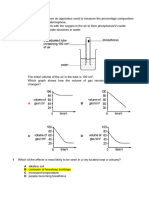
Академический Документы
Профессиональный Документы
Культура Документы
AL-CHEM Chemistry of Carbon Compounds (97-02)
Загружено:
AmyLinИсходное описание:
Оригинальное название
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
AL-CHEM Chemistry of Carbon Compounds (97-02)
Загружено:
AmyLinАвторское право:
Доступные форматы
AS/AL-CHEM (1997-2002)
Part XI
Chemistry of carbon compounds
NOTE: Questions with an * are NOT required by the HKDSE syllabus. They are included so as to enrich your knowledge and broaden your understanding in chemistry.
1997-AL-CHEM 1
5. (b) Consider the following reactions:
conc.H2SO 4 heat
HBr
CH3CH2CH2OH
CH3CH2COCl
NHNH2 NO2
Na2Cr2O 7 + H3O
Give structures for J, K, L, M and N.
NO2
N (a red precipitate)
(5 marks)
Mark Scheme ..............................................................................................................................................
5. (b) J K : : CH3CH=CH2 1 1
CH3CHCH3 Br
L M N
: : :
CH3CH2COOCH2CH2CH3 CH3CH2CHO
O2N H C CH3CH2 N NH NO2
1 Served as a chemical test for carbonyl group 1 1
(a condensation reaction by elimination of H2O: equation
DSE)
Please identify the types of reactions involved in forming J, K, L and M.
[5]
1997-AL-CHEM 2 5. (b) The following equation represents the acid hydrolysis of a dipeptide D to produce compounds E and F, one of which is a chiral compound.
H3C O H2NCHC NHCH2CO 2H
D (i) (ii) (iii) Name all functional groups in D. Give one structure for E and one for F.
H3O +
Draw a suitable representation for the chiral product.
Suggest a method to separate E and F from the reaction mixture. (5 marks)
5.
(d)
Give a systematic name to each of the following compounds:
O (ii) CH3CH2 C CH(CH3)CH2CH3
(iii) HO 2C
C C
CH3 H
(2 marks)
Exam Practice Marking Scheme 5. (b) (i) amino group / amine
Functional groups
Do Brilliantly Mark 1
amide group / peptide linkage carboxyl group / carboxylic acid (ii) E:
CH3 O H2N CH C OH
(deduct marks for each extra functional group) F: H2NCH2CO2H
CH3 NH2 H C
and
(Also accept protonated forms of the amino acids)
1 (L or D isomer) 1 [5]
Stereo-structure of E: (iii)
CO2H
paper chromatography / thin layer chromatography / column chromatography / electrophoresis
5.
(d)
(ii) (iii)
4-methylhexan-3-one trans-but-2-enoic acid / E-but-2-enoic acid
1 1 [2]
-------------------------------------------------- Take a break ----------------------------------------------------6. (a) The following conversion can be completed in not more than three steps. Use equations to show how you would carry out each conversion in the laboratory and for each step, give the reagent(s), conditions, and structure of the product.
(ii) NH2 NHCH2
(3 marks) 6. (c) Compound H, C3H6O2, does not react with NaBH4 and displays the following infra-red spectrum. Deduce all possible structures of H.
(4 marks)
Mark Scheme ..............................................................................................................................................
6. (a) (ii)
NOTE: Step 1 amide formation (in basic medium); Step 2 Reduction of amide to give amine (in dry ether)
AS/AL-CHEM (1997-2002)
Part XI
-1
Chemistry of carbon compounds
6.
(c)
IR peak at ~ 1750 cm indicates the presence of a carbonyl group (C=O) No broad OH absorption band implies that H is not a carboxylic acid No reaction with NaBH4 implies H is not ketone or aldehyde / may be an ester Possible structures of H:
O CH3 C OCH3 ;
O H C OCH2CH3
1+1
(Deduct 1 mark for each extra structure.) NOTE: NaBH4 is a milder reducing agent compared with LiAlH4. It is more selective in use and can only reducing carbonyl compounds to alcohols but not carboxylic acids (and their derivatives, e.g. esters).
[4]
-------------------------------------------------- Take a break ----------------------------------------------------7. (a) Suggest a chemical test to distinguish one compound from the other in each of the following pairs. Each test should include the reagent(s), the expected observation with each compound and the chemical equation(s).
(i)
and
O (iii) C2H5 C O(CH2)2CH3 and C2H5
O C CH2OC 2H5
O (iv) C2H5 C C2H5 and CH3(CH2)3
O C H
(9 marks)
Mark Scheme ..............................................................................................................................................
7. (a) (i) Treat compound with Br2 (in CCl4 or in CH3CCl3) 1
rapidly decolorizes the red-orange Br2 solution while
CCl4 Br Br
does not
Br2
(Also accept Baeyers test or using acidified potassium permanganate solution) (iii) Treat compound with a solution of 2,4-dinitrophenylhydrazine in methanol/Bradys reagent
O
Red/orange/yellow ppt. is observed with CH3CH2CCH2OCH2CH3
O
No ppt. with CH3CH2C OCH2CH2CH3
O2N O2N O O H2NNH NO2 N NH O NO2
( + H 2O )
1 1
(iv)
Treat compound with Tollens reagent/ammoniacal silver nitrate A silver mirror / silver deposit is observed with CH3(CH2)3CHO; No silver mirror / silver deposit with CH3CH2COCH2CH3
CH3(CH2)3CHO
[Ag(NH3)2]+ / OH-
CH3(CH2)3COO- + Ag(s) silver mirror
1 9 marks
Exam Practice
Functional groups
Do Brilliantly
1998-AL-CHEM 1
4. Alcohol E has the structure CH3CH(OH)C2H5. (a) (i) (ii) (b) (i) *(ii) Draw a three-dimensional representation of E. What type of isomerism can be exhibited by E? (2 marks) Draw the structures of three structural isomers of E, all of which are alcohols.
(c)
Describe how the reagent Zn/concentrated HCl can be used to distinguish E from the three structural isomers. (3 marks) When heating with concentrated H2SO4(l), E gives mainly two isomeric compounds, F and G, both of which have the formula C4H8. On treatment with bromine, both F and G give a product H with formula C4H8Br2. (i) Draw structures for F, G and H. What is the isomeric relationship between F and G? Outline the mechanism for the formation of H from either F or G. (5 marks) Mark
(Accept any correct representation of butan-2-ol showing the arrangement of groups at the chiral carbon)
(ii) *(iii)
4.
Marking Scheme (a) (i)
H C CH3
(ii) (b) (i)
CH3 CH3CH2CH2CH2OH CH3CHCH2OH CH3 CH3 C OH CH3
C2H5 OH
1 1
2 marks
optical isomerism / enantiomerism
++
(ii)
NOTE:
Upon reaction with Zn/conc.HCl, E gives turbidity (or cloudiness) at a slower rate than (CH3)3COH, but at a faster rate than CH3(CH2)3OH and (CH3)2CHCH2OH. ,
This test is a modified version of Lucas test (conc.HCl/ZnCl2) used to distinguish 1, 2 and 3 ROH. Please refer to 1999-AL-CHEM 2 Q.7(a) for further information. conc.HCl/ZnCl2 ROH RCl (RCl formed is insoluble in water) 3 marks
(c)
(i)
F and G:
H H C CH3
Br CH3 Br C H H C Br CH3
1+1
H and CH3 CH3 C C H CH3
and
H: (ii) (iii)
Br
1 1
Geometrical isomerism / cis-trans isomerism () ()
Br Br Br or,
Electrophilic addition mechanism DSE
+
Br Br Br
Br
5 marks
AS/AL-CHEM (1997-2002)
Part XI
Chemistry of carbon compounds
1998-AL-CHEM 2 6. (a) Consider the structures of the two synthetic polymers shown below:
CH2CH2 n poly(ethene)
(i) (ii)
HN(CH2)6NHCO(CH2)4CO n nylon-6,6
Suggest an explanation for the fact that nylon-6,6 has a higher tensile strength than poly(ethene). Briefly explain why aqueous acids can more readily attack nylon-6,6 than poly(ethene) inducing degradation. Apart from acidic conditions, state one other condition under which nylon-6,6 degrades more readily than poly(ethene). (5 marks)
(iii)
(c)
2-Ethanoyloxybenzoic acid (aspirin) is one of the most common substances used to relieve pain.
COOH OCOCH3
2-ethanoyloxybenzoic acid
*(i) (ii) Outline a synthetic route for preparing 2-ethanoyloxybenzoic acid from 2-methylphenol. Suggest how you could show the presence of the two functional groups in 2-ethanoyloxybenzoic acid. (4 marks) (d) Identify J, K, L and M in the following reactions.
CONH2 (ii)
CN K
(iii)
H2C=CH Cl
CH2
CH Cl
n
(4 marks)
Mark Scheme ..............................................................................................................................................
6. (a) (i) The interaction between polymer chain of PE is van der Waals forces while that between Nylon 6.6 is hydrogen bond. 1
The higher tensile strength of nylon is due to its stronger intermolecular attraction. (ii) CC linkage in PE is non-polar. not readily attacked by acids/has no reaction with acids. The amide linkage in nylon hydrolyse in acids to give NH3+ and COOH groups causing shortening of the chain. (iii) alkaline conditions / enzyme 1
, 1 5 marks
Exam Practice 6. (c) (i)
Functional groups
Do Brilliantly 2
OH CH3 (1/2) KMnO4/H+ heat (1/2)
OH CO2H
(1/2) CH3COCl or, (CH3CO)2O
OCOCH3 CO2H
Note:
(1/2) In carrying out the ethanoylation of phenol, we usually convert the phenol to phenoxide ion (by adding NaOH) before adding ethanoyl chloride (or ethanoic anhydride) for the ethanoylation.
Use IR spectroscopy: Absorption at 2500 to 3300 cm-1 indicates the presence of OH of the carboxylic acid
(ii)
[Note that extensive H-bonding in the acid gives rise to the very broad characteristic absorption band.] Absorption at 1680 to 1750 cm-1 indicates the presence of C=O of the acid and the ester. or, Chemical test: treat compound with Na2CO3(aq) / NaHCO3(aq) evolution of (colourless) gas bubbles, CO2(g), indicates the presence of CO2H or, ,
() ()
warm compound with dilute acid. Smell of vinegar indicates the presence of an ethanoyl ester. (,) 4 marks P2O5 (a dehydrating agent), heat
NOTE: P2O5 is the empirical formula of P4O10. 1 Although this dehydration reaction is NOT mentioned in DSE, the reagent is worth learning.
(d)
(ii) (iii)
H2O2, or other initiators (e.g. ROOR, i.e. organic peroxide)
1 4 marks
-------------------------------------------------- Take a break ----------------------------------------------------7. (a) Briefly explain why each of the following proposed reactions cannot lead to the target molecule. In each case, using the same starting molecule, outline a feasible synthetic route to obtain the target molecule, with no more than three steps. In each step, give the reagent(s) used, the conditions required and structure of the product. Starting molecule Target molecule
O (i) C NHCH3 CH3OH
O C OCH3
O (iii) CH3CH2 C OH LiAlH4, H3O
O CH3CH2 C H
Br (iv) CH3CH2CH CH3 NaOH H3CC CCH3
(9 marks) (c) Give a systematic name to each of the following compounds. (ii) CH3CH=CHCH2CHO (1 mark)
AS/AL-CHEM (1997-2002)
Part XI
Chemistry of carbon compounds
Mark Scheme ..............................................................................................................................................
7. (a) (i) No reaction between the reactants A feasible synthetic route is shown below: 5
NOTE: The functional group transformation required is from amide to ester.
or,
O C NHCH 3 H3O+
O C OH SOCl2
O C Cl CH3OH
O C OCH 3
NOTE:
Step 1 acid hydrolysis of amide; Step 2 conversion to acyl chloride from acid; Step 3 ester formation
(iii)
LiAlH4 will react with H3O+; or, LiAlH4 reduces carboxylic acids to alcohols, not aldehydes
NOTE: LiAlH4 + 4H2O LiOH + Al(OH)3 + 4H2 The bases formed will be neutralised by the acid H3O+
A feasible synthetic route is shown below:
in dry ether
Step 1: Reduction of COOH to CH2OH Step 2: Mild oxidation of 1 ROH to aldehyde
or, using Cr2O72-/H+ to oxidize the alcohol and distilling the aldehyde out from the reaction mixture as the aldehyde is being produced. or, SOCl2 H2, Pd/BaSO4 CH3CH2COOH CH3CH2COCl CH3CH2CHO (for reference only) (iv) Only alkene / but-2-ene will be formed. or, butan-2-ol will be formed. A feasible synthetic route is shown below:
Br
2) alcoholic NaOH heat
NOTE: Elimination reaction NOTE: Substitution reaction
() 4
(1/
(1/
( /2)
( /2) Br2 / CCl4 Br
Br
2) alcoholic NaOH CH3 C C CH3 heat or, using a stronger base, e.g. NaOC2H5 or NaNH2 instead of NaOH
7.
(c)
(ii)
pent-3-enal / 3-pentenal
9 marks 1 1 mark
1999-AL-CHEM 1
5. (a) Under certain conditions, methane reacts with chlorine to give chloromethane as the major product. (i) (ii) (iii) State the conditions for the reaction. Outline the mechanism and name the mechanistic steps of the reaction. Is the reaction of methane with chlorine an appropriate method for the preparation of dichloromethane? Explain. (5 marks)
7
Exam Practice
Functional groups
Do Brilliantly Mark , 3
Marking Scheme 1999-AL-CHEM 1 5. (a) (i) diffused light; excess methane (ii) mechanism:
(iii)
No. The reaction of CH4 with Cl2 gives a mixture of CH3Cl, CH2Cl2, CHCl3 and CCl4.
1
5 marks
1999-AL-CHEM 2
5. (a) Identify D, E, F, G and J in the following reactions.
(i)
NNH
CH2CH3 (ii) O2N
E O 2N
CO2H
CH2CH2OH (iii) HO
O NH (v) H3O heat
+
Na2Cr2O7/H3O heat CH3
(Hint: J is a polymer.)
(c)
(4 marks)
Dacron is the most common of the group of polymers known as polymers. A segment of the polymer chain is shown below.
O C
(i) (ii)
O C OCH2CH2O
C OCH2CH2O C
Suggest two types of material which can be made from polyesters. Draw the structures of the two monomers used in manufacturing Dacron. Name the type of polymerization involved. How can Dacron be degraded in the environment? (5 marks)
8
(iii)
AS/AL-CHEM (1997-2002)
Part XI
Chemistry of carbon compounds
Marking Scheme 5. (a) (i) D:
1999-AL-CHEM 2
Mark 1
NHNH2
(ii) (iii) E: F: MnO4-/H3O+; heat
CO2H OR HO CH3 HO CH3 CHO
1 1
(v)
J:
HN
O (CH2)5 C n
[4] (c) (i) Any TWO of the following: fibres, plastics, coatings, clothing, etc. (ii)
O HO C O C OH or
(Accept other correct uses of Dacron)
O CH3O C O C OCH3
, 1 1 1 1 [5]
and HOCH2CH2OH condensation polymerization (iii) Hydrolysis of the ester group by enzymes /bacteria
-------------------------------------------------- Take a break ----------------------------------------------------6. (a) (i) Suggest a reagent for the following conversion:
O Cl NH2 Cl NH CCH3
4-chlorophenylamine
(ii)
N-(4-chlorophenyl)ethanamide
Based on the fact that the amino group of 4-chlorophenylamine is more basic than the amide group of N-(4-chlorophenyl)ethanamide, outline a procedure to separate a mixture of the two compounds. Suggest how to show the presence of the amide group in N-(4-chlorophenyl)ethanamide by (I) (II) a chemical test, and a spectroscopic method. (6 marks)
(iii)
6.
(c)
State the relationship between each pair of structures shown below: CH2CH3 CH2CH3
(i) Cl Cl Cl and Cl
CH3 (ii) H C C
H and CH2CH3
Br
CH3CH2 C H
H and CH2
9
CH3 C H
(iii)
CH2
CH
C CH 3 H
CH
C CH 3 Br
(3 marks)
Exam Practice Marking Scheme 6. (a) (i) (ii) (CH3CO)2O / CH3COCl
Functional groups
Do Brilliantly Mark 1,
1999-AL-CHEM 2
Shake the mixture with dilute HCl and ether in a separating funnel. The amine will form the aminium salt and dissolve in the aqueous solution. The amide is not basic enough to be protonated by HCl and dissolves in the ether. Separate the aqueous layer from the ethereal layer. Treated the aqueous layer with NaOH(aq) to liberate the free amine. Use ether (or an appropriate solvent, e.g. THF, hexane, etc.) to extract amine from aqueous layer. (Distil off the organic solvent to obtain amine.) Distil off ether from the ethereal layer to obtain the amide. (I) Chemical test: Heat the amide with acid, smell of vinegar can be detected.
(iii)
(II)
Spectroscopic method: IR: strong absorption at 1680 1750 cm-1 indicates the presence of C=O functional group / at 3350 3500 cm-1 indicates the presence of NH group. ,
6 marks
(c)
(i) (ii) (iii)
identical compound geometrical isomers /cis-trans isomers / Z-E isomers enantiomers /optical isomers
1 1 1
3 marks
-------------------------------------------------- Take a break ----------------------------------------------------7. *(a) Lucas reagent, a mixture of ZnCl2 and concentrated HCl, can be used to distinguish the following alcohols from one another:
(CH3)2CHCH2OH,
(i) (ii)
CH3CH2CH(OH)CH3
and
(CH3)3COH
State the expected observation when these alcohols are separately treated with Lucas reagent. Suggest why these alcohols behave differently towards Lucas reagent.
(Hint: The zinc ion binds strongly with the oxygen atom of an alcohol, weakening the CO bond and creating a better leaving group.)
(4 marks) 7. (c) Compound M is a hydrocarbon isolated from oranges and lemons. (i) M contains 88.2% by mass of carbon and has a relative molecular mass of 136.2. Deduce the molecular formula of M. Based on the reactions given below, deduce the structure of M.
*(ii)
M + 2H2
(1) O 3 (2) Zinc dust
Pt
O
CH3
O H
CH(CH3)2
CH3 C(CH2)2CHCH2C O C CH3
HCHO
Marking Scheme 7. (a) (i)
1999-AL-CHEM 2 (CH3)2CHCH2OH : 1 ROH clear solution CH3CH2CH(OH)CH3 : 2 ROH cloudiness/turbidity appears after about five minutes (CH3)3COH : 3 ROH cloudiness/turbidity appears immediately ( marks for the appearance of cloudiness; 1 mark for the difference in rate.)
(5 marks) Mark
(ii)
The appearance of cloudiness is due to the formation of haloalkanes which are immiscible with water. Due to the formation of the relatively stable 3 carbocations, 3 alcohols reacts rapidly with Lucas reagent. The reaction mechanism is SN1. 1 carbocation is relatively unstable. The reaction of 1 alcohol with Lucas reagent proceeds through a mechanism which is not favoured in acidic conditions. (The reaction rate of 2 alcohols with Lucas reagent is moderate.)
4 marks
10
AS/AL-CHEM (1997-2002)
Part XI
Chemistry of carbon compounds
7.
(c)
(i) C Mass (g) no. of moles 88.2 H 11.8
88.2 = 7.34 12.01 7.34 =1 7.34
5
11.8 = 11.71 1.008 11.71 = 1.60 7.34
8
Mole ratio
Simple whole no. ratio Empirical formula of M is C5H8.
Relative molecular mass of M = 136.2. Molecular formula of M is C10H16. (For the empirical/molecular formula, award 1 mark for the answer + deduction) (ii) M reacts with H2 in mole ratio of 1 : 2. M possesses two C=C bond /one CC bond. Upon ozonolysis, M gives HCHO and a compound with 3 carbonyl groups. M possesses a terminal C=C bond and a cycloalkene/cyclohexene structure. Structure of M: NOTE: , 1 1
limonene
Note that limonene is optically active; the (+) form being present in citrus oils whilst the (-) form is found in pine needle oil. The racemic mixture, called dipentene, is a constituent of turpentine.
limonene isoprene
7 marks
2000-AL-CHEM 1
5. (a) Consider the reaction:
Br HBr
(i) *(ii) (iii) (iv)
Name the type of the reaction. Outline a mechanism of the reaction. Draw the structures of all possible stereoisomers of the product. Would the product rotate the plane of a beam of plane polarized light? Explain your answer. (5 marks)
11
Exam Practice 5. (b) (i)
Functional groups
Do Brilliantly
Give the reagents and conditions for the two steps of the conversion:
O CH3CH2 C N(CH3)2 OH Step 1 CH3CH2 CH N(CH3)2
Step 2
CH3CH CH
N(CH3)2
Step 1: Step 2: (ii) (iii) Suggest a chemical test for the carbonyl group in compound A. State the expected observation when HCl(aq) is added to compound A. 2000-AL-CHEM 1 (4 marks) Mark 1 2
5.
Marking Scheme (a) (i) electrophilic addition (ii)
(iii)
(iv)
No. The product is a mixture of the enantiomers in mole ratio of 1 : 1 / a racemic mixture Step 1: LiAlH4/ether, followed by acid hydrolysis or, NaBH4 / H2, Pd; high pressure Step 2: Heat with concentrated H2SO4 / Al2O3 / H3PO4 / P4O10
1 [5] 1 (,) , () () 1
4 marks
(b)
(i)
(ii)
Reagent: 2,4-dinitrophenylhydrazine / Bradys reagent Observation: yellow / orange / red precipitate or Reagent: hydroxylamine / phenylhydrazine / semicarbazide Observation: white (colourless) crystals A colourless solution is formed / only one layer is observed.
(iii)
2000-AL-CHEM 2
5. (d) A mixture of two organic products, G and H, was obtained in the reaction :
The mixture was separated by paper chromatography using a mixture of hexane and ether as solvent. The Rf values of G and H were found to be 0.84 and 0.55 respectively. (i) (ii) (iii) Deduce the structures of G and H. Suggest a chemical test to distinguish between G and H. State how the use of infra-red spectroscopy can provide further proof for the structures of G and H. (8 marks)
12
AS/AL-CHEM (1997-2002)
Part XI
Chemistry of carbon compounds
Marking Scheme 5. (d) (i) G:
O
2000-AL-CHEM 2
O C O(CH2)5O C
Mark
O C O(CH2)5OH
H:
1,1
G is less polar than H (or H has a polar OH group) and is therefore less tightly bound to the stationary phase and hence moves faster. (ii) or, or, or, (iii) Reagent: PCl5 / PCl3 / SOCl2 Only H will give misty fumes of HCl
NOTE: ROH + SOCl2 RCl + SO2 + HCl
1,1 1 1 (1) (1)
Reagent: Na Only H will react with Na to give gas bubbles (H2)
2+
NOTE: 2ROH + 2Na 2RO-Na+ + H2
Reagent: Cr2O7 /H Only H will induce a colour change from orange to green Reagent: MnO4-/H+ Only H will induce a colour change from purple to colourless Compound H shows an IR absorption at 3200-3700 cm-1 but G does not, because H has an OH functional group whereas G does not. 1, [8]
-------------------------------------------------- Take a break ----------------------------------------------------6. *(d) The conversion below can be completed in not more than four steps.
Use equations to show how you would carry out the conversion in the laboratory. For each step, give the reagent(s), conditions and structure of the product.
(4 marks) (e) Identify L, M, N, and P in the following reactions:
(4 marks)
13
Exam Practice Marking Scheme 6. (d)
Functional groups
Do Brilliantly Mark 4
2000-AL-CHEM 2
heat
heat
4 marks
6.
(e)
(i) (ii) *(iii)
L: M: N:
Cl2 and light or hv / N-chlorosuccinimide (NBS) and light H2 and transition metal catalyst (e.g. Pt or Ni) + pressure
1 1 1
(elimination reaction) *(iv) P: HOOC(CH2)4COOH (hydrolysis of ester group and nitrile group (CN) 1
5 marks
-------------------------------------------------- Take a break ----------------------------------------------------7. (a) An acyclic compound R (C7H12O) has a linear structure. R can be converted to S and then to T:
Given that R exists as a mixture of geometrical isomers, S has a chiral carbon centre, and T does not have any chiral carbon centre, deduce all possible structures of R, S and T. (8 marks) 7. (b) Aspartame, a sweetener, has the structure below:
(i) (ii) (iii)
Name all functional groups in aspartame. Upon hydrolysis, aspartame gives two amino acids. Draw their structures. Two electrodes are dipped into an aqueous solution containing the two amino acids in a pH 12 buffer, and are connected to the two poles of a battery. Which species derived from these amino acids will move faster towards the anode? Explain your answer. (7 marks)
14
AS/AL-CHEM (1997-2002)
Part XI
Chemistry of carbon compounds
7.
(c)
The compounds below are the monomers of some polymers.
O2N
COCl
H2N
CO2H
These compounds react in the presence of an inorganic reagent U to give compound V.
(i) (ii)
What is reagent U? The flow diagram below illustrates the reaction sequence for the transformation of V to compound W with four aromatic rings. What are reagents X and Y?
In the reaction sequence as in (ii), V is replaced by W, i.e., W is allowed to react with reagents X and Y, and the products react in the presence of U to form a polymer Z. Draw the structure Z. (5 marks) Marking Scheme 2000-AL-CHEM 2 Mark 7. (a) T (C7H16O) is an alkanol / alcohol. 1 R has 2 double bonds and S has 1 double bond. 1
(iii)
R can be reduced by LiAlH4 and S can undergo catalytic hydrogenation. R possesses the >C=O and >C=C< functional groups and S is an unsaturated alcohol. ,
S has a chiral carbon centre while T has not. Possible structures of R:
O
or Possible structures of S:
O
2
OH *
T is heptan-4-ol:
OH * or 2+1 (2 marks for structures; 1 mark for a clear indication of the chiral centre.)
OH
1 (For each of R, S and T, deduct marks for each additional structure.)
8 marks 15
Exam Practice 7. (b) (i)
Functional groups
Do Brilliantly
(max. 4 guessing, phenyl group not counted as a guess) amine/amino carboxylic acid/carboxyl/alkanoic acid amide/peptide (link) ester/alkyl alkanoate
COOH H2N COOH
H2N COOH
(ii)
(iii)
1+1 (accept structures in form of Zwitterion.)
At pH 12, the amino acids exist as anions:
COO H2N COO
-
H2N
COO-
1+1 ,
7 marks
(I) (II) (I) is doubly (more highly) charged, and will moves faster towards the anode. 7. (c) (i) (ii) U: X: Y: (iii) Z:
O O 2N C N H 7 COOH
NaOH/KOH/Na2CO3/K2CO3 SOCl2 / PCl3 / PCl5 Sn/HCl or Fe/HCl, or Zn/HCl or H2/Pd-C or H2/PtO2 or Na + alcohol or
1 1 1
O O 2N C N H COOH 7
2
5 marks
2001-AL-CHEM 1
5. (b) Give the structures of the organic compound E.
(ii) (1 mark) 6. (b) After some lessons in organic chemistry, a student remarked, Alkanes are more stable than alkenes, therefore alkanes do not react with chlorine but alkenes do. Do you agree with the student ? Explain. (3 marks)
Marking Scheme
5. (b) (ii) E:
2001-AL-CHEM-1
Mark
1
NOTE: IT is a condensation polymerisation reaction. 6. (b) or, Alkanes are thermodynamically/energetically more stable than alkenes because hydrogenation of alkenes is exothermic.
[1] 1 (1)
H2
Pt C C
H < O
H H
Alkanes do react with chlorine under sunlight to give chloroalkanes. 1
16
AS/AL-CHEM (1997-2002)
Part XI
Chemistry of carbon compounds
e.g.
CH3CH3 + Cl2
hv
CH3CH2Cl + HCl
Comment on the relationship of the first and second statement.
(1) 1 [3]
2001-AL-CHEM 2
5. (a) Compound A has the following structure :
*(i)
The carbon atom indicated by arrow a is more reactive towards nucleophilic substitution (SN2) reaction with C2H5ONa than that indicated by arrow b. Explain. 5.56 g of A is treated with 1.36 g of C2H5ONa. B. 5.56 g of A is treated with 3.00 g of C2H5ONa. molecular formula C11H14O. (I) (II) (III) Give the structure of D. If the yield of D is 82%, calculate the mass of D that can be obtained. Give the structure of E and name the types of reactions involved in the formation of E. (8 marks) Mark 1 Deduce the structure of the major substitution product
(ii)
(iii)
The reaction gives two products, D and E.
E has the
Marking Scheme
5. (a) (i) (ii)
2001-AL-CHEM 2
The reaction involves the attack of C2H5O- on the C atom of the substrate. Carbon atom b is more crowded. it is less reactive. (Accept equivalent answers, such as steric hindrance, etc.) No. of moles of A =
5.56 = 0.02 (12.01 9 + 1.008 10 + 79.9 2) 1.36 = 0.02 (12.01 2 + 1.008 5 + 16 + 22.99)
No. of moles of C2H5ONa =
A : C2H5ONa = 1 : 1
Structure of B:
Br O
(iii)
(I)
Structure of D:
O
(II)
C2H5ONa is in excess. Molar mass of D (C13H20O2) = 12.01 13 + 1.008 20 + 16 2 = 208.3 g mol-1 Mass of D obtained = 0.02 208.3 0.82 = 3.42 g
(Deduct marks for wrong/no unit.)
1 1 1
(III)
Structure of E:
Types of reactions: elimination and substitution
, [8]
17
Exam Practice
Functional groups
Do Brilliantly
-------------------------------------------------- Take a break ----------------------------------------------------5. (b) *(i) Give the reagent(s) and conditions for Step 1 and Step 2 in the reaction sequence below :
(ii)
4-nitrobenzoic acid can be converted to benzocaine, an anaesthetic, in not more than three steps.
Marking Scheme
5. (b) (i)
Use equations to show how you would carry out the conversion. In each step, give the reagent(s), conditions and structure of the product. (5 marks) 2001-AL-CHEM 2 Mark Step 1: conc. HNO3 + conc. H2SO4 (); 0-60C / heat/warm () Step 2: MnO4-/H3O+ (); heat () ( marks for reagents; marks for conditions.) 1 1 3
(ii)
c:
Alternative answers:
H2/Pt, or Na/EtOH, or NaBH4 (1) (3)
d: H2/Pt, or Sn/HCl + heat
e: EtOH/H3O+ + heat
f: OH-(aq)
-------------------------------------------------- Take a break ----------------------------------------------------5. (c) Consider the information below concerning the production of low density polyethene from ethene.
*(i) (ii) (iii) (iv)
Outline a mechanism for the polymerization and name each mechanistic step. Explain why benzoyl peroxide is used. Why is high pressure needed for the polymerization ? Is the product a single compound ? Explain. (7 marks)
18
AS/AL-CHEM (1997-2002)
Part XI
Chemistry of carbon compounds
Marking Scheme 5. (c) (i)
2001-AL-CHEM 2
Initiation ( )
Mark 2
Propagation ( )
Termination ( )
(Fish-hooks should be shown in the mechanism; otherwise max. deduction 1 mark) (ii) or, (iii) Benzoyl peroxide readily undergoes homolytic cleavage / decompose to give free radicals Benzoyl peroxide is a free radical initiator Concentration of reactants is high. 1 (1)
chance of collision between ethene and free radical increases /higher reaction rate.
(iv) No, because the product is a mixture of polymer molecules with different chain lengths.
1 1
-------------------------------------------------- Take a break ----------------------------------------------------7. (a) For each of the following reactions, suggest a possible reactant :
19
Exam Practice Marking Scheme
7. (a) (i)
Functional groups
Do Brilliantly Mark
1
2001-AL-CHEM 2
O
(ii)
O
COCl
(iii)
COOCOR
(iv) (v)
O OH
CO2CH3
OH Cl
Each: 1 mark
-------------------------------------------------- Take a break ----------------------------------------------------7. (b) Study the following information: Aromatic compounds T and U have the same molecular formula C10H12O.
T gives positive result in iodoform test. U reacts with hydrogen in the presence of a palladium catalyst to give compound V (C10H14O). obtained from the reaction of T with LiAlH4. V can also be
Treatment of U with ozone followed by acidified KMnO4 gives two products, one of which is benzoic acid. (i) (ii) Deduce the structures of T, U and V. For each compound, give all possible stereoisomers.
Suggest how infra-red spectroscopy can be used to distinguish between T and U.
Marking Scheme
7. (b) (i)
2001-AL-CHEM 2
T gives positive result in the iodoform test and has the structural characteristic
(9 marks) Mark 1
O C CH3 or
OH CHCH3
( marks for each characteristic.)
U reacts with H2-Pd U is an alkene / has a C=C group T can be reduced by LiAlH4 T is a ketone / has a C=O group Reaction of U with O3 following by KMnO4 gives benzoic acid. The double bond in U is adjacent to the benzene ring. Structures: (Four possible structures; 1 mark each for the 1 and 2 structures, and marks for each of the 3rd and 4th structures) T:
st nd
U:
OH *
1,1
V:
OH *
(two stereoisomers; marks for each structure)
NOTE:
Also accept the following structures for T, U and V:
(ii)
T has a sharp absorption peak in the wavenumber range from 1680 to 1750 cm-1 but U has not. U has a broad absorption peak in the wavenumber range from 3230 to 3670 cm-1 but T has not.
, , [9]
20
AS/AL-CHEM (1997-2002)
Part XI
Chemistry of carbon compounds
2002-AL-CHEM 1
5. (a) Two acyclic structural isomeric compounds have the molecular formula C3H6O3. Both are optically active and have infrared absorptions at 3400 cm-1 and 1700 cm-1. Neither possesses an alkoxy group. (i) (ii) Draw the structure of each of the compounds. Label one of these structures A and the other B.
Suggest a chemical test, giving the expected observation, to distinguish between the compounds represented by A and B. Give the structure of the major organic product formed when the two compounds are treated separately with excess LiAlH4. Product from the compound represented by A Product from the compound represented by B
(iii)
(6 marks)
Marking Scheme
5. (a) (i)
CO 2H OH HO
2002-AL-CHEM 1
CHO OH
Mark
1,1
(ii)
Treat compounds with Na2CO3(aq). Only the carboxylic acid will give colourless gas bubbles, CO2(g). Treat compounds with 2,4-dinitrophenylhydrazine / Fehlings solution Only the aldehyde will give a yellow precipitate / red precipitate (Accept other tests for the carbonyl group and the corresponding expected observations)
1 1 (1) (1)
(iii)
product from the carboxylic acid OH
OH
product from the aldehyde OH
HO OH
1,1
[6]
-------------------------------------------------- Take a break ----------------------------------------------------5. (b) Suggest reagent(s) to accomplish each of the following single-step transformations:
O
(i)
OH
CH3C(CH2)4COCH3
CHO
CH3CH(CH2)4COCH3
CO 2H
(iii)
COCH3 OH
Marking Scheme
5. (b) (i) (iii)
COCH3 OH
2002-AL-CHEM 1
(3 marks) Mark 1 1
NaBH4
or
H2/Pt (Accept other correct answers.)
Cr2O72-/H3O+
3 marks 21
Exam Practice
Functional groups
Do Brilliantly
2002-AL-CHEM 2
5. (b) Suggest a synthetic route, in not more than four steps and using LiAlD4 or NaBD4 as a source of deuterium (isotope of hydrogen), for the following transformation.
OH D
Marking Scheme
2002-AL-CHEM 2
(4 marks) Mark
-------------------------------------------------- Take a break ----------------------------------------------------5. (c) Consider the substances listed below: butane ethanoic acid propanone benzoic acid hexane tetrachloromethane dichlorodifluoromethane polystyrene triethylamine
For each of the descriptions of physical properties from (i) to (viii) below, choose from the above list, one substance which best fits the description. (i) (ii) (iii) (iv) (v) (vi) (vii) (viii) a colourless, flammable gas a colourless liquid with a sour odour a colourless, water miscible, flammable liquid a colourless, non-flammable liquid a colourless liquid with a fishy smell a colourless, water immiscible, flammable liquid a white solid which is insoluble in both cold and hot water a white solid which is insoluble in cold water, but soluble in hot water (8 marks)
22
AS/AL-CHEM (1997-2002)
Part XI
Chemistry of carbon compounds
Marking Scheme
5. (c) (i) (ii) (iii) (iv) (v) (vi) (vii) (viii)
2002-AL-CHEM 2 butane - a colourless, flammable gas ethanoic acid - a colourless liquid with a sour odour propanone / ethanoic acid - a colourless, water miscible, flammable liquid tetrachloromethane - a colourless, non-flammable liquid triethylamine - a colourless liquid with a fishy smell hexane - a colourless, water immiscible, flammable liquid polystyrene - a white solid which is insoluble in both cold and hot water benzoic acid - a white solid which is insoluble in cold water, but soluble in hot water
Mark
1 1 1 1 1 1 1 1 [8]
-------------------------------------------------- Take a break ----------------------------------------------------7. (b) For each of the following pairs of molecules, identify their relationship as identical, enantiomeric, geometrical isomeric or structural isomeric.
Marking Scheme
7.
2002-AL-CHEM 2
Mark
23
Exam Practice
Functional groups
Do Brilliantly
-------------------------------------------------- Take a break ----------------------------------------------------7. (c) A mixture of 2.8 g of butane-1,4-diol and 6.3 g of benzene-1,3-dicarbonyl chloride was heated at 215C for 30 minutes to give 6.4 g of a polymer M.
(i) (ii) (iii)
Draw the repeating unit of M. What type of polymerization is involved in the formation of M ? Calculate the percentage yield of M.
Marking Scheme
7.
2002-AL-CHEM 2
(5 marks) Mark
-------------------------------------------------- Take a break ----------------------------------------------------7. (d) Suggest a synthetic route, in not more than three steps, for the transformation of 3-methylbenzoic acid to N,N-diethyl-3-methylbenzamide, a substance commonly used in mosquito repellent.
Marking Scheme
7.
2002-AL-CHEM 2
(2 marks) Mark
24
Вам также может понравиться
- AL-CHEM 97-06 Chemistry and Society PDFДокумент18 страницAL-CHEM 97-06 Chemistry and Society PDFAmyLinОценок пока нет
- AL-CHEM Chemistry of Carbon Compounds (03-06)Документ24 страницыAL-CHEM Chemistry of Carbon Compounds (03-06)AmyLinОценок пока нет
- NSS Chemistry Part 11 Chemistry of Carbon CompoundsДокумент47 страницNSS Chemistry Part 11 Chemistry of Carbon CompoundsFelix YueОценок пока нет
- International Indian School Chemistry Worksheet on Carbon CompoundsДокумент4 страницыInternational Indian School Chemistry Worksheet on Carbon CompoundsRaghav GuptaОценок пока нет
- 2013 s5 Chem Supple Paper 1a (All)Документ16 страниц2013 s5 Chem Supple Paper 1a (All)梁山伯Оценок пока нет
- Cs Chem Hkdse Mock Section B EeДокумент12 страницCs Chem Hkdse Mock Section B Eeleung_ting_2Оценок пока нет
- HKDSE Chem FX ExamS5 2011 Set1 EngДокумент27 страницHKDSE Chem FX ExamS5 2011 Set1 Eng12376590Оценок пока нет
- F6 Physics Mock P1 AEXДокумент14 страницF6 Physics Mock P1 AEXporter100% (1)
- HKDSE Chem FX Mock Exam Paper 1 2012 Set 1 EngДокумент28 страницHKDSE Chem FX Mock Exam Paper 1 2012 Set 1 Engleung_ting_2100% (1)
- HKDSE Chem FX Mock Exam Paper 1 2012 Set 1 Eng AnsДокумент10 страницHKDSE Chem FX Mock Exam Paper 1 2012 Set 1 Eng Ansleung_ting_2Оценок пока нет
- NSS Chemistry Part 13 Industrial Chemistry - IIДокумент21 страницаNSS Chemistry Part 13 Industrial Chemistry - IIFelix YueОценок пока нет
- Physics Paper 2: Question BookДокумент12 страницPhysics Paper 2: Question BookporterОценок пока нет
- NSS Chemistry Part 4 Acids and Bases - LQДокумент40 страницNSS Chemistry Part 4 Acids and Bases - LQFelix YueОценок пока нет
- NSS Chemistry Part 4 Acids and Bases - MCДокумент35 страницNSS Chemistry Part 4 Acids and Bases - MCFelix YueОценок пока нет
- 2015 F6 Mock P2 PDFДокумент7 страниц2015 F6 Mock P2 PDFKaylie WongОценок пока нет
- NSS Chemistry Part 12 Patterns in Chemical WorldДокумент7 страницNSS Chemistry Part 12 Patterns in Chemical WorldFelix YueОценок пока нет
- Queen's College Mock Exam SolutionsДокумент16 страницQueen's College Mock Exam Solutionsym5c2324Оценок пока нет
- F5 BAFS Final - 1920 - DraftДокумент3 страницыF5 BAFS Final - 1920 - Draft4E-27 Tsoi Cheuk Ying (Ada)Оценок пока нет
- NSS Chemistry Part 9 Rate of ReactionsДокумент26 страницNSS Chemistry Part 9 Rate of ReactionsFelix YueОценок пока нет
- Microscopic World I - Atomic Structure and PropertiesДокумент40 страницMicroscopic World I - Atomic Structure and PropertiesRyanОценок пока нет
- Past Paper - Acids and Alkalis - LQДокумент10 страницPast Paper - Acids and Alkalis - LQapi-3739994100% (2)
- Previous HSE Questions From The Chapter "Alcohols, Phenols and Ethers"Документ3 страницыPrevious HSE Questions From The Chapter "Alcohols, Phenols and Ethers"alan ChackoОценок пока нет
- WS2 Redox Reaction AnsДокумент2 страницыWS2 Redox Reaction AnsAndyОценок пока нет
- Air and AtmosphereДокумент12 страницAir and Atmospherebob leowОценок пока нет
- NSS Chemistry Part 3 Metals - MCДокумент20 страницNSS Chemistry Part 3 Metals - MCFelix YueОценок пока нет
- Past Paper Micro World I II 2019 20 PDFДокумент19 страницPast Paper Micro World I II 2019 20 PDF779720 cОценок пока нет
- Chemical Bonding: Ionic Bonding and Metallic Bonding: Learning GoalДокумент36 страницChemical Bonding: Ionic Bonding and Metallic Bonding: Learning GoalRyanОценок пока нет
- Exam Paper - M1 (By Topic)Документ19 страницExam Paper - M1 (By Topic)Henry Leung100% (1)
- Redox Reactions, Chemical Cells and Electrolysis (Less than 40 chars: 35 charsДокумент38 страницRedox Reactions, Chemical Cells and Electrolysis (Less than 40 chars: 35 charsミーチェルОценок пока нет
- MSS 1718MockPaper1AДокумент11 страницMSS 1718MockPaper1AKelvin Chow100% (1)
- Po Leung Kuk No.1 W.H.Cheung College Yearly Examination (2021-2022) FORM 6 CHEMISTRY PAPER 2 Suggested AnswersДокумент4 страницыPo Leung Kuk No.1 W.H.Cheung College Yearly Examination (2021-2022) FORM 6 CHEMISTRY PAPER 2 Suggested AnswersChun Kit LauОценок пока нет
- NSS Chemistry Part 2 Structural Questions and AnswersДокумент22 страницыNSS Chemistry Part 2 Structural Questions and AnswersFelix YueОценок пока нет
- Chem 6 (2nd) PDFДокумент28 страницChem 6 (2nd) PDFRyanОценок пока нет
- Something Old and Something New: A Comparison of Michelangelo and DuchampДокумент11 страницSomething Old and Something New: A Comparison of Michelangelo and DuchampD HernandezОценок пока нет
- 2021 DSE Phy Mock Marking SchemeДокумент45 страниц2021 DSE Phy Mock Marking SchemeDavid LouОценок пока нет
- NSS Chemistry Part 3 Metals - LQДокумент25 страницNSS Chemistry Part 3 Metals - LQNicole ChanОценок пока нет
- 3B - ch07 - LQ - E: (A) Do Not Direct The Laser Beam To The Eyes. 1AДокумент10 страниц3B - ch07 - LQ - E: (A) Do Not Direct The Laser Beam To The Eyes. 1AVincent haОценок пока нет
- A4 QB-MC Ch03 Movement of Substances Across Cell MembraneДокумент22 страницыA4 QB-MC Ch03 Movement of Substances Across Cell MembraneReg ChooОценок пока нет
- Chem 2 (2nd) PDFДокумент28 страницChem 2 (2nd) PDFRyanОценок пока нет
- Respiration: Energy Release in Cycling RaceДокумент56 страницRespiration: Energy Release in Cycling RaceBernardОценок пока нет
- Biology HKDSEДокумент19 страницBiology HKDSERoy SzeОценок пока нет
- Instructions:: Part-A I. Answer ALL The Questions (Each Question Carries One Mark) 10x1 10Документ3 страницыInstructions:: Part-A I. Answer ALL The Questions (Each Question Carries One Mark) 10x1 10anon_850201470Оценок пока нет
- A4 QB-MC Ch08 Transport in HumansДокумент23 страницыA4 QB-MC Ch08 Transport in HumansReg ChooОценок пока нет
- Periodic Table SQДокумент17 страницPeriodic Table SQNg Swee Loong StevenОценок пока нет
- (A) (I) Period 0.02 S 1M Frequency 50 HZ 1A (Ii) I 5 A 1A (Iii) Effective Value I 1M 3.54 A 1A (B) (I) P I 1M 5 5 125 W 1AДокумент11 страниц(A) (I) Period 0.02 S 1M Frequency 50 HZ 1A (Ii) I 5 A 1A (Iii) Effective Value I 1M 3.54 A 1A (B) (I) P I 1M 5 5 125 W 1AVincent haОценок пока нет
- IT Chem F5 Mid-Year Examination (E)Документ10 страницIT Chem F5 Mid-Year Examination (E)Norzawati NoordinОценок пока нет
- The Ultimate Question Bank: Dse Chem MasteryДокумент48 страницThe Ultimate Question Bank: Dse Chem MasteryYip AvaОценок пока нет
- Afterschool Mole Calculation Exercise Ans.Документ32 страницыAfterschool Mole Calculation Exercise Ans.J TОценок пока нет
- HSC Chemistry Module 9.3 SummaryДокумент51 страницаHSC Chemistry Module 9.3 SummarySwonderhОценок пока нет
- NSS Chemistry Part 7 Redox Reactions Chemical Cells and Electrolysis - LQДокумент38 страницNSS Chemistry Part 7 Redox Reactions Chemical Cells and Electrolysis - LQFelix YueОценок пока нет
- Part I Introducing Chemistry MCДокумент6 страницPart I Introducing Chemistry MCDavid LouОценок пока нет
- Structured Questions: Core SectionДокумент24 страницыStructured Questions: Core Sectionapi-19650882Оценок пока нет
- 20 - 21 f5 My Math CP BДокумент8 страниц20 - 21 f5 My Math CP BWaSx3lyОценок пока нет
- 2012 CJC CH h2 p2 PromoДокумент12 страниц2012 CJC CH h2 p2 PromoDaniel ChuОценок пока нет
- Ionic vs Covalent Bonding in RbF and GeF4 CompoundsДокумент7 страницIonic vs Covalent Bonding in RbF and GeF4 CompoundsLee HollidayОценок пока нет
- 962/2 2006 Trial Examinations Upper 6 Panitia Daerah Johor Bahru Chemistry Paper 2 (2 Hours)Документ12 страниц962/2 2006 Trial Examinations Upper 6 Panitia Daerah Johor Bahru Chemistry Paper 2 (2 Hours)sherry_christyОценок пока нет
- HSC June 2009 Paper and Marking Scheme On Same Paper Word DocumentДокумент26 страницHSC June 2009 Paper and Marking Scheme On Same Paper Word DocumentreekoyeОценок пока нет
- Organometallic TurotialДокумент2 страницыOrganometallic TurotialkhemrajmahadewОценок пока нет
- Chapter 1 ReviewДокумент2 страницыChapter 1 ReviewGmat PrepОценок пока нет
- P24 Answers Kweyete AlbertДокумент7 страницP24 Answers Kweyete AlbertMuhammad HashirОценок пока нет
- Llcmhlss S1 Summer Foundation Course English NotesДокумент22 страницыLlcmhlss S1 Summer Foundation Course English NotesAmyLinОценок пока нет
- Phy SamplePaper Paper1 2 e PDFДокумент48 страницPhy SamplePaper Paper1 2 e PDFAmyLinОценок пока нет
- 3D Printing Applications and Impacts SurveyДокумент12 страниц3D Printing Applications and Impacts SurveyAmyLinОценок пока нет
- 2012 Maths Practice Paper M2Документ16 страниц2012 Maths Practice Paper M2AmyLinОценок пока нет
- 2012 Maths Practice Paper M1Документ6 страниц2012 Maths Practice Paper M1AmyLinОценок пока нет
- DSE 2012 - Marking and ReportДокумент20 страницDSE 2012 - Marking and ReportAmyLinОценок пока нет
- 1 Conductor Characteristics PDFДокумент20 страниц1 Conductor Characteristics PDFjaimeОценок пока нет
- Microsoft Word - Dangerous Goods Declaration PDFДокумент1 страницаMicrosoft Word - Dangerous Goods Declaration PDFAlfian AnasОценок пока нет
- EndPl MomConn LSDДокумент54 страницыEndPl MomConn LSDTony RoseОценок пока нет
- 5 Things To Know About DB2 Native EncryptionДокумент4 страницы5 Things To Know About DB2 Native EncryptionAnup MukhopadhyayОценок пока нет
- Shear Forces and Bending Moments: Understanding Structural AnalysisДокумент2 страницыShear Forces and Bending Moments: Understanding Structural AnalysisKang Lee76% (25)
- Steam Sterilization PrinciplesДокумент8 страницSteam Sterilization PrinciplesBhavik Thakar100% (1)
- Calculation and Design of Critical Speed and Power AgitatorДокумент4 страницыCalculation and Design of Critical Speed and Power AgitatorFrendy RianОценок пока нет
- Amman Master PlanДокумент205 страницAmman Master Planomaroyoun100% (9)
- Temporary Horizontal Lifeline Rope A6Документ3 страницыTemporary Horizontal Lifeline Rope A6tanu00Оценок пока нет
- 12.fire Safety Operations BookletДокумент14 страниц12.fire Safety Operations Bookletdkpushpdk0% (1)
- Bernini PDFДокумент13 страницBernini PDFChris AntoniouОценок пока нет
- Design Optimization of The Control System For The Powertrain of An Electric Vehicle A Cyber-Physical System ApproachДокумент6 страницDesign Optimization of The Control System For The Powertrain of An Electric Vehicle A Cyber-Physical System ApproachHamaad RafiqueОценок пока нет
- Math4220 PDFДокумент10 страницMath4220 PDFjhonmt7Оценок пока нет
- Serway Physics II Example Questions Chapter 8Документ1 страницаSerway Physics II Example Questions Chapter 8AizuddinОценок пока нет
- Shrinkage Cracking in Restrained Reinforced Concrete MembersДокумент93 страницыShrinkage Cracking in Restrained Reinforced Concrete MembersPeter MsomaОценок пока нет
- CRP900 Benchtop PRO Assembly Instructions v2019Q1 1Документ86 страницCRP900 Benchtop PRO Assembly Instructions v2019Q1 1glamura100% (1)
- Make Noise Soundhack Erbe-Verb: Reverb FX ModuleДокумент1 страницаMake Noise Soundhack Erbe-Verb: Reverb FX ModuleAghzuiОценок пока нет
- Manual Intel D815EEA2-D815EPEA2 P3 Socket370Документ146 страницManual Intel D815EEA2-D815EPEA2 P3 Socket370Raul MejicanoОценок пока нет
- Perfect Gas Law Lab ReportДокумент9 страницPerfect Gas Law Lab ReportTan Zu Kuan50% (2)
- The Waterfall Model: Testing ModelsДокумент6 страницThe Waterfall Model: Testing ModelsanilОценок пока нет
- ResistanceДокумент72 страницыResistanceParisDelaCruzОценок пока нет
- 14EEMME00 - Darshna RamawatДокумент31 страница14EEMME00 - Darshna Ramawatdatshna ramawatОценок пока нет
- Breathing Air System Performance QualificationДокумент11 страницBreathing Air System Performance QualificationCHALLA ANITHA100% (1)
- Asme Pcc-2-2015 Article 3.12Документ9 страницAsme Pcc-2-2015 Article 3.12munawarОценок пока нет
- DC-Lec-03 & 04 (Physical Structure of Network)Документ44 страницыDC-Lec-03 & 04 (Physical Structure of Network)tabby919100% (4)
- The Garrison Border Town of Elvas and Its FortificationsДокумент850 страницThe Garrison Border Town of Elvas and Its FortificationsAndré SerraОценок пока нет
- The Iron Carbon Phase DiagramДокумент2 страницыThe Iron Carbon Phase Diagramsinha.subhasis1417Оценок пока нет
- Train Backyard ToyДокумент12 страницTrain Backyard ToyJim100% (17)
- Chapter OverviewДокумент57 страницChapter OverviewWajiha SharifОценок пока нет
- Fire Protection For LithiumДокумент2 страницыFire Protection For LithiumSofiqОценок пока нет