Академический Документы
Профессиональный Документы
Культура Документы
Nuclear Chem
Загружено:
anon_177392401Исходное описание:
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
Nuclear Chem
Загружено:
anon_177392401Авторское право:
Доступные форматы
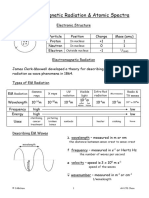
Holiday Research Tom Bradbury 1. (a) Distinguish between stable and radioactive isotopes with reference to a stability graph.
. Radioactive isotopes actively decay due to the instability of their nucleus, emitting radiation as the nucleus breaks down to a more stable state. Due to the consistency of atomic decay for each isotope, the time it takes for the original radioisotope to decay halfway to its final stable state can be measured and applied as the half-life. In the graph below, radioactive isotopes occupy the space above the stability zone. On the other hand, a stable isotope is an isotope that does not undergo radioactive decay, or if it does, cannot have its half-life measured because it takes too long for any measurable decay to actually occur. Refering to the graph below, it becomes clear that the most stable isotopes form in the stability zone.
(b) Describe the conditions under which a nucleus is unstable. A nucleus is comprised of protons and neutrons held together by nuclear binding energy, which dissipates as the mass of the nucleus increases. As such, heavier isotopes and elements such as uranium are unstable as the binding energy cannot hold the large, energetic nucleus together, resulting in radioactive decay of protons and neutrons until it reaches a stable state. Furthermore, a nucleus can be unstable if the nucleus is too proton or neutron heavy, causing for the excess subatomic particles to be released as radiation.
2. (a) What is a transuranic element? A Transuranic element is any element that has an atomic number greater than that of Uranium, 92. Typically, all transuranic elements dont occur naturally on Earth anymore, or can only be found as trace particles from the natural decay of other radioactive isotopes.
(b) Describe how transuranic elements are produced. Transmutation is the process of changing one element into another. For the production of heavy transuranic elements, nuclear bombardment of smaller atoms is used, with techniques differing for the desired product. For transuranic elements with an atomic number of 93, 94 or 95 neutrons collide with a large nucleus (such as that of Uranium) and form a heavier isotope. These neutrons make the nucleus unstable, and decay into a proton and an electron, forming a new element. For heavier transuranic elements, particle accelerators are used to accelerate small atoms of elements such as carbon into a target of larger atoms to form a new transuranic element with a proton count higher than 95.
(C) Describe 3 recent discoveries of elements. Since 2000 five transuranic elements, each with an atomic number greater than 100, have been synthesised by research labs in Russia and America. Ununpentium, Ununtrium and Ununhexium have all been discovered through the process of colliding Calcium isotopes with a transuranic target element and subsequently measuring the product of the collision and the state they decayed to. Unupentium and Ununtrium were in fact discovered in the same experiment, as the collision of Calcium ions with an Americanium target yielded Ununpentium which decayed in 100 milliseconds to form ununtrium. This experiment was a joint effort between Russian and American scientists who confirmed the discovery and published their results. Ununhexium was discovered in a similar fashion, with calcium ions colliding with a Curium target to form ununoctium, which rapidly decayed to form Ununhexium, which in turn decayed to form more stable Transuranic elements.
3. Describe how commercial radioisotopes are produced. Radioisotopes can be produced through the methods mentioned above; either by bombarding a transuranic target with smaller atoms or by exposing a heavy element to neutron emissions (that are originating from nuclear fission) which subsequently gains a neutron which decays into a proton and electron. Cyclotrons are also used to accelerate hydrogen atoms through electric fields into target atoms to form useful commercial isotopes.
4. Identify Instruments and processes used to detect radiation and name the type of radiation they can detect. Broadly speaking, radioactive emissions can be classed as alpha radiation, the release of two protons and two neutrons bound together; beta radiation, the release of neutrons or electrons; and gamma radiation, which are ionising electromagnetic waves. The most common device used to detect radiation is the Geiger counter, which detects the ionising effects of radiation on a tube of argon gas and relays the subsequent electrical emissions from the ionisation up to a counter which registers the fact that radiation is present. Variations of the gases used in the Geiger counter design mean that alpha, beta and gamma radiation can all be detected but not differentiated between one another. Another device is the Scintillation Counter, which can be used to gamma radiation and how strong they are using the principle that certain elements fluoresce when exposed to ionising radiation. In use, gamma radiation causes a scintillation crystal to fluoresce, with the light being picked up by a photomultiplier tube and processed to give a reading of how strong the radiation is.
5. (A) Name a radioisotope used in industry Perhaps the most widespread radioactive isotope in the western world is Americanium-241, a vital component of all smoke alarms. (B) Describe how it is used based on its properties. Americanium-241 is favoured in smoke alarms as it emits small levels of ionising radiation into a contained chamber, causing the molecules in the air to ionise and be attracted to the charged electrodes in the chamber, emitting small electrical impulses. When smoke enters the chamber from a fire, the air molecules attach to the particles and reduce their ability to carry a current. This drop in activity is detected by a set of electronics in the alarm which sets itself off. Because of its low toxicity and radiation emissions, it is an ideal industrial isotope to be used domestically.
6. (A) Name a radioisotope used in medicine. Iodine -125 is used in the field of nuclear medicine in the form brachytherapy to treat prostate cancer and brain tumours. (B) Describe how it is used based on its properties. Removing cancers that cannot be operated on is difficult and is usually when brachytherapy is called into play. Placing a source of radiation local to the cancerous growth, an isotope with a minimal halflife is required so that trace amounts will not stay in the patient after extraction. Iodine-125 is the ideal candidate as it is not radioactive enough to cause cancer (in the fight against cancer), primarily emitting low levels of gamma radiation that will only affect the local tissue, meaning that the rest of the body will remain unaffected. Solid at room temperature and with a half-life of 59 days,
implantation is easy and as such has become a common procedure in combatting prostate cancer and brain tumours.
7. Analyse the benefits and problems associated with the use of radioactive isotopes in identified industries and medicine. Radioisotopes have already permanently impacted on our daily lives in several ways, with no viable alternatives available to deliver the services and quality they can provide for use. Put simply, society has become dependent on them for the quality of living we are used to. Industrially, radioisotopes have been used to raise the quality of products in a variety of fields. Metal-working is has been improved by radioactive isotopes which measure the thickness of metal sheeting and impurities on a molecular scale, resulting in a high quality material with a greater amount of applications and longer durability. The cost of using the radioisotope is negligible on an industrial scale and with increased sales due to the demand of the quality of the material, the consumer will only buy it for a fraction of the cost more. However, there are drawbacks, and the current supply of radioisotopes is probably the most restricting, as a majority of the worlds supply comes from a handful of nuclear reactors spread out across the globe. Considering that nuclear isotopes are an integral part of all industry and medicine, it is disturbing to think that there are only 130 reactors in the world capable of producing them with only a tiny fraction of these able to produce pure medical radioisotopes. Their short half-life (required so that they dont accumulate in human tissue) means that they can only be transported over relatively short distances, with producers needing to be geographically close to the medical consumers.
Вам также может понравиться
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeОт EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeРейтинг: 4 из 5 звезд4/5 (5794)
- The Yellow House: A Memoir (2019 National Book Award Winner)От EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Рейтинг: 4 из 5 звезд4/5 (98)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryОт EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryРейтинг: 3.5 из 5 звезд3.5/5 (231)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceОт EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceРейтинг: 4 из 5 звезд4/5 (895)
- The Little Book of Hygge: Danish Secrets to Happy LivingОт EverandThe Little Book of Hygge: Danish Secrets to Happy LivingРейтинг: 3.5 из 5 звезд3.5/5 (400)
- Never Split the Difference: Negotiating As If Your Life Depended On ItОт EverandNever Split the Difference: Negotiating As If Your Life Depended On ItРейтинг: 4.5 из 5 звезд4.5/5 (838)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureОт EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureРейтинг: 4.5 из 5 звезд4.5/5 (474)
- The Emperor of All Maladies: A Biography of CancerОт EverandThe Emperor of All Maladies: A Biography of CancerРейтинг: 4.5 из 5 звезд4.5/5 (271)
- Team of Rivals: The Political Genius of Abraham LincolnОт EverandTeam of Rivals: The Political Genius of Abraham LincolnРейтинг: 4.5 из 5 звезд4.5/5 (234)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaОт EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaРейтинг: 4.5 из 5 звезд4.5/5 (266)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersОт EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersРейтинг: 4.5 из 5 звезд4.5/5 (344)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyОт EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyРейтинг: 3.5 из 5 звезд3.5/5 (2259)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreОт EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreРейтинг: 4 из 5 звезд4/5 (1090)
- The Unwinding: An Inner History of the New AmericaОт EverandThe Unwinding: An Inner History of the New AmericaРейтинг: 4 из 5 звезд4/5 (45)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)От EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Рейтинг: 4.5 из 5 звезд4.5/5 (121)
- Modern Physics Target QuestionДокумент2 страницыModern Physics Target QuestionAnkur GoyalОценок пока нет
- Notes - Radioactivity and Nuclear EnergyДокумент28 страницNotes - Radioactivity and Nuclear EnergyUlwindass Victor Gorge100% (1)
- NuclearproblemskeyДокумент1 страницаNuclearproblemskeyAubrey Ortilla CabralОценок пока нет
- Lecture On Nuclear ChemistryДокумент58 страницLecture On Nuclear Chemistrysadia SultanaОценок пока нет
- Name: Christina Eleanor Martin Subject: Hygiene Group: 3 Course: 4 Year 1 SemДокумент21 страницаName: Christina Eleanor Martin Subject: Hygiene Group: 3 Course: 4 Year 1 SemSuthan KaveriОценок пока нет
- Nuclear TransmutationДокумент14 страницNuclear TransmutationDaniel Baylosis AsongОценок пока нет
- 20190814173010Документ5 страниц20190814173010BОценок пока нет
- Canal TopДокумент2 страницыCanal TopJP GUPTAОценок пока нет
- Radioactivity 3Документ4 страницыRadioactivity 3wangks1980Оценок пока нет
- 13 BIS Certificate - MBB Half Cut Single GlassДокумент1 страница13 BIS Certificate - MBB Half Cut Single GlassHarish GovindОценок пока нет
- Penentuan Nilai Koefisien Serapan BahanДокумент6 страницPenentuan Nilai Koefisien Serapan BahancozmoanandkОценок пока нет
- Day 2 - Q2M2 - PropertiesEMwavesДокумент49 страницDay 2 - Q2M2 - PropertiesEMwavesMarilyn Castro LaquindanumОценок пока нет
- Victoreen Geiger-Mueller and Scintillation Probe Selection GuideДокумент5 страницVictoreen Geiger-Mueller and Scintillation Probe Selection GuideJorge LopezОценок пока нет
- En 15Документ9 страницEn 15AntonellaОценок пока нет
- RPDIR-P12.1 Shielding Calculation WEBДокумент23 страницыRPDIR-P12.1 Shielding Calculation WEBPici-pici BaliОценок пока нет
- Radiation Safety Policy AND ProceduresДокумент55 страницRadiation Safety Policy AND ProceduresFiza Nnazlan100% (1)
- Em Wave PDFДокумент4 страницыEm Wave PDFRajuPrabaharОценок пока нет
- Evs Project 12thДокумент12 страницEvs Project 12th1163 Om JadhavОценок пока нет
- Nuclear Radiation Lab CHEM136Документ3 страницыNuclear Radiation Lab CHEM136NatОценок пока нет
- Sources of Radiant EnergyДокумент1 страницаSources of Radiant EnergyRjvm Net Ca Fe100% (1)
- 07 PETImagingДокумент61 страница07 PETImagingLouis FortunatoОценок пока нет
- Lecture 2.1 - EM WavesДокумент8 страницLecture 2.1 - EM WavesAPPLE CAGUNGUN-BELGICAОценок пока нет
- 08 Lessons Learned (Industry)Документ77 страниц08 Lessons Learned (Industry)Michael Murillo BaraquioОценок пока нет
- Anib 46 3-4Документ491 страницаAnib 46 3-4Nicholas AlmslawyОценок пока нет
- 1.1 AH CfE Chemistry NotesДокумент7 страниц1.1 AH CfE Chemistry Noteskira.zavyalova24Оценок пока нет
- DecommissioningДокумент10 страницDecommissioningNia TowneОценок пока нет
- Test Bank For Frommers Radiology For The Dental Professional 10th Edition by Stabulas SavageДокумент36 страницTest Bank For Frommers Radiology For The Dental Professional 10th Edition by Stabulas Savageooezoapunitory.xkgyo4100% (44)
- Radiation Protection Course: Bangi Ray Services Sdn. BHDДокумент2 страницыRadiation Protection Course: Bangi Ray Services Sdn. BHDAali .iimranОценок пока нет
- Chemistry A Volatile History Episode Three HandoutДокумент2 страницыChemistry A Volatile History Episode Three HandoutMauricio CarranzaОценок пока нет
- Radiation Safety of Sealed Sources and Equipment Containing ThemДокумент16 страницRadiation Safety of Sealed Sources and Equipment Containing Themist93993Оценок пока нет