Академический Документы
Профессиональный Документы
Культура Документы
25 Water
Загружено:
gore_11Исходное описание:
Оригинальное название
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
25 Water
Загружено:
gore_11Авторское право:
Доступные форматы
HSC Chemistry 6.
0
Peter Bjorklund August 10 2006
25 - 1
06120-!"C-#
25. $A#%"
&igure 1' Pressure-Temperature calculator for water. The Pressure and Temperature calculator enables a complete thermodynamic description for a species, by allowing the user to specify not only the temperature, but also the pressure (partial). This calculator is currently only available for water, since the properties of steam in particular, deviate significantly from those of the ideal gas, i.e. the heat capacity is purely a temperature-dependent function. In letting the user specify water/steam pressure, cases where pressure is of e treme importance can easily be calculated. This calculator is a very useful replacement for ste(m t()le )ooks and *ollier di(gr(ms. !y directly typing the pressure and temperature of the process points, or by simply clic"ing on the diagram, the process enthalpy and entropy are calculated along with several other useful thermodynamic data. The module is also available through the #eat and $aterial !alances module (%hapter 11. Heat and Material Balance), where the pressure correction may be inserted into the wor"sheet as a cell formula.
HSC Chemistry 6.0
Peter Bjorklund August 10 2006
25 - 2
06120-!"C-#
25.1
B(si+ C(l+ul(tion Pro+edure
The calculator lets the user specify two process points& point ' being the starting point and point ( the end point. The calculator then calculates the absolute values for the two points and then the difference between them. The absolute values are mostly useful for density and heat capacity data, whereas the difference or delta values are useful for enthalpy and entropy data. ) line between the two points may be drawn by selecting Show/Line from the menu. #owever, this line does not necessaril represent the actual process path , instead it ma"es the visuali*ation of the two points easier. There are two basic ways, which may also be combined, of specifying the process points+ S,e+i-ying ,oints using the mouse' 1. ,pecify the appropriate units from the combobo es and the amount ("mol or "g) in the te tbo . 2. )ctivate one of the points by clic"ing on the appropriate bo in the diagram. ) yellow bo indicates the active point and a white bo the inactive point. .. %lic" on a position in the diagram and the selected point will move to that position and the data will automatically be recalculated. -ote that if a saturated state is selected, the point will .ump to the appropriate saturated curve if possible. It is also possible to use the drag-and-drop techni/ue to move the selected point. /. 0epeat steps ( and 1 for the other point. 5. If a point is on a saturated curve, the user may specify the $i ture 2, i.e. the percentage of the first of the two phases in the mi ture shown in the combobo on row 1 of the wor"sheet. The default value is 34 2. S,e+i-ying ,oints m(nu(lly' 1. ,pecify the appropriate units from the combobo es and the amount ("mol or "g) in the te tbo . 2. ,pecify pressures and temperatures for the two points by simply typing them into the cells on columns ' and (. -ote that if a saturated state is selected, the temperature or pressure will automatically be calculated. The values that are automatically calculated are indicated in a blac" font. .. If a point is on a saturated curve, the user has to specify the $i ture 2, i.e. the percentage of the first of the two phases in the mi ture given in the combobo on row 1. The default value is 34 2. /. If a point is on a saturated curve, the user may specify the $i ture 2, i.e. the percentage of the first of the two phases in the mi ture shown in the combobo on row 1 of the wor"sheet. The default value is 34 2. %0,l(n(tion o- the , #-di(gr(m' The p,T-diagram to the right of the window contains a few labels. These are e plained in the following points+ 1. #ri,le Point The triple point refers to the point where all three phases of water may e ist simultaneously. This point is at T 5 4.4' 6% and p 5 4.447' bar. $ost steam tables use this as the reference point, i.e. the absolute enthalpy and absolute entropy are set to *ero. !y selecting !dit/Point " # Triple Point from the menu, the second process point will automatically be set to the triple point, which is also the default setting. This means that
HSC Chemistry 6.0
Peter Bjorklund August 10 2006
25 - .
06120-!"C-#
the enthalpy and entropy differences will have the same values as those found in most steam tables if the phase is set to li$uid at the triple point. 2. Criti+(l Point 8hen the temperature rises above the critical point, it is no longer possible to clearly distinguish between the li/uid and the gaseous phase. ,uch a condition is referred to as supercritical. The critical point for water is at T 5 191.:;7 6% and p 5 ((4.7; bar. It is important to "now that above this point the program still specifies the phase of the point, although there are no longer any actual phase transitions between the phases. .. S(tur(ted Cur1es There are three saturated curves in the diagram. These are %as/li$uid, %as/solid and li$uid/solid. ) point located on any one of these curves indicates that the two phases are in e/uilibrium. The three curves .oin at the triple point, which is where all three phases can e ist simultaneously. The user may force a point onto the saturated curve by selecting either S(t. 2g l3, S(t. 2g s3 or S(t. 2l s3 from one of the phase combobo es on row 1. /. Solid 4+e ) stability area consisting of several solid ice phases, with different crystal structures, of which some are shown on the diagram. <ata is not, however, available for the high pressure solid phases. C(l+ul(tion 5orksheet' The calculation wor"sheet is to the left of the window. The first four rows are input rows and the last si rows show the results. The input rows are e plained in the following points+ 1. #em,er(ture The temperature unit may be either in 6C or in 7, depending on the temperature unit combobo . The temperature is automatically calculatad when the S(t. 2l s3 state is selected. 2. Pressure The pressure unit may be either in )(r or in *P(, depending on the pressure unit combobo . The pressure is automatically calculatad when the S(t. 2g l3 or the S(t. 2g s3 state is selected. .. Ph(se There are three different pure phases available for water+ Solid 2s3, 8i9uid 2l3 and :(s 2g3. These three phases may be combined on the saturated curves and they are specified as+ S(t. 2g l3, S(t. 2g s3 and S(t. 2l s3. )t the triple point a mi ture of all of these is possible, however, only a mi ture of two is possible in this module. 8hen a pure phase is selected, the actual phase is automatically selected according to the user input (manually or with the mouse). 8hen a saturated state is selected, the temperature or pressure is automatically calculated, which is then indicated in a blac" font. =or the gas/li/uid and gas/solid mi tures the pressure is automatically calculated and for the li/uid/solid mi ture the temperature is automatically calculated. -ote that when moving outside the limits of the saturation curve, the phase will automatically change into the corresponding pure phase of the specified point. /. *i0ture ; It is possible here to enter the mi ture percentage of the saturated state. This is the same as the dr ness percenta%e of steam when the point is on the saturated gas/li/uid curve. The percentage always refers to the amount of the first phase in the total mi ture, e.g. the gas percentage in a gas/li/uid mi ture. This is set to 34 2 by default and only visible when a saturated state is specified.
HSC Chemistry 6.0
Peter Bjorklund August 10 2006
25 - /
06120-!"C-#
The results of the calculation are shown in the same table below the input. The data calculated includes+ 1. %nth(l,y 2H3 (nd %ntro,y 2S3 The enthalpies and entropies for the two process points are calculated using the reference point T 5 (3 6% and p 5 ' bar. This differs from what is normally found in steam tables, where the reference point is T 5 4.4' 6% and p 5 4.447' bar (triple point for water). This means that only the differences in enthalp and entrop are of importance, since they are independent of the choice of reference points. Point ( is therefore b default set to the triple point , which means that the enthalpy and entropy differences, indicated by a yellow bac"ground, give the values normally found in steam tables. 2. S,e+i-i+ %nth(l,y 2H s,e+i-i+3 The specific enthalpies are the enthalpies when the phase transformations are ignored. In other words the enthalpy released/gained when water is cooled/heated to (3 6% at constant pressure. Therefore the specific enthalpy is *ero at (3 6%. .. :i))s %nergy 2:3 These cells show the >ibbs ?nergy for the two points. /. He(t C(,(+ity 2C,3 These cells show the specific heat capacity at constant pressure for the two points. 5. <ensity These cells give the densities for the two points. The densities are interpolated or e trapolated from the ? cel file #(@.AB, located in the .C#,%3CPT%alc directory. Please do not modify this file. !ther im,ort(nt terms' 1. 4sentro,i+ ,ro+ess If the entropy is constant through a process, i.e. the entropy difference is *ero, the process is called isentropic. )n isentropic process is an ideal process and real processes are often compared to the corresponding isentropic process. 2. 4sentro,i+ e--i+ien+y )n isentropic process generates no entropy and may therefore be considered an ideal process. It is useful to compare real processes to that of the isentropic through the isentropic efficiency. The isentropic efficiency for a compressor or pump is defined as
s &c =
hs , h
D'E
where h is the real enthalp difference and hs is the isentropic enthalp difference. The isentropic efficiency for a turbine is defined as
s &t = h . hs
D(E
)n isentropic process is always adiabatic, however, an adiabatic process is not necessarily isentropic.
25.2
C(l+ul(tion e0(m,les
HSC Chemistry 6.0
Peter Bjorklund 25.2.1 4+e melting (t high ,ressure August 10 2006
25 - 5
06120-!"C-#
&igure 2' Meltin% of ice at a pressure of '(( bar. The e ample in =igure ( shows the enthalpy and entropy change of ' "g solid ice melting at a constant pressure of 344 bar, until :4 2 of the total mi ture is li/uid water. The enthalpy difference is F(:4 "G and the process therefore endothermic. The entropy difference is F'.49: "G/6% and the process therefore generates entropy. The melting point of ice at 344 bar is automatically calculated, since the point is on the saturated li/uid/solid curve, and it is roughly H;.3 6%. This pressure is achieved, for e ample, when pressing a sharp ob.ect against an icy surface, which then melts due to the decreased melting point temperature.
HSC Chemistry 6.0
Peter Bjorklund 25.2.2 August 10 2006
25 - 6
06120-!"C-#
%1(,or(tion ,ro+ess in ( he(t re+o1ery )oiler
&igure .' !vaporation process. The e ample in =igure 1 shows the evaporation process in a heat recovery boiler, when the mass flow is 344 t/h ()mount+ 344444 "g, Init+ $8h). The temperature is (J3 6% and the pressures are automatically calculated, since the saturated gas/li/uid state is selected. The transferred heat is F(' $8, when '4 2 of the total water flow evaporates. This is e/uivalent to a water/steam ratio of :42/'42 5 :. The entropy generation of F4.41J $8/6% indicates that the process is not isentropic.
HSC Chemistry 6.0
Peter Bjorklund 25.2.. Ste(m tur)ine ,ro+ess August 10 2006
25 - =
06120-!"C-#
&igure /' Steam turbine process. ) simple steam turbine process is shown in =igure ;. The calculation shows that the net enthalpy for a mass flow of '4 "g/s is H'4711 "8 and thus e othermic, i.e. the enthalpy is released in the form of wor" on the shaft. The entropy generation is 9.3 "8/6% and the process is therefore not isentropic. !y decreasing the $i ture 2 (in this case FJ'.9 2) the process may be compared with the ideal isentropic process for which there is no entropy generation. The enthalpy released in the isentropic process is H'(:41 "8 and the isentropic efficiency is therefore, according to e/uation (, s,t 5 -'4711 "8 / -'(:41 "8 4.J(.
HSC Chemistry 6.0
Peter Bjorklund August 10 2006
25 - >
06120-!"C-#
25..
25...1
<et(iled <es+ri,tion
Pressure +orre+tion in the d(t()(se The pressure-corrected calculations are based on new pressure-specific species in the database. They are of the form S(XbarP), where S denotes the species, X the pressure (partial) in bars and P the phase. The phase is only specified for gaseous species. The only currently available species is #(@ (water), and the pressure-corrected species are found in the database as #(@(4.4'bar), #(@(4.4'barg), #(@('44bar), #(@('44barg), etc. -ote that if the phase is not specified, it may be either solid or li/uid depending on the temperature. =or supercritical steam, that is for T 191.:;7 6%, a gaseous phase is used for p K ((4.7; bar and a li/uid phase for p ((4.7; bar, although the two phases are impossible to separate in reality. Table ' shows the pressure-corrected water species currently found in the #,% database.
Pressure ?)(r@
4.4' 4.43 4.' 4.3 ' 3 '4 (4 14 ;4 34 74 94 J4 :4 '44 (44 144 L ;44 L 344 L 744 L 944 L J44 L :44 L '444 L
L ,upercritical pressures
SolidAli9uid s,e+ies 2i+eA5(ter3
#(@(4.4'bar) #(@(4.43bar) #(@(4.'bar) #(@(4.3bar) #(@('bar) #(@(3bar) #(@('4bar) #(@((4bar) #(@(14bar) #(@(;4bar) #(@(34bar) #(@(74bar) #(@(94bar) #(@(J4bar) #(@(:4bar) #(@('44bar) #(@((44bar) #(@(144bar) L #(@(;44bar) L #(@(344bar) L #(@(744bar) L #(@(944bar) L #(@(J44bar) L #(@(:44bar) L #(@('444bar) L
:(seous s,e+ies 2ste(m3
#(@(4.4' barg) #(@(4.43 barg) #(@(4.' barg) #(@(4.3 barg) #(@(' barg) #(@(3 barg) #(@('4 barg) #(@((4barg) #(@(14barg) #(@(;4barg) #(@(34barg) #(@(74barg) #(@(94barg) #(@(J4barg) #(@(:4barg) #(@('44barg) #(@((44barg)
#()le 1' Pressure-corrected species currentl available in the database. ,ince water is in a li/uid form at the reference temperature of (3 6% for pressures higher than 4.41( bar, the %p functions for steam follow the saturated steam curve below the boiling point. This means that the pressure is not )ept constant below this point and thus the pressure value in the species name, i.e. B in #(@(Bbarg), is no longer valid for these temperatures. This may be seen from =igure 3, where the %p functions of steam at different pressures are shown. 8hen the temperature drops below the boiling point the %p functions follow the saturation curve, where the partial pressure also reduces with the temperature. This also means that the total enthalp and entrop curves below the boiling point will not .oin as e pected and therefore these curves are comparable with each other onl for values above or e$ual to the boilin% point . This is, however, not an issue when studying only one
HSC Chemistry 6.0
Peter Bjorklund August 10 2006
25 - C
06120-!"C-#
pressure-corrected species at a time since the enthalpy/entropy differences are then still valid. -aturally this is never a problem in the Pressure and Temperature calculator, since it automatically chooses the li/uid phase when the temperature drops below the boiling point for a constant pressure. =or the solid/li/uid phases, i.e. #(@(Abar), this reference point does not cause any problems, since at least one of the two phases (li/uid and/or solid) e ist below (3 6% and at pressure A. They can therefore easily be e trapolated up to (3 6% when necessary. ? trapolation above the boiling/sublimation points at different pressures are shown as dotted curves in diagrams, which also means that the li/uid/solid phase does not e ist at these temperatures. =igure 3 also shows that the ideal gas appro imation, indicated by the label #(@(g), is generally more accurate for low pressures and high temperatures. The ideal gas curve can be seen below the constant pressure curves. The rule of thumb is that the accuracy of the ideal gas appro imation increases the further away you move from the critical point.
&igure 5' *p functions for steam at different pressures.
HSC Chemistry 6.0
Peter Bjorklund 25...2 B(si+ theory August 10 2006
25 - 10
06120-!"C-#
In order to calculate the enthalpy and entropy at different pressures, slight modifications have to be made to the enthalpy of formation and standard entropy at (3 6%, i.e. the # f -term of e/uations ' and 1 in %hapter +. ,ntroduction. $ost steam tables use the triple point of water (T 5 4.4' 6%, p 5 4.447' bar), instead of the standard reference point used in #,% (T 5 (3 6%, p 5 ' bar), therefore the absolute enthalpies and entropies will be different from those found in steam tables. #owever, since point ( is by default set to the triple point, the enthalpy and entropy differences (cells %3 and %7) are the same as the absolute values found in steam tables and diagrams. This is achieved by selecting !dit/Point " # Triple Point from the menu and choosing the li$uid phase. The pressure corrected enthalpy at temperature T and pressure p is calculated as
T
H 0 T & p / = H .f 0 (:J.'3 - & p / +
(:J.'3 -
*p(T & p ) dT + H
tr
, D1E
where #Mf ((:J.'3N, p) is the modified enthalpy of formation at pressure p and # tr is the enthalpy of a phase transformation. The entropy at pressure p may be calculated in a similar manner as
. S0 T & p / = S 4 0 (:J.'3 - & p / +
H *p ( T & p ) dT + tr , T Ttr (:J.'3 T
D;E where ,M4 ((:J.'3N, p) is the modified standard entropy at pressure p and # tr is the enthalpy of a phase transformation at temperature T tr. The %p function still utili*es the Nelley e/uation, but the coefficients are now pressuredependent according to
*p ( T & p ) = 2( p ) + B ( p ) '4 1 + * ( p ) '4 3 T ( + 1( p ) '4 7 T ( ,
D3E
where )(p), !(p), %(p) and <(p) are the coefficients fitted at different pressures p. ,ee Table ' for a list of the current pressures available. The temperature or pressure of a saturated curve is calculated through either one of the e/uations 7-J. The pressure for a saturated %as/li$uid mi3ture is
p0 T / = 4.' e3p(''.9J( T 19(.9:) / ( T ;1.'3) ) ,
D7E
where T is the temperature in N and p the pressure in $Pa. The pressure for a saturated %as/solid mi3ture is
p0 T / = '4'4.31J4::9 - (771.:' / ( T O 4.4') / '4444 ,
D9E
where T is the temperature in N and p the pressure in $Pa. The temperature for a saturated li$uid/solid mi3ture is
HSC Chemistry 6.0
Peter Bjorklund
T 0 p / = 4.44: p + (91.'7 ,
25 - 11
06120-!"C-# DJE
August 10 2006
where T is the temperature in N and p the pressure in $Pa. These e/uations correspond to the saturated curves in the p,T-diagram and are utili*ed in the module whenever a saturated state is selected. In order to calculate the influence of pressure on the enthalpy and entropy below the free*ing point, a pressure correction term is used. The correction term is
hT =const = (' T f v &ice )ice ( p f p 4 ) ,
D:E where Tf is the temperature at the free*ing point, p f is the pressure at the free*ing point, v &ice is the coefficient of thermal e pansion and ice is the specific volume.
25./
8imit(tions
The current pressure range is H(44 6% to (444 6% and 4.44' bar to '444 bar. )ccuracy in cp-values and density values decreases close to the critical point. )ccuracy in saturated li/uid/gas pressure decreases close to the critical point. Incertainties in cp-values for ice at different pressures. The high temperature and pressure region (J44 6% - (444 6% and '44 bar - '444 bar) is e trapolated. The low pressure region (4.44' bar - 4.4' bar) is e trapolated.
Вам также может понравиться
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeОт EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeРейтинг: 4 из 5 звезд4/5 (5794)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreОт EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreРейтинг: 4 из 5 звезд4/5 (1090)
- Never Split the Difference: Negotiating As If Your Life Depended On ItОт EverandNever Split the Difference: Negotiating As If Your Life Depended On ItРейтинг: 4.5 из 5 звезд4.5/5 (838)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceОт EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceРейтинг: 4 из 5 звезд4/5 (894)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureОт EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureРейтинг: 4.5 из 5 звезд4.5/5 (474)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersОт EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersРейтинг: 4.5 из 5 звезд4.5/5 (344)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)От EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Рейтинг: 4.5 из 5 звезд4.5/5 (119)
- The Emperor of All Maladies: A Biography of CancerОт EverandThe Emperor of All Maladies: A Biography of CancerРейтинг: 4.5 из 5 звезд4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingОт EverandThe Little Book of Hygge: Danish Secrets to Happy LivingРейтинг: 3.5 из 5 звезд3.5/5 (399)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyОт EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyРейтинг: 3.5 из 5 звезд3.5/5 (2219)
- The Yellow House: A Memoir (2019 National Book Award Winner)От EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Рейтинг: 4 из 5 звезд4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaОт EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaРейтинг: 4.5 из 5 звезд4.5/5 (265)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryОт EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryРейтинг: 3.5 из 5 звезд3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnОт EverandTeam of Rivals: The Political Genius of Abraham LincolnРейтинг: 4.5 из 5 звезд4.5/5 (234)
- Solutions - Callen H.B. - Exercicios Resolvidos (Cap (01-06) )Документ61 страницаSolutions - Callen H.B. - Exercicios Resolvidos (Cap (01-06) )Leonardo Xavier Neves79% (14)
- The Unwinding: An Inner History of the New AmericaОт EverandThe Unwinding: An Inner History of the New AmericaРейтинг: 4 из 5 звезд4/5 (45)
- Bar PDFДокумент18 страницBar PDFgore_11Оценок пока нет
- Beta Distribution Fitting: Reliability FunctionДокумент15 страницBeta Distribution Fitting: Reliability Functiongore_11Оценок пока нет
- Repeated Measures Analysis of Variance: An ExampleДокумент32 страницыRepeated Measures Analysis of Variance: An Examplegore_11Оценок пока нет
- Pass User'S Guide: PASS 2000 Power Analysis and Sample Size For WindowsДокумент7 страницPass User'S Guide: PASS 2000 Power Analysis and Sample Size For Windowsgore_11Оценок пока нет
- PASS 2000: Power Analysis and Sample Size Software From NCSSДокумент2 страницыPASS 2000: Power Analysis and Sample Size Software From NCSSgore_11Оценок пока нет
- Set Up MineralsДокумент1 страницаSet Up Mineralsgore_11Оценок пока нет
- 3dscat PDFДокумент10 страниц3dscat PDFgore_11Оценок пока нет
- 40 Sim FlowsheetДокумент74 страницы40 Sim Flowsheetgore_11Оценок пока нет
- Auc PDFДокумент7 страницAuc PDFgore_11Оценок пока нет
- Set Up MineralsДокумент1 страницаSet Up Mineralsgore_11Оценок пока нет
- Set Up ParticlesДокумент1 страницаSet Up Particlesgore_11Оценок пока нет
- Set Up ParticlesДокумент1 страницаSet Up Particlesgore_11Оценок пока нет
- Set Up FractionsДокумент1 страницаSet Up Fractionsgore_11Оценок пока нет
- Set Up MineralsДокумент1 страницаSet Up Mineralsgore_11Оценок пока нет
- Set Up FractionsДокумент1 страницаSet Up Fractionsgore_11Оценок пока нет
- Set Up ParticlesДокумент1 страницаSet Up Particlesgore_11Оценок пока нет
- Set Up ParticlesДокумент2 страницыSet Up Particlesgore_11Оценок пока нет
- Whats New in HSC 6 - 1Документ4 страницыWhats New in HSC 6 - 1Manojlovic VasoОценок пока нет
- Tutorial For Simple Flotation Example PDFДокумент28 страницTutorial For Simple Flotation Example PDFgore_11Оценок пока нет
- Tutorial For Mass Blancing and Data ReconciliationДокумент9 страницTutorial For Mass Blancing and Data Reconciliationgore_11Оценок пока нет
- Cannington ModalДокумент1 страницаCannington Modalgore_11Оценок пока нет
- Cannington ExampleДокумент2 страницыCannington Examplegore_11Оценок пока нет
- Setup FractionsДокумент1 страницаSetup FractionscontretrasОценок пока нет
- Setup ParticlesДокумент2 страницыSetup ParticlescontretrasОценок пока нет
- Setup MineralsДокумент1 страницаSetup MineralscontretrasОценок пока нет
- Tutorial For Calculating Modal CompositionДокумент28 страницTutorial For Calculating Modal Compositiongore_11Оценок пока нет
- Setup MineralsДокумент1 страницаSetup MineralscontretrasОценок пока нет
- Installation To Hard Disk 7.1 System Requirements: HSC Chemistry® 6.0 7 - 1Документ6 страницInstallation To Hard Disk 7.1 System Requirements: HSC Chemistry® 6.0 7 - 1gore_11Оценок пока нет
- 27 Excel Add-InsДокумент10 страниц27 Excel Add-Insgore_11Оценок пока нет
- 26 Units ModuleДокумент3 страницы26 Units Modulegore_11Оценок пока нет
- FinalДокумент2 страницыFinalZahraa A. NadeemОценок пока нет
- Condair Dehumidifiers Brochure en RTДокумент16 страницCondair Dehumidifiers Brochure en RTMuhammad Faheem ShahbazОценок пока нет
- Muhga35vb PDFДокумент36 страницMuhga35vb PDFMihaela CondratОценок пока нет
- Shell and Tube Heat ExchangerДокумент27 страницShell and Tube Heat ExchangerDeepak K NambiarОценок пока нет
- Energy-Changes-And-Rates-Of-Reaction CheatsheetДокумент4 страницыEnergy-Changes-And-Rates-Of-Reaction CheatsheetMaryam SameerОценок пока нет
- Creative English EGCIM UNДокумент64 страницыCreative English EGCIM UNANSELME DEZOUMBE PALAIОценок пока нет
- C W PDV DV V V W PV VДокумент4 страницыC W PDV DV V V W PV VRizwan Ullah BaigОценок пока нет
- Enthalpy of Vaporization LabДокумент5 страницEnthalpy of Vaporization LabDaniel LieОценок пока нет
- Analytic CombustionДокумент435 страницAnalytic Combustionsheila_faustino_3Оценок пока нет
- Information Theory in Molecular Biology: Christoph AdamiДокумент30 страницInformation Theory in Molecular Biology: Christoph AdamiNONAME69Оценок пока нет
- Concealed Duct Spec Sheet A4 - Print CompressedДокумент2 страницыConcealed Duct Spec Sheet A4 - Print CompressedJames HillОценок пока нет
- Boost Boiler Efficiency with Proper Fuel SelectionДокумент15 страницBoost Boiler Efficiency with Proper Fuel SelectionOm Prakash DubeyОценок пока нет
- Atmospheric Water Generator - ReportДокумент38 страницAtmospheric Water Generator - ReportArif AliОценок пока нет
- Density of Dry Air, Water Vapor and Moist Humid AirДокумент4 страницыDensity of Dry Air, Water Vapor and Moist Humid AirLeonardo CostaОценок пока нет
- DUPLEX 500 - 11000 Multi EN - 2018 - 03Документ8 страницDUPLEX 500 - 11000 Multi EN - 2018 - 03Constantin CilibiuОценок пока нет
- Lunaire Steady State StabilityДокумент2 страницыLunaire Steady State StabilityDanОценок пока нет
- The Distillation Process ExplainedДокумент13 страницThe Distillation Process ExplainedIsna NurhidayatiОценок пока нет
- General Physics A1: Ho Chi Minh City University of TechnologyДокумент9 страницGeneral Physics A1: Ho Chi Minh City University of TechnologyKhoa NguyenОценок пока нет
- LM335 Therometer Using The Arduino: Sensor TemperaturaДокумент7 страницLM335 Therometer Using The Arduino: Sensor TemperaturaEnrikeAguilarОценок пока нет
- Physics Project On ThermoelectricityДокумент10 страницPhysics Project On ThermoelectricityAbdul SamiОценок пока нет
- Evaporative Condenser Engineering Manual: Technical ResourcesДокумент18 страницEvaporative Condenser Engineering Manual: Technical ResourcesMohamed IbrahimОценок пока нет
- Advanced Design and Analysis of BOG Treatment Process in LNG Fueled ShipДокумент12 страницAdvanced Design and Analysis of BOG Treatment Process in LNG Fueled Shipsameh tawfeekОценок пока нет
- 4 Way Reversing Valve Series SHF L Data PDFДокумент3 страницы4 Way Reversing Valve Series SHF L Data PDFDharani PathyОценок пока нет
- Bimetallic Thermometer PDFДокумент3 страницыBimetallic Thermometer PDFHesti Nur AiniОценок пока нет
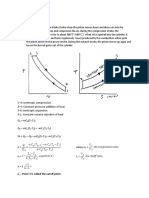
- Diesel Cycle: R - Point 3 Is Called The Cutoff PointДокумент7 страницDiesel Cycle: R - Point 3 Is Called The Cutoff PointJethro Briza GaneloОценок пока нет
- Psychrometric Chart PDFДокумент2 страницыPsychrometric Chart PDFvitaliskcОценок пока нет
- Vessel Heat LossДокумент2 страницыVessel Heat LossakisdassasОценок пока нет
- Quiz - Thermochem PRACTICE ANSWERSДокумент2 страницыQuiz - Thermochem PRACTICE ANSWERSliana.mirlohi4Оценок пока нет
- Engineering Mechanics (Statics) by Meriam (Two Chapters)Документ74 страницыEngineering Mechanics (Statics) by Meriam (Two Chapters)AbdulОценок пока нет