Академический Документы
Профессиональный Документы
Культура Документы
Reservoir Hydrocarbons
Загружено:
ndlr81Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
Reservoir Hydrocarbons
Загружено:
ndlr81Авторское право:
Доступные форматы
Reservoir Fluid Properties Course (1
st
Ed.)
1. Reservoir Fluid Course
2. HC Alteration
3. Properties of Natural Gases
4. Properties of Crude Oils
A. density
B. Gas Solubility
2013 H. AlamiNia Reservoir Fluid Properties Course: Reservoir Hydrocarbons (Bo & Bt & Constants) 2
1. Formation Volume Factor
A. Oil
B. Total (two phase)
2. Property Constants
2013 H. AlamiNia Reservoir Fluid Properties Course: Reservoir Hydrocarbons (Bo & Bt & Constants) 3
Laboratory Determination of
the PVT Relationships
In determining the PVT relationships (including the
gas solubility-pressure relationship) in the
laboratory, it is necessary to record the volume of
oil and volume of liberated gas as the pressure is
reduced below saturation pressure.
The manner in which the solution gas is liberated
from the oil will significantly affect all the PVT
relationships. There are two types of separation
(liberation, vaporization) process, namely:
Flash liberation
Differential liberation
2013 H. AlamiNia Reservoir Fluid Properties Course: Reservoir Hydrocarbons (Bo & Bt & Constants) 5
2013 H. AlamiNia Reservoir Fluid Properties Course: Reservoir Hydrocarbons (Bo & Bt & Constants) 6
Recombination
Courtesy IPE, Tehran, 2012
By transferring an oil mixture from reservoir conditions
to standard conditions, most of the gaseous
components dissolved in the oil at reservoir conditions
are lost.
It, therefore, seems as though the oil shrinks during
production.
The volumetric changes taking place
In the reservoir,
During passage of the well and
In the process plant,
Can be studied by performing PVT experiments on the
reservoir fluid.
2013 H. AlamiNia Reservoir Fluid Properties Course: Reservoir Hydrocarbons (Bo & Bt & Constants) 7
The oil formation volume factor, Bo, is defined as
the ratio of the volume of oil (plus the gas in
solution) at the prevailing reservoir temperature
and pressure (bbl) to the volume of oil at standard
conditions (STB).
Evidently, Bo is always greater than or equal to
unity. The oil formation volume factor can be
expressed mathematically as
2013 H. AlamiNia Reservoir Fluid Properties Course: Reservoir Hydrocarbons (Bo & Bt & Constants) 8
=
,
Oil Formation Volume Factor
vs. P Diagram
2013 H. AlamiNia Reservoir Fluid Properties Course: Reservoir Hydrocarbons (Bo & Bt & Constants) 9
As the pressure is reduced below the initial
reservoir pressure, pi, the oil volume increases due
to the oil expansion.
This behavior results in an increase in the oil formation
volume factor and will continue until the bubble-point
pressure is reached.
At Pb, the oil reaches its maximum expansion and
consequently attains a maximum value of Bob for
the oil formation volume factor.
2013 H. AlamiNia Reservoir Fluid Properties Course: Reservoir Hydrocarbons (Bo & Bt & Constants) 10
As the pressure is reduced below Pb, volume of the
oil and Bo are decreased as the solution gas is
liberated.
When the pressure is reduced to atmospheric
pressure and the temperature to 60F, the value of
Bo is equal to one.
2013 H. AlamiNia Reservoir Fluid Properties Course: Reservoir Hydrocarbons (Bo & Bt & Constants) 11
2013 H. AlamiNia Reservoir Fluid Properties Course: Reservoir Hydrocarbons (Bo & Bt & Constants) 12
Numerical Value of the Bo
As in the case of the gas solubility
determination, the numerical value
of the oil formation volume factor at
different pressures will depend upon
the method of gas liberation.
There is some mathematical and graphical
correlations as:
Standing's Correlation
Vasquez and Beggs' Correlation
Glaso's Correlation
Marhowi's Correlation
2013 H. AlamiNia Reservoir Fluid Properties Course: Reservoir Hydrocarbons (Bo & Bt & Constants) 13
Oil Formation Volume Factor for
Undersaturated Oils
With increasing p above the Pb, the oil formation
volume factor decreases due to the compression of
the oil.
To account for the effects of oil compression on Bo,
bob is first calculated by using any of the methods.
The calculated Bo is then adjusted to account for
the effect of increasing the pressure above the Pb.
This adjustment step is accomplished by using the
isothermal compressibility coefficient as described
below.
2013 H. AlamiNia Reservoir Fluid Properties Course: Reservoir Hydrocarbons (Bo & Bt & Constants) 14
The isothermal compressibility coefficient (as
expressed mathematically by Co=-1/v ( V/ p) T)
can be equivalently written in terms of the oil
formation volume factor:
2013 H. AlamiNia Reservoir Fluid Properties Course: Reservoir Hydrocarbons (Bo & Bt & Constants) 15
=
1
To describe the pressure-volume relationship of
hydrocarbon systems below their bubble-point
pressure, it is convenient to express this
relationship in terms of the total formation volume
factor as a function of pressure. The total formation
volume factor defines the total volume of a system
regardless of the number of phases present.
The total formation volume factor, as denoted by
Bt, is defined as the ratio of the total volume of the
hydrocarbon system at the prevailing pressure and
temperature per unit volume of the stock-tank oil.
2013 H. AlamiNia Reservoir Fluid Properties Course: Reservoir Hydrocarbons (Bo & Bt & Constants) 18
Because naturally occurring hydrocarbon systems
usually exist in either one or two phases, the term
"two-phase formation volume factor" has become
synonymous with the total formation volume.
Mathematically, Bt is defined by the following
relationship:
2013 H. AlamiNia Reservoir Fluid Properties Course: Reservoir Hydrocarbons (Bo & Bt & Constants) 19
=
,
+
2013 H. AlamiNia Reservoir Fluid Properties Course: Reservoir Hydrocarbons (Bo & Bt & Constants) 20
It should be noted that Bo and Bt are identical at
pressures above or equal to the bubble-point
pressure because only one phase, the oil phase,
exists at these pressures.
It should also be noted that at pressures below the
bubble-point pressure, the difference in the values
of the two oil properties represents the volume of
the evolved solution gas as measured at system
conditions per stock-tank barrel of oil.
2013 H. AlamiNia Reservoir Fluid Properties Course: Reservoir Hydrocarbons (Bo & Bt & Constants) 21
The Concept of the Two-Phase
Formation Volume Factor
2013 H. AlamiNia Reservoir Fluid Properties Course: Reservoir Hydrocarbons (Bo & Bt & Constants) 22
Consider a crude oil sample placed in a PVT cell at
its Pb and reservoir temperature. Assume that the
volume of the oil sample is sufficient to yield one
stock-tank barrel of oil at standard conditions. Let
Rsb represent the gas solubility at Pb
By lowering the cell pressure to p, a portion of the
solution gas is evolved and occupies a certain
volume of the PVT cell. Let Rs and Bo represent the
corresponding gas solubility and oil formation
volume factor at p.
2013 H. AlamiNia Reservoir Fluid Properties Course: Reservoir Hydrocarbons (Bo & Bt & Constants) 23
2013 H. AlamiNia Reservoir Fluid Properties Course: Reservoir Hydrocarbons (Bo & Bt & Constants) 24
PVT Cell
Courtesy IPE, Tehran, 2012
Obviously, the term (Rsb - Rs) represents the
volume of the free gas as measured in scf per stock-
tank barrel of the oil. The volume of the free gas at
the cell conditions is then
The volume of the remaining oil at the cell
condition is ((V) p, T=Bo)
From the definition of the two-phase formation
volume factor
2013 H. AlamiNia Reservoir Fluid Properties Course: Reservoir Hydrocarbons (Bo & Bt & Constants) 25
,
=
Total System Isothermal
Compressibility Coefficient
All solutions of transient fluid-flow problems
contain a parameter called the total system
isothermal compressibility, written as Ct.
This property of the reservoir fluids and the porous
rock is a measure of the change in volume of the
fluid content of porous rock with a change in
pressure, and it may vary considerably with
pressure.
The total system isothermal compressibility is defined
mathematically by the following relationship
2013 H. AlamiNia Reservoir Fluid Properties Course: Reservoir Hydrocarbons (Bo & Bt & Constants) 26
The bubble-point pressure Pb of a hydrocarbon system
is defined as the highest pressure at which a bubble of
gas is first liberated from the oil.
This important property can be measured
experimentally for a crude oil system by conducting a
constant-composition expansion test (i.e., flash
liberation test).
In the absence of the experimentally measured Pb,
Several graphical and mathematical correlations for
determining Pb have been proposed.
These correlations are essentially based on the
assumption that the bubble-point pressure is a strong
function of gas solubility, gas gravity, oil gravity, and
temperature, or Pb = f (Rs, g, API, T)
2013 H. AlamiNia Reservoir Fluid Properties Course: Reservoir Hydrocarbons (Bo & Bt & Constants) 27
Pure Component Property Constants
Many of the physical properties of pure components
have been measured and compiled over the years.
These properties provide essential information for
studying the volumetric behavior and determining the
thermodynamic properties of pure components and
their mixtures. The most important of these properties
are:
Pc, Tc, Vc, Zc, , MW
Pure component property constants are often used as
the basis for models such as corresponding states
correlations for PVT equations of state.
They are often used in composition-dependent mixing
rules for the parameters to describe mixtures.
2013 H. AlamiNia Reservoir Fluid Properties Course: Reservoir Hydrocarbons (Bo & Bt & Constants) 29
. Petroleum engineers are usually interested in the
behavior of hydrocarbon mixtures rather than pure
components.
However, the above characteristic constants of the
pure component can be used with the independent
state variables such as pressure, temperature, and
composition to characterize and define the physical
properties and the phase behavior of mixtures.
2013 H. AlamiNia Reservoir Fluid Properties Course: Reservoir Hydrocarbons (Bo & Bt & Constants) 30
Generalized Correlations for
Estimating
There are numerous correlations for estimating the
physical properties of petroleum fractions. Most of
these correlations use the specific gravity and the
boiling point Tb as correlation parameters.
Selecting proper values for the above parameters is
very important because slight changes in these
parameters can cause significant variations in the
predicted results.
2013 H. AlamiNia Reservoir Fluid Properties Course: Reservoir Hydrocarbons (Bo & Bt & Constants) 31
Riazi-Daubert Generalized Correlations
They developed a simple two-parameter equation
for predicting the physical properties of pure
compounds and undefined hydrocarbon mixtures.
Where: =any physical property, Tb = normal boiling
point, R =specific gravity and a, b, c = correlation
constants are given in Table
2013 H. AlamiNia Reservoir Fluid Properties Course: Reservoir Hydrocarbons (Bo & Bt & Constants) 32
=
Numerous graphical correlations have been proposed
over the years for determining the physical and critical
properties of petroleum fractions. Most of these
correlations use the normal boiling point as one of the
correlation parameters.
There are five different methods of defining the normal
boiling point:
Volume Average Boiling Point (VABP)
Weight Average Boiling Point (WABP)
Molal Average Boiling Point (MABP)
Cubic Average Boiling Point (CABP)
Mean Average Boiling Point (MeABP)
2013 H. AlamiNia Reservoir Fluid Properties Course: Reservoir Hydrocarbons (Bo & Bt & Constants) 33
2013 H. AlamiNia Reservoir Fluid Properties Course: Reservoir Hydrocarbons (Bo & Bt & Constants) 34
Molecular Weight
Figure shows a convenient graphical
correlation for determining the
molecular weight of petroleum
fractions from their mean average
boiling points (MeABP) and API
gravities.
2013 H. AlamiNia Reservoir Fluid Properties Course: Reservoir Hydrocarbons (Bo & Bt & Constants) 35
Critical Temperature
The critical temperature of a
petroleum fraction can be
determined by using the graphical
correlation shown in Figure.
2013 H. AlamiNia Reservoir Fluid Properties Course: Reservoir Hydrocarbons (Bo & Bt & Constants) 36
Critical Pressure
Figure is a graphical correlation of
the critical pressure of the
undefined petroleum fractions as a
function of the mean average
boiling point (MeABP) and the API
gravity.
Pseudo critical constants are necessary if one is to
use most corresponding states correlations to
estimate mixture PVT{y} or derived properties.
However, these pseudo critical constants often differ
considerably from the true critical points for mixtures.
There is Estimation techniques for the latter can be
evaluated by comparison with experimental data.
2013 H. AlamiNia Reservoir Fluid Properties Course: Reservoir Hydrocarbons (Bo & Bt & Constants) 37
1. Tarek, A. (1989). Hydrocarbon Phase Behavior
(Gulf Publishing Company, Houston). Ch2 &
Ch3 & Ch4.
2013 H. AlamiNia Reservoir Fluid Properties Course: Reservoir Hydrocarbons (Bo & Bt & Constants) 38
1. Constant-mass expansion Experiment
2. Constant-Volume Depletion Experiment
3. Differential Liberation Experiment: Procedure
2013 H. AlamiNia Reservoir Fluid Properties Course: Reservoir Hydrocarbons (Bo & Bt & Constants) 39
Вам также может понравиться
- Working Guide to Reservoir Rock Properties and Fluid FlowОт EverandWorking Guide to Reservoir Rock Properties and Fluid FlowРейтинг: 3 из 5 звезд3/5 (1)
- Reservoir-Fluid Properties Course: Properties of Crude Oil SystemsДокумент10 страницReservoir-Fluid Properties Course: Properties of Crude Oil Systemsكهلان البريهيОценок пока нет
- Petroleum Engineering: Principles, Calculations, and WorkflowsОт EverandPetroleum Engineering: Principles, Calculations, and WorkflowsРейтинг: 5 из 5 звезд5/5 (1)
- SC RE Chap6 LiquidsДокумент54 страницыSC RE Chap6 LiquidsweldsvОценок пока нет
- Black Oil Model Fluid PropertiesДокумент48 страницBlack Oil Model Fluid PropertiesCaleb Jonatan Montes VelardeОценок пока нет
- PDPU Lecture PVT SeparatorДокумент37 страницPDPU Lecture PVT Separatorayush gandhiОценок пока нет
- Reservoir Fluid Volumetric Properties: Formation Volume Factors, Solution Gas-Oil RatioДокумент7 страницReservoir Fluid Volumetric Properties: Formation Volume Factors, Solution Gas-Oil RatioSawsen CheboukiОценок пока нет
- Material Balance EquationДокумент10 страницMaterial Balance EquationMurtez100% (1)
- Identification of Pitfalls in PVT Gas Condensate Modeling Using Modified Black-Oil FormulationsДокумент13 страницIdentification of Pitfalls in PVT Gas Condensate Modeling Using Modified Black-Oil FormulationsChávez Ibarra Sergio Kevin100% (1)
- DF3 - PVT DataДокумент10 страницDF3 - PVT Dataadnan0307Оценок пока нет
- PVT - DR M Idrees - Parts 1 and 2Документ49 страницPVT - DR M Idrees - Parts 1 and 2Vigna Ruban RamОценок пока нет
- Properties of Black Oils LectureДокумент21 страницаProperties of Black Oils Lecturemohameda20Оценок пока нет
- Feb 8 WeekДокумент51 страницаFeb 8 WeekMohammad Iqbal Mahamad Amir100% (2)
- PVT Analysis Parameters for Relating Oil Reservoir and Surface VolumesДокумент27 страницPVT Analysis Parameters for Relating Oil Reservoir and Surface Volumesel hadi100% (1)
- Petroleum Reservoirs Engineering Third Stage Assist Prof. Dr. Ahmed K. AlsharaДокумент20 страницPetroleum Reservoirs Engineering Third Stage Assist Prof. Dr. Ahmed K. AlsharaWanambwa SilagiОценок пока нет
- The Oil Compressibility Below Bubble Point Pressure Revisited - Formulations and EstimationsДокумент20 страницThe Oil Compressibility Below Bubble Point Pressure Revisited - Formulations and EstimationsMuezzElerebyОценок пока нет
- UOZ Petroleum Engineering DL Test ReportДокумент20 страницUOZ Petroleum Engineering DL Test ReportShaaban HassanОценок пока нет
- Trisakti 2012 PVT DasarДокумент30 страницTrisakti 2012 PVT DasarJason RojasОценок пока нет
- Phase Behavior Key to Oil ProductionДокумент3 страницыPhase Behavior Key to Oil ProductionDuc TranОценок пока нет
- Material Balance - IAPДокумент205 страницMaterial Balance - IAPBessam MeghdouriОценок пока нет
- Oil Properties - 1Документ13 страницOil Properties - 1Driss EddeniaОценок пока нет
- Properties of Reservoir LiquidsДокумент48 страницProperties of Reservoir Liquidsyimam aliОценок пока нет
- Reservoir EstimationДокумент11 страницReservoir EstimationRizwan FaridОценок пока нет
- Oil Formation Volume Factor - An Overview - ScienceDirect Topics PDFДокумент27 страницOil Formation Volume Factor - An Overview - ScienceDirect Topics PDFVinod KumarОценок пока нет
- 4d - Reservoir Fluid Sampling and PVT Analysis and Rs CorrelationsДокумент23 страницы4d - Reservoir Fluid Sampling and PVT Analysis and Rs CorrelationsTHE TERMINATORОценок пока нет
- PVT ExperimentsДокумент45 страницPVT Experimentsndlr81Оценок пока нет
- Differential Liberation Test PDFДокумент19 страницDifferential Liberation Test PDFSimone SanОценок пока нет
- Black Oil Properties ExplainedДокумент7 страницBlack Oil Properties ExplainedPhuc TruongОценок пока нет
- Res Eng CH 15Документ29 страницRes Eng CH 15weldsvОценок пока нет
- L23 24 Density Compr ViscosityДокумент15 страницL23 24 Density Compr ViscosityLuis Morales CanedoОценок пока нет
- L3-Reservoir Fluids ClassificationДокумент91 страницаL3-Reservoir Fluids ClassificationadeeyoОценок пока нет
- Lecture-08 - Properties of Reservoir Fluid LiquidsДокумент70 страницLecture-08 - Properties of Reservoir Fluid LiquidsJoy Prokash RoyОценок пока нет
- PVT AnalysisДокумент40 страницPVT AnalysisBrian CbtngnОценок пока нет
- MccainДокумент23 страницыMccainCarlos Orley Gil AmayaОценок пока нет
- Advancement of Gas Lift in Production OpДокумент61 страницаAdvancement of Gas Lift in Production OpGODWIN ANYIMAHОценок пока нет
- Reservoir Fluid Properties Course Differential Liberation TestДокумент19 страницReservoir Fluid Properties Course Differential Liberation Testndlr81100% (1)
- Fluid and Rock Properties 2014Документ11 страницFluid and Rock Properties 2014sausmanovОценок пока нет
- Material Balance Estimation For OilДокумент4 страницыMaterial Balance Estimation For OilPraveen Ran SubheОценок пока нет
- Unit 2: Phase Behaviour of Hydrocarbons: Hrishikesh Chavan Hrishikesh - Chavan@mitpune - Edu.inДокумент66 страницUnit 2: Phase Behaviour of Hydrocarbons: Hrishikesh Chavan Hrishikesh - Chavan@mitpune - Edu.inSuvishal ReddyОценок пока нет
- Reservoir Fliud Phase BehavoirДокумент50 страницReservoir Fliud Phase BehavoirBrian OmbogoОценок пока нет
- Classifying Hydrocarbon Reservoirs Based on Phase BehaviorДокумент19 страницClassifying Hydrocarbon Reservoirs Based on Phase BehaviorWanambwa SilagiОценок пока нет
- Thermodynamics 4Документ38 страницThermodynamics 413670319Оценок пока нет
- Differential Liberation - LabДокумент12 страницDifferential Liberation - LabAhmed AmirОценок пока нет
- Reserves Estimation MethodsДокумент20 страницReserves Estimation MethodsRanim HishamОценок пока нет
- Reservoir Fluid Properties Lab TestsДокумент29 страницReservoir Fluid Properties Lab TestssereptОценок пока нет
- Five Fluid PDFДокумент14 страницFive Fluid PDFاحمد ابوبكر اشقيفهОценок пока нет
- SC-RE Chap6-LiquidsДокумент54 страницыSC-RE Chap6-Liquidsb mОценок пока нет
- 2 - Oil PropДокумент38 страниц2 - Oil PropIksan Adityo MulyoОценок пока нет
- Introduction To Reservoir EngineeringДокумент28 страницIntroduction To Reservoir EngineeringChijioke Zion OkabieОценок пока нет
- 4a - Phase Behaviour of Hydrocarbon, Ideal and Non-Ideal SystemДокумент12 страниц4a - Phase Behaviour of Hydrocarbon, Ideal and Non-Ideal SystemTHE TERMINATORОценок пока нет
- Material Balance Method ExplainedДокумент22 страницыMaterial Balance Method ExplainedBrahim LetaiefОценок пока нет
- SPE 77386 Analysis of Black Oil PVT Reports Revisited: B B B at P PДокумент5 страницSPE 77386 Analysis of Black Oil PVT Reports Revisited: B B B at P PJuan MartinezОценок пока нет
- Black Oil DefinitionsДокумент21 страницаBlack Oil Definitionsmanish.7417Оценок пока нет
- NB (R R) B (W WB) N (1 m) (S c c) p B B B mB 1 B B (1 S) ⎡ ⎤ + − − − ⎣ ⎦ = ⎛ ⎞ + + Δ − + − + ⎜ ⎟ ⎜ ⎟ − ⎝ ⎠Документ3 страницыNB (R R) B (W WB) N (1 m) (S c c) p B B B mB 1 B B (1 S) ⎡ ⎤ + − − − ⎣ ⎦ = ⎛ ⎞ + + Δ − + − + ⎜ ⎟ ⎜ ⎟ − ⎝ ⎠Ali HijaziОценок пока нет
- Reservoir Engineering I: Crude Oil Properties GuideДокумент25 страницReservoir Engineering I: Crude Oil Properties GuideJoseph YepezОценок пока нет
- Properties of Crude Oil SystemsДокумент32 страницыProperties of Crude Oil SystemsEl CoolОценок пока нет
- Abdullwahid Ahmed EXPДокумент14 страницAbdullwahid Ahmed EXPAbdullwahid AhmedОценок пока нет
- Differential Liberation TestДокумент5 страницDifferential Liberation Testtahsim laptopОценок пока нет
- PVT Experiments: Differential Liberation:: Reservoir Fluid Properties LabДокумент8 страницPVT Experiments: Differential Liberation:: Reservoir Fluid Properties LabMohammed MohammedОценок пока нет
- VALVES - Are Used To Control Flow, Pressure, and Direction ofДокумент3 страницыVALVES - Are Used To Control Flow, Pressure, and Direction ofJohari Valiao AliОценок пока нет
- Basic Artificial LiftДокумент9 страницBasic Artificial LiftlabrujisОценок пока нет
- 12 Drilling PreventersДокумент82 страницы12 Drilling PreventersDavid Kusuma100% (1)
- RazorДокумент3 страницыRazorB Deyse FernandesОценок пока нет
- We Work: Worldwide Petroleum Exploration and Engineering ServicesДокумент8 страницWe Work: Worldwide Petroleum Exploration and Engineering ServicesB Deyse FernandesОценок пока нет
- PERFORATING GUN INCIDENT ON NOBLE PIET VAN EDE RIGДокумент5 страницPERFORATING GUN INCIDENT ON NOBLE PIET VAN EDE RIGB Deyse FernandesОценок пока нет
- Casing Design ExampleДокумент43 страницыCasing Design ExampleSug E QalanderОценок пока нет
- We Work: Worldwide Petroleum Exploration and Engineering ServicesДокумент8 страницWe Work: Worldwide Petroleum Exploration and Engineering ServicesB Deyse FernandesОценок пока нет
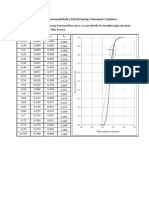
- A) by Preparing The Following Fractional Flow Curve, We Can Identify The Breakthrough Saturation As 0.6 (Indicated With Blue Arrows)Документ2 страницыA) by Preparing The Following Fractional Flow Curve, We Can Identify The Breakthrough Saturation As 0.6 (Indicated With Blue Arrows)B Deyse FernandesОценок пока нет