценок пока нет
- CV - Gaurav BahlДокумент17 страницCV - Gaurav BahlTanveerОценок пока нет
- Procedure Qualification Records (PQR)Документ2 страницыProcedure Qualification Records (PQR)amine algОценок пока нет
- Photocolorimetry and SpectrophotometryДокумент11 страницPhotocolorimetry and SpectrophotometryRay Mondy100% (1)
- Cyano Compounds, Inorganic: 1. Hydrogen CyanideДокумент38 страницCyano Compounds, Inorganic: 1. Hydrogen CyanideRasoulОценок пока нет
- Jotafloor SL UniversalДокумент6 страницJotafloor SL UniversalrogandatambunanОценок пока нет
- Me22 E02 Tumangan M2a2Документ3 страницыMe22 E02 Tumangan M2a2Jihoo JungОценок пока нет
- 15 BergstromДокумент40 страниц15 BergstromAdolfo Gálvez VillacortaОценок пока нет
- Greentech - Isolatek SBK-113 TDSДокумент2 страницыGreentech - Isolatek SBK-113 TDSRAОценок пока нет
- Cbiescss 09Документ7 страницCbiescss 09Ayush BeheraОценок пока нет
- Vsa 032 - Vsa 068 - Vsa 100 - AuДокумент9 страницVsa 032 - Vsa 068 - Vsa 100 - AuMariana CardosoОценок пока нет