Академический Документы
Профессиональный Документы
Культура Документы
(E Z) Isomerization of Unsaturated Esters X CH CH COOCH3
Загружено:
falobaitОригинальное название
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
(E Z) Isomerization of Unsaturated Esters X CH CH COOCH3
Загружено:
falobaitАвторское право:
Доступные форматы
(E- Z)-ISOMERIZATION OF UNSATURATED ESTERS
X--CH= CH-COOCH
3
Vaclav VSETECKA, Jaroslav PECKA and Milos PROCHAZKA
Department of Organic Chemistry,
Charles University, 12840 Prague 2
277
Received November 6th, 1980
Total energy differences for a series of (E) and (Z) isomers of unsaturated esters X- CH=CH-
- COOCH3 have been calculated by the CNDO/ 2 method. The applicability of this method
to the prediction of relative stabilities of (E) and (Z) isomers and their optimal conformations
was checked by comparison with the experimental data .
The (E - Z) isomerization equilibria for many methyl propenoate derivatives
(X-CH= CH-COOCH
3
) were investigated by us
l
(X = CI, Br, I, CN, OCH
3
,
SCH
3
, NO
z
), as well as other authors (X = CH
3
, ref.
Z
-
4
, X = CzH
s
, ref.
4
; X =
= C
6
H
s
, ref.
s
; X = OCH
3
, ref.
4
; X = COOCH
3
, ref.
6
7
). We have now attempted
to compare the experimentally determined enthalpy differences with those calculated
by the CNDO/2 method and to consider the possible use of this method in predicting
the relative stabilities of the (E) and (Z) isomers and their optimal conformations.
We performed quant um-chemical calculations using the MO-LCAO-CNDO/2
methodS in the Santry-Segal parametrization
9
for compounds X-CH= CH-
-COOCH3 where X was CI, CN, OCH
3
, SCH
3
, CH
3
and COOCH
3
. Geometric
parameters, used in these calculations, were taken from the experimentally deter-
mined values for analogous compounds. According to preliminary calculations,
in the optimum conformation of (E) as well as (Z)-isomers of methyl 3-chloropro-
penoate the C= C- C= O system is planar and the O= C-O- C grouping adopts
an sp arrangement (Scheme 1, Table I) . In both the (E) and (Z) isomers the sp con-
formation of the C= C- C= O grouping is slightly preferred (about 3 kJ mol - I),
any conformation of the (E) isomer being more stable than that of the (Z)-isomer
by about 8 kJ mol - 1. Calculation, using the spd-basis, does not agree with the experi-
mental datal because it predicts greater stability of the (Z)-isomer.
Planar arrangement of the C= C- C= O system was also assumed in the calcula-
tion of total energy of methyl 3-cyanopropenoate. We studied also the dependence
of f1E on rotation of the CH
3
0 group (Table II and III). According to the calcula-
tion, the sp conformation of the CH
3
0 group relative to the C= O bond is preferred.
The energy difference between the optimal conformations, f1E(Z - E), was cal-
culated to be 112 kJ mol-I. The experimental values are f1G
473
= -8,3 kJ mol -
l
Collection Czechosl ovak Chem. Commun. [Vol. 47] [1982]
278 Vsetecka, Pecka, Prochazka:
and 11H = -40 kJ mol-l, the (Z)-isomer predominating
1
As calculated for the
sp and ap conformations of the C=C-C= O system in the (Z)- and (E)-isomers,
in both compounds the sp forms predominate (11E = 40 kJ mol-
1
and 52 kJ mol-
1
,
TABLE I
Total energy calculation (in kJ mol- 1) for methyl 3-chloropropenoate
Parameter
spa
apa
tJ.E(sp-ap)
spa
apa
tJ.E(sp-ap)
a Conformation C=C-C=O.
TABLE II
(Z)-Isomer
1a
CNDO/2
- 128489
- 128460
-29
(E)-Isomer
1b
-128569
-128538
-31
CNDO/2 with d-orbitals
-131965 -13 1682
-131916 -13 1650
-49 -32
tJ.E(E- Z)
- 80
- 78
283
266
Total energy calculation (in kJ mol-
1
) for methyl 3-cyanopropenoate
Rotation of -OCH3
(Z)-Isomer
(E)-Isomer
(Z)-Isomer
(E)-Isomer
sp conformation of C=C- C=O
-162488 -162264
-16237-6 -162153
ap conformation of C=C-C= O
- 162448 -162389 -161130
-162324 -162268 -161918
Collection Czechoslovak Chern. Commun. [Vol. 47] [1982]
(E- Z)-Isomerization
2.9 kJ mol-
1
3.1 kJ mol - I
18. 0 kJ mol-I
10.7 kJ mol -
1
5.2 U mol - I
19. 6 kJ mol-
1
6.4 kJ mol- I
Collection Czechoslovak Chern. Cornrnun. [Vol. 47] [19821
"'c "",,'
/ '
o
"-
H C=O
"C=C/
"- / "
C H
1\
279
(fa)
(1b)
(2a)
(2b)
(3a)
(3b)
(4)
280 Vsetecka, Pecka, Prochazka:
respectively). Although the barrier of rotation in this system is not known, the dif-
ference between standard heats of hydrogenation of (E)-2-butene and (E)-2-butenal
shows that the conjugation energy is at most 10 kJ mol-I. We can therefore assume
a practically free rotation in the system C=C-C= O at 293 K, with a slight excess
of the sp conformer. In both the conformations, the (Z)-isomer is calculated to be
more stable mainly due to the attractive interaction C=OC=N.
TABLE III
One-center and two-center contributions to the total energy in methyl 3-cyanopropenoate
(in kJ mol-I)
TABLE IV
Energy Monocentric Bicentric Total
sp conformation of C= C- C= O
(Z)-Isomer
(E)-Isomer
llE(Z-=- E)
55\00
5 509 5
05
-21758,8
-21 7471
- 11,7
-16248,8
-16237,6
-11,2
ap conformation of C=C-C=O
(Z)-Isomer
(E)-Isomer
llE(Z- E)
55081
5510'5
-2,4
-21 752' 9 - 16244,8
- 217429 - 16232'4
-100 - 12-4
One-center and two-center contributions to the total energy of methyl 2-butenoate (in kJ mol-I)
Energy Monocentric Bicentric Total
(Z)-Isomer
a
5131 9 - 215693 - 16437,4
(E)-Isomer
a
51432 - 21575'4 - 16432' 2
llE(Z-E)
-11,3
6 1
-5,2
(Z)-Isomer
b
51256 - 21542-4 - 16416,8
(E)-Isomer
b
51411 - 215775 - 16436-4
llE(Z- E) -15' 5 351 1%
a Conformation 3a; b conformation 3b.
Collection Czechoslovak Chern. Cornrnun. [Vol. 47] [1982]
(E- Z)-Isomerization 281
Optimal conformations obtained for the isomeric methyl 2-butenoates are given
in Scheme 2 (Table IV). A 30 deviation of the CH30-groUP from the O- C=O plane
increases the energy of the system mainly by suppressing the C(9f' 0(7) bonding
interactions and reducing the C(6)- 0(8) bond strength. The CH
3
COO- group
adopts therefore the planar sp arrangement (Scheme 3). In analogous arrangements
differing in rotation of the CH
3
COO- group by 180, the calculation prefers for
the sp conformation of the HCH
2
- C= grouping an sp arrangement of the C= C--C
- C= O system (72 kJ mol -1 for the (Z)-isomer and 56 kJ mol -
1
for the (E)-isomer).
For the ap conformation of HCH
2
-C= , the sp arrangement of C=C- C= O is
again preferred (11 kJ mol-
1
in the (Z)-isomer and 55 kJ mol -
1
in the (E)-isomer).
The ap conformation of the segment HCH
2
-C= is preferred by 24-4 kJ mol-
1
or 206 kJ mol -
1
for the (Z)-isomers, however, for the (E)-isomers the sp conforma-
tion of the HCH
2
- C= segment is by 42 or 43 kJ mol -
1
more stable, depending
on the C=C-C= O conformation. If we compare conformations of lowest energy
-Z.r-t a '-
1\
1\
-17330
r
E
-16944
- 17360
z
E
-17410 '-----,0..,..-L--=6'=- 0.".. -'-----''---'---' 1''' 8"'''0. :-'
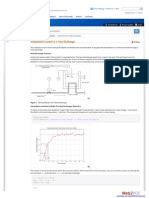
FIG. 1
Dependence of total energy of methyl 3-me-
thoxypropenoate on rotation of the CH
3
0
group (synperiplanar conformation of C=C
-C=O)
Collection Czechoslovak Chern. Cornrnun. [Vo l. 47] [1982J
:;zan
)(0-(_1-(-
/\
/\
- 16039
t
-"14772
E
-16047
-16055 '--Ob.,..-.L..--L--;; 9:'::: 0;'-. --'
FIG. 2
Dependence of total energy of methyl 3-me-
thylpropenoate on rotation of the CH
3
S
group (synperiplanar conformation of C=C
-C=O)
282 Vsetecka, Pecka, Prochazka:
(Scheme 4), we see that the (Z)-isomer should be more stable by 64 kJ mol-
l
which
does not agree with the experimental data. According to equilibration measure-
ments
3
, !lG < 9 kJ mol-I; this vaiue corresponds rather to the !lE value according
to Scheme (2a) or (3b), i.e. + 18-19 kJ mol-I.
In the case of methyl 3-methoxypropenoate we studied dependence of the energy
content on rotation of the -OCH3 group. As seen from Fig. 1, conformations with
180 and 120 angles between the planes are operating in both isomers (Scheme 5).
Calculation shows that an sp conformation of the CH
3
0- group is not advantageous
in either isomer. Equilibration measurements I found that !lG
403
= 157 kJ mol-
l
and !lH = 25 kJ mol -
l
with a significant contribution of the entropy term.
Also for methyl 3-methylthiopropenoate the dependence of the total energy
on rotation of the - SCH3 group was calculated (in the sp conformation of the
C=C-C=O system; Fig. 2). For the (E)-isomer two stable conformations with
torsion angles 0 and 180 are possible (Scheme 6) whereas iI! the (Z)-isomer only
conformation with a 0 angle is stable and rotation of the SCH
3
group results in
a steep energy increase. If we compare conformers with sp and ap arrangement
of the C=C-C=O system and with the same conformation of the -SCH3 group,
we see that the compounds should exist in both these conformations because
!lE(sp-ap) values for the (Z) and (E) isomers are 53 kJ mol-
l
and 18 kJ mol - I,
respectively (Scheme 7). The experimental value of !lG
423
(99 kJ mol-I) and !lH
(2'6 kJ mol-I, with a high entropy term) fairly agree with the value of !lE (0-4 kJ .
. mol-I).
(5a)
2 kJ rnol-
I
I kJ rnol-
1
( 5b )
(6(/)
Collection Czechoslovak Chern. Commun. [Vol. 47J [1982]
(E-Z)-Isomerization
283
0.4 kJ mo]-l
(6h)
3.9 kJ mo(1
(7)
33.6 kJ mol - I
(8a)
TABLE V
Total energy calculation for dimethyl butenedioate (in kJ mol- 1)
Conformation (8a) (8b) (8e)
(Z)-Isomer - 20524' 3 - 20494-4 - 20522,1
(E)-Isomer - 20557,9 -20548,2
-20553'3
/>"E(Z-E) 336 538 312
TABLE VI
Comparison of the experimental enthalpy differences with the calculated total energy differences
for selected su bstituents (kJ mol- 1)
X />"H />"E (/>"H-/>"E)
CI 108 80 20
CN
--4,0 -11,2
72
CH
3
18- 19
<9
CH
3
0 2' 5
0
- 20 4'5
CH
3
S 2'6
0
0-4 22
COOCH
3
322 33-6
-1,4
o The value suffers from a considerable experimental error.
Collection Czechoslovak Chern. Cornrnun. [Vol. 47] [19821
284 Vsetecka, Pecka, Prochazka :
For (Z) and (E) dimethyl butanedioate, total energy was calculated for conforma-
tions depicted in Scheme 8 (Table V). Calculation shows that deviation of both the
-COOCH3 groups from planarity by 20 results in an energy increase of about
34 kJ mol-
1
for the (Z)-isomer but 182 kJ mol-
1
for the (E)-isomer. We cannot
therefore exclude that, contrary to the (E)-isomer, the (Z)-isomer is not planar.
The value f,.E = 336 kJ mol - 1 agrees very well with the experimental value of f,.H
(32'2 kJ mol-I; Table VI).
We can conclude that the experiment ally found values of f,.H agree well with the
calculated f,.E values, except the data calculated for the methoxy group which wrongly
predict a predominance of the (Z) -isomer.
53.8 kJ mol -
1
31.2 kJ mol-I
EXPERIMENTAL
CH
J
0 /
H "C= O
" /
C=C"
/ " O= C H
"
/
HJC
(8b)
( 8e )
(9)
The geometric parameters of the molecules were based on the experimental data found for
analogous compounds
lO
. Basic data for methyl 2-propenoate (Scheme 9): C(I )-C(2) 1' 34;
C(2)- C(6) 1-46; C(6)-0(7) 1' 22; C(6)-0(8) 1'36; 0(8)- C(9) 1' 43; C(l)- H(3) 1'08; C(1)- H(4)
1'08; C(2)- H(5) 1' 08; C(9)- H(lO) 1' 09; C(9)-H(1l) 1'09; C(9)- H(12) 1' 09; C(6)- 0(8)-
- C(9) 109'5.
Data for substituents: 3-CH3: C(l)- C 1' 51; C-H 109; 3-Cl: C(l)- Cl 1'72; 3-CN: C(I)- C
1'45, C-N 116; 3-CH
3
0: C(l)- O 1' 36, C- O 1-43, C- H 1,08, C(I)-O-C 109' 5; 3-CH
3
S:
C(l)- S 1' 748, S- C 1-81, C-H 1' 09, C(l)- S-C 104'5.
Collection Czechoslovak Chern. Cornrnun. [Vol. 47] [1982]
(E- Z)-Isornerization 285
REFERENCES
1. Topek K., Vsetecka V., Prochazka M. : This Journal 43, 2395 (1978).
2. Uchytilova V.: Thesis. Charles University, Prague 1969.
3. Butler J. N., Small G. J. : Can. J. Chern. 41, 2492 (1963).
4. Rhoads S. J., Jitendra K., Chattopadhyay, WaaJi E. E.: J. Org. Chern. 35, 3352 (1970).
5. Hocking M. B.: Can. J. Chern. 47, 4567 (1969).
6. Davies M., Evans F. P., Trans . Faraday Soc. 61, 1506 (1955).
7. Nelles M.: Z. Phys. Chern. (Leipzig) 1931, 369.
8. Pople J . A., Beveridge D. L.: Approximate Molecular Orbital Theory . McGraw-Hili, New
York 1970.
9. Santry D. P., Segal G. A. : J . Chern. Phys. 47, 158 (1967) .
10. Int eratomic Distances Supplement (L. E. Sutton, Ed.), Spec. Pub!. 18. The Chemical Society,
London 1965.
Translated by M. Tichy.
Collection Czechosl ovak Chem. Commun. [Vol. 47J [1982J
Вам также может понравиться
- Homework Questions On Introductory ChemistryДокумент3 страницыHomework Questions On Introductory ChemistryfalobaitОценок пока нет
- New Student Orientation ManualДокумент27 страницNew Student Orientation ManualfalobaitОценок пока нет
- Molecular Orbital TheoryДокумент62 страницыMolecular Orbital TheoryfalobaitОценок пока нет
- Exm ThermoДокумент6 страницExm ThermofalobaitОценок пока нет
- Challenges Facing Democracy in NigeriaДокумент6 страницChallenges Facing Democracy in NigeriafalobaitОценок пока нет
- TOEFL - GrammarДокумент221 страницаTOEFL - Grammarcharlesbl100% (4)
- Learner Centered TeachingДокумент39 страницLearner Centered TeachingKlaribelle Villaceran0% (1)
- Physical Chemistry Level 2 LabДокумент34 страницыPhysical Chemistry Level 2 LabfalobaitОценок пока нет
- Making Chemistry Fun To LearnДокумент5 страницMaking Chemistry Fun To LearnLeela JayabalanОценок пока нет
- Alternative FuelsДокумент10 страницAlternative FuelsfalobaitОценок пока нет
- Teaching Chemistry in Secondary Schools - A Case For Cooperative Instructional StrategyДокумент7 страницTeaching Chemistry in Secondary Schools - A Case For Cooperative Instructional StrategyfalobaitОценок пока нет
- Gaussian 03 ManualДокумент592 страницыGaussian 03 ManualfalobaitОценок пока нет
- The Antibacterial, Antiviral Activities and Phytochemical Screening of Some Sudanese PlantsДокумент9 страницThe Antibacterial, Antiviral Activities and Phytochemical Screening of Some Sudanese PlantsfalobaitОценок пока нет
- Ethanol Internal Combustion EnginesДокумент6 страницEthanol Internal Combustion EnginesfalobaitОценок пока нет
- Ethanol and Internal Combustion EnginesДокумент20 страницEthanol and Internal Combustion EnginesfalobaitОценок пока нет
- What Is BiodieselДокумент2 страницыWhat Is BiodieselfalobaitОценок пока нет
- Unix - VI - EditorДокумент2 страницыUnix - VI - Editorapi-3749401Оценок пока нет
- Arl TR 5088Документ36 страницArl TR 5088falobaitОценок пока нет
- Effective Core Potential - by DolgДокумент35 страницEffective Core Potential - by DolgfalobaitОценок пока нет
- Reduced Scaling Electron Correlation MethodsДокумент47 страницReduced Scaling Electron Correlation MethodsfalobaitОценок пока нет
- Excited States and Electronic Spectroscopy - Edward C. Lim Notes2008Документ26 страницExcited States and Electronic Spectroscopy - Edward C. Lim Notes2008falobaitОценок пока нет
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceОт EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceРейтинг: 4 из 5 звезд4/5 (894)
- The Yellow House: A Memoir (2019 National Book Award Winner)От EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Рейтинг: 4 из 5 звезд4/5 (98)
- The Little Book of Hygge: Danish Secrets to Happy LivingОт EverandThe Little Book of Hygge: Danish Secrets to Happy LivingРейтинг: 3.5 из 5 звезд3.5/5 (399)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeОт EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeРейтинг: 4 из 5 звезд4/5 (5794)
- Never Split the Difference: Negotiating As If Your Life Depended On ItОт EverandNever Split the Difference: Negotiating As If Your Life Depended On ItРейтинг: 4.5 из 5 звезд4.5/5 (838)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureОт EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureРейтинг: 4.5 из 5 звезд4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryОт EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryРейтинг: 3.5 из 5 звезд3.5/5 (231)
- The Emperor of All Maladies: A Biography of CancerОт EverandThe Emperor of All Maladies: A Biography of CancerРейтинг: 4.5 из 5 звезд4.5/5 (271)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreОт EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreРейтинг: 4 из 5 звезд4/5 (1090)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyОт EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyРейтинг: 3.5 из 5 звезд3.5/5 (2219)
- Team of Rivals: The Political Genius of Abraham LincolnОт EverandTeam of Rivals: The Political Genius of Abraham LincolnРейтинг: 4.5 из 5 звезд4.5/5 (234)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersОт EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersРейтинг: 4.5 из 5 звезд4.5/5 (344)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaОт EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaРейтинг: 4.5 из 5 звезд4.5/5 (265)
- The Unwinding: An Inner History of the New AmericaОт EverandThe Unwinding: An Inner History of the New AmericaРейтинг: 4 из 5 звезд4/5 (45)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)От EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Рейтинг: 4.5 из 5 звезд4.5/5 (119)
- Slides-Developing Owner Cost Estimate PDFДокумент117 страницSlides-Developing Owner Cost Estimate PDFDedi JuherdiОценок пока нет
- AFM13 Preface Solutions ManualДокумент5 страницAFM13 Preface Solutions ManualArshad33% (3)
- Training Seminar ON GSM Held at BSNL: Presented By: Charli Bagga Kriti Rastogi (AEI-08)Документ51 страницаTraining Seminar ON GSM Held at BSNL: Presented By: Charli Bagga Kriti Rastogi (AEI-08)Ankita BharadwajОценок пока нет
- Lsi/Csi: 24-Bit Dual-Axis Quadrature CounterДокумент12 страницLsi/Csi: 24-Bit Dual-Axis Quadrature CounterWilliam Méndez PérezОценок пока нет
- 11.rolling Disc SolutionДокумент12 страниц11.rolling Disc SolutionAlimin AnniОценок пока нет
- Crowd Puller FinalДокумент10 страницCrowd Puller FinalJanette Tibayan CruzeiroОценок пока нет
- Installation and Setup Guide For The Cisco Active Directory Agent, Release 1.0Документ62 страницыInstallation and Setup Guide For The Cisco Active Directory Agent, Release 1.0Madelaine Velasquez Blacutt100% (1)
- LCD Interfacing PDFДокумент37 страницLCD Interfacing PDFPeeyush Kp100% (1)
- J Gen Physiol-1952-Hershey-39-56Документ18 страницJ Gen Physiol-1952-Hershey-39-56api-277839406Оценок пока нет
- Record results of toy soldier experiment times with different massesДокумент42 страницыRecord results of toy soldier experiment times with different massesTeoh Han Jie100% (1)
- Abstract Load Balancing 1Документ3 страницыAbstract Load Balancing 1Naveen AbhiОценок пока нет
- Grand Vitara 2012 2013Документ193 страницыGrand Vitara 2012 2013Favio Alejandro Herrera ZapataОценок пока нет
- The Windows Process Journey v6 0 Aug2023 1691726739Документ53 страницыThe Windows Process Journey v6 0 Aug2023 1691726739blakboukiОценок пока нет
- Ldp-105m150 Moso Test ReportДокумент17 страницLdp-105m150 Moso Test ReportzecyberОценок пока нет
- Operations Management 1St Edition Cachon Test Bank Full Chapter PDFДокумент36 страницOperations Management 1St Edition Cachon Test Bank Full Chapter PDFwayne.martin885100% (11)
- MinePlan Release NotesДокумент14 страницMinePlan Release NotesJuanJo RoblesОценок пока нет
- WPMD 3002Документ6 страницWPMD 3002Adilson LucaОценок пока нет
- Using LD - PreloadДокумент4 страницыUsing LD - Preloadmr z3iyaОценок пока нет
- Data MiningДокумент48 страницData MiningJAYASREE K.KОценок пока нет
- WWW Mathworks inДокумент7 страницWWW Mathworks inRagini SharmaОценок пока нет
- Convert Decimal To Binary Sunday ClassДокумент14 страницConvert Decimal To Binary Sunday ClassLaila HammadОценок пока нет
- Eb 20 11Документ408 страницEb 20 11henryОценок пока нет
- Effort Distribution On Waterfall and AgileДокумент12 страницEffort Distribution On Waterfall and Agileanandapramanik100% (2)
- Homework Lesson 6-10Документ9 страницHomework Lesson 6-10Valerie YenshawОценок пока нет
- Basic Chromatography Notes 1Документ27 страницBasic Chromatography Notes 1Aufa InsyirahОценок пока нет
- Pritchett Clock Repair Shop Breakeven Analysis ExcelДокумент138 страницPritchett Clock Repair Shop Breakeven Analysis ExcelMohd Yousuf MasoodОценок пока нет
- Noc18 cs48 Assignment3Документ4 страницыNoc18 cs48 Assignment3shweta100% (1)
- Dynamics and Bifurcations-HaleДокумент577 страницDynamics and Bifurcations-Halealaa alqurasheyОценок пока нет
- Monico Gen. 2 Gateway Datasheet PDFДокумент2 страницыMonico Gen. 2 Gateway Datasheet PDFRicardo OyarzunОценок пока нет