Академический Документы
Профессиональный Документы
Культура Документы
P9 Natural Gas - Removal of Hydrates
Загружено:
omeshchemИсходное описание:
Оригинальное название
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
P9 Natural Gas - Removal of Hydrates
Загружено:
omeshchemАвторское право:
Доступные форматы
TOPIC II
HYDRATES- INHIBITION-
DEHYDRATION
Controlling and/or Removal of Water
in Natural Gas
Hydrates Inhibition
Hydrates Removal
Glycol Absorption (high quantity of water)
Description of process
Field of application
Unit design:
a. foaming;
b. glycol degradation/corrosion
Adsorption (low to moderate amount of water)
Description of process
Adsorbent characteristic
Unit design:
a. choice of adsorbent
b. Dryer arrangement
c. Restricting the quantity of adsorbent required
Hydrates
Hydrates are:
crystal (solid) formed.
physical combination of water and light hydrcarbon
molecules.
Characteristics
resemble packed, wet snow flakes
density =0.9 kg/dm
3
regular cubic structure in shape
only small gas molecules can form hydrates
(i.e: CH
4
,C
2
H
6
,C
3
H
8
,C
4
H
10
, CO
2
,H
2
S and N
2
)
- hydrocarbon tend to be trapped in cavities of water molecule structures
large proportion of water (CH
4
.6H
2
0 methane hydrate)
Hydrates contain a very large proportion of water (CH4.6H2O for methane hydrate;
C3H8.17H2O for propane hydrate)
high concentration entails presence of liquid water
Conditions for Hydrates Formation : Primary cause
in presence of liquid water
temperature does not exceed the water dew point specification
(dew point=lowest temperature at which vapour starts to condense/liquefy)
Effects of hydrate formation
- increase processing difficulty; caused disruption of process during natural gas
treatment
- blockage; accumulation of hydrates at orifice plates or valves, reduce the
cross-sectional area.
- corrosion of equipment and lines by acid gases (CO
2
, H
2
S) in presence of
water
How to prevent hydrates formation
1. Operating Condition
- modify the operating condition of natural gas treatment.
at P=60 bar, hydrates formed at T <16
o
C
at P=100 bar, hydrates formed at T =20
o
C
2. Injecting a hydrate inhibitor into the natural gas, designed to lower hydrate
formation temperature.
3. Removing part of water contained in the natural gas.
These preliminary treatment is necessary for
1. To prevent hydrate formation
2. To prevent corrosion of equipment and lines by acids in gas (CO2, H2S) in the
presence of water
3. To meet dew point specification
HYDRATES INHIBITION
Methanol
Advantages:
effective in case of polar products especially water.
attractive due to low crystallization temperature, low viscosity
Disadvantages:
not economical
volatile product
high losses of vaporization in gas
Ethylene Glycol
Advantages:
more preferred than methanol.
used on intermittent basis as curative rather than preventive measurement.
important in drying natural gas.
loss through vaporization in natural gas can be neglected
Types of Glycols widely used:
monoethylene glycol, MEG (HOC
2
H
4
OH) for low T
diethylene glycol, DEG (HO(C
2
H
4
O)
2
H)
triethylene glycol, TEG (HO(C
2
H
4
O)
3
H) for high T
* depend on operating temperature that influence on viscosity
Properties of Hydrate Inhibitors
Quantity of Inhibitor to Inject
Ethylene Glycol
Properties:
MEG used for temperature below 5 to 10
o
C
TEG too viscous at T = -5 /-10
o
C
Hammerschmidt Formula
Quantity of inhibitor:
W = weight percentage of inhibitor in aqueous phase
dx = decrease in temperature of hydrate formation
M = molecular weight of inhibitor
K
i
= constant related inhibitor
Operation
remove free water from circuits first before start up process
drain the lower points
blow the circuits with dry air / nitrogen
monitor the variation in operating parameters (i.eP, T,deltaP)
DRYING GAS BY HYDRATE-INHIBITION
Type: MEG, DEG
T=-5 to -10
o
C
P=
T=
DEHYDRATION
Definition :
Dehydration is
a process of water removal or drying of natural gas.
compliance with a water dew point specification.
Purposes
to avoid the risk of condensation of water in the pipeline.
to avoid the formation of liquid slugs.
to prevent gradual plugging of the circuits by ice.
Types of dehydration processes
1. Glycol absorption dehydration
most suited is TEG (Triethylene Glycol)
TEG has low vapor pressure hence minimum losses in gas
high concentration of TEG give low gas dew point
temperature
2. Dehydration by adsorption
used adsorbent in dehydration process
principle based on porous solid with specific property,
able to fix water molecules on the surface of pores where
water vapor condensed.
Glycol Absorption Dehydration
Principle:
wet gas dehydrated by glycol in absorber
water contained glycol reconcentrated in regenerator
water removed from natural gas, recycled back at top of the column
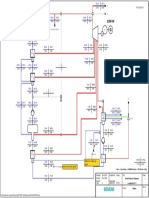
Process Description (refer to Fig 2):
wet natural gas (NG) pass through separator
on leaving the separator, the gas is fed into bottom of absorber
lean glycol solution is fed on top of absorber
rich glycol expanded in expansion drum to degas the light
hydrocarbons, H
2
S and CO
2
entering regenerator and reboiler to minimize risks of corrosion
exchanger is used to condense the regenerator reflux
the gas free water leaves the top of the column
DRYING GAS BY DEHYDRATION: GLYCOL ABSORPTION PROCESS
T
glycol
=-6 to -10
o
C(low dew point)
T
regenerate
=204
o
C
P =high
Glycol Absorption Dehydration
Main Operating Parameter:
Glycol concentration has to be highly concentrated to lower the dew point
The lower the dew point to be reached, the greater the degree of regeneration
of glycol required
The actual dew point reached in the absorber is bet. 6-10 deg C higher the
equilibrium dew point. The gap is called the approach
cannot obtain directly by reboiling-rectification, need to regenerate at high
temperature (204
o
C)
high T can caused glycol breakdown and formed of corrosive compound
Alternative Solution to Operate at T>204 deg
low pressure by vacuum operation (risk of oxygen inducing flammable
mixture)
fuel gas or natural gas (to decrease the water partial pressure , thus >ing
vaporisation
iso-octane (non miscible substance)
Phenomena for Optimal Dehydration Unit Design Operation:
Foaming
Glycol Degradation
Phenomena for Optimal Dehydration Operation
1. Foaming
i. Glycol has a tendency to foam when in contact with foaming promoters such as
liquid HC, solid particles (compound produced by thermal degradation of
glycol)
ii. develop in absorber, and can fill the entire column
iii. top of column no effect on foam
iv. the onset of foaming in absorber reflected by increase pressure drop in absorber
Prevention of foaming
i. Installation of separator (to remove free water, liq. HC, solid particles)
ii. Lean glycol inlet temperature need to be higher than temperatureof natural gas
to prevent condensation of HC
iii. TEG regeneration not exceeding its thermal degradation, 206
o
C
iv. Glycol filtering to extract foaming promoters:
i. Catridgefilter (polypropylene) to remove solid particles
ii. Activated carbon filter to remove HC and products of glycol degration
v. pH of solution maintain between 6 to 8; >8 there is a risk foaming
vi. Injection of small quantity of an anti-foaming agent
Phenomena for Optimal Dehydration Operation
2. Glycol degradation
thermal degradation of TEG at 206
o
C onwards
produce highly corrosive organic acid
glycol solution absorbs acid gases, lead to decrease pH
corrosion occur caused by presence of salt water
lead to give erosion-corrosion that caused by high rate of glycol
circulation in piping/bends.
Injection of a small amount of corrosion inhibitor, e.g. amines
Prevention of glycol degradation
limit the temperature in reboiler to 204oC
prevent air from enter unit
pH need to be maintained above 6 to avoid TEG breakdown
ensure satisfactory separation of free water
injection a small amount of corrosion inhibitor
Dehydration by Adsorption
Principle:
A physical process whereby a suitable porous solid with specificproperty is
able to fix water molecules on the surface of pores where water vapor
condensed
Is the fixation of molecules by reversible reaction on the surface of a solid.
Three different phenomena of adsorption:
Chemisorption
forming the first layer at low partial pressures.
Physisorption
due to the formation of multiple layers by hydrogen bonding in the adsorbent pores.
Capillary condensation
where localized condensation takes place at temperatures above that of the bulk
fluids dew point.
Characteristic of Adsorbents
have very large internal contact, 250-850 m2/g
Possess a strong affinity for water vapor and a high capacity for adsorption
Be easily and economically regenerable
Undergo slight pressure drop under flow of gas
Possess good mechanical strength
Adsorption Process Description
Water adsorbs and condenses on the surface of adsorbent
Beyond the pure surface adsorption, a secondary mechanism, capillary
condensation kicks-in when pore diameter is comparable to molecular diameter.
Pores in the adsorbent are asymmetrical, i.e. further down into the gel, the
narrower the pore becomes (like a volcano crater).
This capillary condensation is driven by differences of partial pressure outside
and inside of the pore.
To remove the water from the adsorbent surface, energy is used
After regeneration, the adsorbent can be put to use again after cooling to
ambient temperature.
Schematic Representation of Capillary
Condensation
ADSORBENT TYPES
Activated alumina
Alumina is the most widely used adsorbent because of:
The chemical properties of its surface
Its ability to be shaped with well-defined pores defined as follows:
(Ultra) Macropores(>1000 ) to enhance diffusion into the pore system.
Mesopores(30 to 1000 ) to accommodate medium size molecules.
Micropores(<30 ) to accommodate small molecules like water.
Zeolite/Molecular Sieve
Type A zeolite
With sodium cationshas a pore width of 4 , called MS4A.
Replacement of sodium by calcium cationsleads to 5 pores, called MS5A.
Replacement of sodium by potassium leads to molecular sieve MS3A.
Type X Zeolite
Gives pores of 10 and the calcium type corresponds to MS13X.
Silica gel
Activated carbon.
Types of Adsorbent
activated alumina
SA=280 m2/g; pore volume=0.4 m3/g; pore diameter=2-4 nm; density=720-820
kg/m3)
Reduce content by 1 ppmv
Regeneration T=150-220
o
C
silica gel
SA=550-800 m2/g; pore volume=0.35-0.5 m3/g; pore diameter=2.5 nm; density=
720-800 kg/m3)
Reduce content by 10 ppmv
Regeneration T=150-250
o
C
molecular sieves (or zeolites)
Composition oxides of (Si, Al) and Na or K or Ca
Zeolite3A (K), Zeolite4A(Ca); Zeolite5A(Na); ZeoliteX (10 A diameter)
SA=650-800 m2/g; pore volume=0.27 m3/g; pore diameter=3-5 nm; density=690-720
kg/m3)
Reduce content by 1 ppmv
Regeneration T=200-300
o
C
activated carbon
Adsorption of Water to the Alumina
Surface
MOLECULAR SIEVES
Dehydration by Adsorption
Characteristic of a Good Adsorbent:
should posses strong affinity for water vapor
high capacity for adsorption process
capacity of adsorbents depends on their nature
low value of relative humidity gives high capacity of molecular sieve
(ex: adsorbent)
easily and economically re-generable
undergo little drop in pressure
posses good mechanical strength
low dew point is required for cryogenic treatment of natural gas
ability to adsorb heavy HC can show the selectivity of adsorbent
DEHYDRATION UNITS
ADSORPTION PHASE:
Gas Flow: From top to bottom
Adsorbent : Saturated with H2O
Polarity of H2O: Stronger
HC Molecule: Less Stronger
REGENERATION PHASE:
T: 200-300
o
C
P: Low
Adsorbent : Activated Al, Si Gel, Mol Sieves
Stages of Regeneration: Heating Phase
Cooling Phase
Dehydration by Adsorption
Process Description:
using 2 columns called dryers packed with solid adsorbent
involve 2 phase: adsorption phase & regeneration phase
1. Adsorption Phase
gas flow through drier from top to bottom
adsorbent saturated with water
halted phase first before reach breakthrough point
polarity of water much stronger attraction on adsorbent
ejects hydrocarbon molecules that is less stronger
2. Regeneration Phase
regenerate bed of adsorbent
performed operation by increasing in T (200-300
o
C) or lowering the P, or by a
combination of both
can be performed by heating bed of adsorbent
2 stage of regeneration of drier: heating phase & cooling phase
Heating Phase: Hot air desorbthe water from the adsorbent
Cooling Phase: Drier is cooled at the end of the heating phase to the initial condition
Picture of EZ (Equilibrium Zone)
& MTZ (Mass Transfer Zone)
Adsorption and Regeneration for PSA and
TSA Processes
Pressure Swing Adsorption
(PSA)
Temperature Swing Adsorption
(TSA)
Block Diagram of Molecular Sieve Process
Field of Applications
Aging/Deactivation of Solid
Adsorbents
A gradual reduction in adsorption capacity is
caused by aging of the adsorbent.
Two types of aging exist;
1. Hydrothermal aging
- an irreversible change of adsorbent structure caused
by hydrothermal treatment during regeneration,
resulting in reduced active area.
2. Aging from contamination
- caused by co-adsorption of undesired species and
coke formation on the active surface of the adsorbent.
This phenomenon is not completely reversible, and
carbon deposits increase with each regeneration
Isotherms of Activated Alumina, Silica
and Molecular Sieves
Combination of Activated Alumina
and Molecular Sieves
Isotherm Activated Alumina and 4A Molecular Sieves
Life Factor vsNumber of Regenerations for
Al
2
O
3
/ MS 4A /MS 4A in Natural Gas Drying
Industrial Adsorbent Problems
Deep dehydration
Upstream process upsets, leading to dessicant
degradation (reduce adsorption capacity & pressure
drop, hence frequent regeneration)
Causes: liquid (free) water or entrainment of amine
deposition on the adsorbent, high CO2 concentrations,
and heavy metal adsorption
Free water entrainment can lead to caking and
powdering, which leads to >ed pressure drop and poor
gas distribution
Вам также может понравиться
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeОт EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeРейтинг: 4 из 5 звезд4/5 (5794)
- 2 X 22MW 2 HP 105535Документ1 страница2 X 22MW 2 HP 105535omeshchemОценок пока нет
- The Yellow House: A Memoir (2019 National Book Award Winner)От EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Рейтинг: 4 из 5 звезд4/5 (98)
- Abs-Stripper 13septДокумент2 страницыAbs-Stripper 13septomeshchemОценок пока нет
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryОт EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryРейтинг: 3.5 из 5 звезд3.5/5 (231)
- 2 X 22MW 2 HP 105535Документ1 страница2 X 22MW 2 HP 105535omeshchemОценок пока нет
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceОт EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceРейтинг: 4 из 5 звезд4/5 (895)
- 01 - Fluid FlowДокумент76 страниц01 - Fluid FlowMubarak AhmadОценок пока нет
- The Little Book of Hygge: Danish Secrets to Happy LivingОт EverandThe Little Book of Hygge: Danish Secrets to Happy LivingРейтинг: 3.5 из 5 звезд3.5/5 (400)
- Analysis of Condenser Shell Side Pressure Drop Based On The Mechanical Energy LossДокумент8 страницAnalysis of Condenser Shell Side Pressure Drop Based On The Mechanical Energy LossomeshchemОценок пока нет
- 4616 PDFДокумент9 страниц4616 PDFBob SmithОценок пока нет
- Never Split the Difference: Negotiating As If Your Life Depended On ItОт EverandNever Split the Difference: Negotiating As If Your Life Depended On ItРейтинг: 4.5 из 5 звезд4.5/5 (838)
- Glycol Dehydration Unit English LetterДокумент4 страницыGlycol Dehydration Unit English LetterRaulCamachoОценок пока нет
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureОт EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureРейтинг: 4.5 из 5 звезд4.5/5 (474)
- Heat 4e Chap11 LectureДокумент32 страницыHeat 4e Chap11 Lecturepradeepgautam1010198Оценок пока нет
- HVAC SystemДокумент6 страницHVAC SystemomeshchemОценок пока нет
- Natural Gas DehydrationДокумент34 страницыNatural Gas DehydrationJefMusОценок пока нет
- The Emperor of All Maladies: A Biography of CancerОт EverandThe Emperor of All Maladies: A Biography of CancerРейтинг: 4.5 из 5 звезд4.5/5 (271)
- Pressure Relief Valve Engineering HandbookДокумент93 страницыPressure Relief Valve Engineering Handbookakrouti92% (12)
- Heat Transfer InnovatorsДокумент9 страницHeat Transfer InnovatorsomeshchemОценок пока нет
- Team of Rivals: The Political Genius of Abraham LincolnОт EverandTeam of Rivals: The Political Genius of Abraham LincolnРейтинг: 4.5 из 5 звезд4.5/5 (234)
- HVAC System Design: Mark Hydeman, P.E., FASHRAE Taylor Engineering, LLCДокумент42 страницыHVAC System Design: Mark Hydeman, P.E., FASHRAE Taylor Engineering, LLCsardarmkhanОценок пока нет
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaОт EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaРейтинг: 4.5 из 5 звезд4.5/5 (266)
- Pipe Sizing Charts Tables.12890822Документ29 страницPipe Sizing Charts Tables.12890822forevertay2000Оценок пока нет
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersОт EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersРейтинг: 4.5 из 5 звезд4.5/5 (344)
- Heat Exchanger CalculationsДокумент12 страницHeat Exchanger CalculationsMichael J. BaneОценок пока нет
- Sizing Shell and Tube Heat ExchangerДокумент17 страницSizing Shell and Tube Heat ExchangerCallum Biggs100% (3)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyОт EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyРейтинг: 3.5 из 5 звезд3.5/5 (2259)
- Heat Exchanger CalculationsДокумент12 страницHeat Exchanger CalculationsMichael J. BaneОценок пока нет
- HVAC SystemДокумент6 страницHVAC SystemomeshchemОценок пока нет
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreОт EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreРейтинг: 4 из 5 звезд4/5 (1090)
- 2980245C HVAC Handbook 2013 WebДокумент35 страниц2980245C HVAC Handbook 2013 WebomeshchemОценок пока нет
- HSJFKKHFKKJDSGKSFMC Nbbbbzcgumkbwmscn, MLJFC S 544 SДокумент1 страницаHSJFKKHFKKJDSGKSFMC Nbbbbzcgumkbwmscn, MLJFC S 544 SomeshchemОценок пока нет
- ATM Tank DatasheetДокумент1 страницаATM Tank DatasheetomeshchemОценок пока нет
- The Unwinding: An Inner History of the New AmericaОт EverandThe Unwinding: An Inner History of the New AmericaРейтинг: 4 из 5 звезд4/5 (45)
- 2980245C HVAC Handbook 2013 WebДокумент35 страниц2980245C HVAC Handbook 2013 WebomeshchemОценок пока нет
- Hydrostatic Test Pressure PipingДокумент2 страницыHydrostatic Test Pressure PipingnitinchautreОценок пока нет
- Pump TrainingДокумент15 страницPump TrainingdalayeliОценок пока нет
- Api 54Документ2 страницыApi 54almandhari33100% (1)
- Pipe/connection Type Speed Possible Speed ValuesДокумент1 страницаPipe/connection Type Speed Possible Speed ValuesomeshchemОценок пока нет
- Comparison Horizontal Vs Vertical SeparatorДокумент54 страницыComparison Horizontal Vs Vertical SeparatorEng Kim Wei100% (6)
- Crosby Pressure Relief Valve HandbookДокумент93 страницыCrosby Pressure Relief Valve HandbookGlen AshwellОценок пока нет
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)От EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Рейтинг: 4.5 из 5 звезд4.5/5 (121)
- E2 Knockout Drum PDFДокумент2 страницыE2 Knockout Drum PDFKyaw Kyaw AungОценок пока нет
- Guidelines For Utility Impact Assessment For Local Roads in Developed Areas MME 2012 Cross-SectionДокумент81 страницаGuidelines For Utility Impact Assessment For Local Roads in Developed Areas MME 2012 Cross-Sectionirfan mohammedОценок пока нет
- EX - NO:1a Data Definition Languages (DDL) Commands of Base Tables and ViewsДокумент44 страницыEX - NO:1a Data Definition Languages (DDL) Commands of Base Tables and Viewslalit thakur100% (1)
- Chapter 1 AssignmentДокумент4 страницыChapter 1 Assignmenthamster808100% (3)
- 1716,1734,1751,1769 - Highrise and Earthquake Resistant ConstructionДокумент34 страницы1716,1734,1751,1769 - Highrise and Earthquake Resistant ConstructionFAB RAHIОценок пока нет
- 125 Tractor: (Specifications and Design Subject To Change Without Notice)Документ5 страниц125 Tractor: (Specifications and Design Subject To Change Without Notice)Gary LarsonОценок пока нет
- Opposite Corners CourseworkДокумент8 страницOpposite Corners Courseworkpqltufajd100% (2)
- Laboratory Work 1 Computation of Metrics of Productivity of Computer SystemДокумент12 страницLaboratory Work 1 Computation of Metrics of Productivity of Computer SystemHhhhhh75% (4)
- Smart Security Camera System For Video Surveillance Using Open CVДокумент6 страницSmart Security Camera System For Video Surveillance Using Open CVlambanaveenОценок пока нет
- SIF Corporate-Presentatie 2017Документ35 страницSIF Corporate-Presentatie 201766apenlullenОценок пока нет
- Powerflex 525 Devicenet Adapter: User ManualДокумент140 страницPowerflex 525 Devicenet Adapter: User ManualJahir Emerson Tantalean QuiñonesОценок пока нет
- Chapter 9 and 10Документ18 страницChapter 9 and 10billОценок пока нет
- 3g3JX InverterДокумент262 страницы3g3JX InverterdatdttvuОценок пока нет
- Astar - 23b.trace - XZ Bimodal Next - Line Next - Line Next - Line Next - Line Drrip 1coreДокумент4 страницыAstar - 23b.trace - XZ Bimodal Next - Line Next - Line Next - Line Next - Line Drrip 1corevaibhav sonewaneОценок пока нет
- Math 138 Functional Analysis Notes PDFДокумент159 страницMath 138 Functional Analysis Notes PDFAidan HolwerdaОценок пока нет
- (Solution Manual) Fundamentals of Electric Circuits 4ed - Sadiku-Pages-774-800Документ35 страниц(Solution Manual) Fundamentals of Electric Circuits 4ed - Sadiku-Pages-774-800Leo AudeОценок пока нет
- Magnetron PDFДокумент1 страницаMagnetron PDFmytrya debОценок пока нет
- Ratio Worksheet AKДокумент12 страницRatio Worksheet AKChika AuliaОценок пока нет
- New EM Quiz13Документ4 страницыNew EM Quiz13Singh KaranОценок пока нет
- Science July Assignment Grade 8Документ3 страницыScience July Assignment Grade 8G PОценок пока нет
- ME361 - Manufacturing Science Technology: Measurements and MetrologyДокумент20 страницME361 - Manufacturing Science Technology: Measurements and MetrologyKartikeyaОценок пока нет
- m1100 s12 v1.1f en - Fender - DesignДокумент48 страницm1100 s12 v1.1f en - Fender - Designdzul fiqarОценок пока нет
- MB-339A User ManualДокумент196 страницMB-339A User Manualkepakko75% (4)
- 2-Way Doherty Amplifier With BLF888AДокумент27 страниц2-Way Doherty Amplifier With BLF888AerdemsecenОценок пока нет
- A Deep Dive Into The Latest HPC SoftwareДокумент38 страницA Deep Dive Into The Latest HPC SoftwareSundar NilОценок пока нет
- Chapter26to29-Bolt Tightening TorqueДокумент36 страницChapter26to29-Bolt Tightening TorqueEnam SembilanОценок пока нет
- S3 3 TrigonometryДокумент81 страницаS3 3 TrigonometryEugene ChoongОценок пока нет
- Sony - HST 211 - Sen 211 SMДокумент30 страницSony - HST 211 - Sen 211 SMOswaldo CamposОценок пока нет
- VT Directed Io SpecДокумент297 страницVT Directed Io SpechobomanОценок пока нет
- Database Management Systems: Lecture - 5Документ37 страницDatabase Management Systems: Lecture - 5harisОценок пока нет