Академический Документы
Профессиональный Документы
Культура Документы
Ovarian Germ Cell Neoplasms
Загружено:
Ralt MedИсходное описание:
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
Ovarian Germ Cell Neoplasms
Загружено:
Ralt MedАвторское право:
Доступные форматы
28/5/2014 Ovarian germ cell neoplasms: Pathology, clinical manifestations, and diagnosis
file:///C:/Users/Ralt/Medicina%20Humana/Libros/Cl%EDnicas/Internal%20Medicine%20-%20Medicina%20Interna/Libros%20de%20Referencia%20de% 1/12
Ovarian germ cell neoplasms: Pathology, clinical manifestations, and diagnosis
TOPIC OUTLINE
SUMMARY & RECOMMENDATIONS
INTRODUCTION
OVERVIEW OF OVARIAN GERM CELL TUMORS
Histopathology overview
Epidemiology
Clinical manifestations overview
Diagnosis overview
- Tumor markers
Staging and surgical treatment
TERATOMAS
Mature cystic teratoma (dermoid cyst)
- Histopathology
- Clinical manifestations
- Diagnosis
- Treatment
- Malignant transformation
- Monodermal highly specialized teratomas
Mature solid teratoma
Immature teratoma
- Histopathology
- Clinical manifestations
DYSGERMINOMA
Histopathology
Clinical manifestations
ENDODERMAL SINUS (YOLK SAC) TUMOR
Histopathology
Clinical manifestations
EMBRYONAL CARCINOMA
Histopathology
Clinical manifestations
MIXED GERM CELL NEOPLASMS
POLYEMBRYOMA
CHORIOCARCINOMA
SUMMARY AND RECOMMENDATIONS
REFERENCES
GRAPHICS View All
FIGURES
Origins ovarian tumors
28/5/2014 Ovarian germ cell neoplasms: Pathology, clinical manifestations, and diagnosis
file:///C:/Users/Ralt/Medicina%20Humana/Libros/Cl%EDnicas/Internal%20Medicine%20-%20Medicina%20Interna/Libros%20de%20Referencia%20de% 2/12
Tryptophan and serotonin metabolism
PICTURES
Mature teratoma
Dysgerminoma histo
Dysgerminoma
Endodermal sinus tumor histo
Schiller-Duval body
TABLES
Types of germ cell neoplasms
Frequency malignant OGCNs
Ovarian tumor markers
Staging ovarian peritoneal carcinoma
Carcinoid symptoms
RELATED TOPICS
Anatomy and pathology of testicular tumors
Clinical characteristics of carcinoid tumors
Clinical features of the carcinoid syndrome
Clinical manifestations and diagnosis of Turner syndrome (gonadal dysgenesis)
Diagnosis of the carcinoid syndrome and tumor localization
Epithelial carcinoma of the ovary, fallopian tube, and peritoneum: Clinical features and diagnosis
Gestational trophoblastic disease: Pathology
Oophorectomy and ovarian cystectomy
Paraneoplastic and autoimmune encephalitis
Sonographic differentiation of benign versus malignant adnexal masses
Struma ovarii
Treatment of malignant germ cell tumors of the ovary
UpToDate
Official reprint from UpToDate
www.uptodate.com
2013 UpToDate
Print | Back
Ovarian germ cell neoplasms: Pathology, clinical manifestations, and diagnosis
Author
David M Gershenson, MD
Section Editors
28/5/2014 Ovarian germ cell neoplasms: Pathology, clinical manifestations, and diagnosis
file:///C:/Users/Ralt/Medicina%20Humana/Libros/Cl%EDnicas/Internal%20Medicine%20-%20Medicina%20Interna/Libros%20de%20Referencia%20de% 3/12
Barbara Goff, MD
Alberto S Pappo, MD
Rochelle L Garcia, MD
Deputy Editor
Sandy J Falk, MD
Disclosures
All topics are updated as new evidence becomes available and our peer review process is complete.
Literature review current through: Mar 2013. | This topic last updated: Aug 8, 2012.
INTRODUCTION Ovarian germ cell neoplasms are derived from primordial germ cells of the ovary (
figure 1 ). They may be benign or malignant. These neoplasms comprise approximately 20 to 25 percent of
ovarian neoplasms overall, but account for only about 5 percent of all malignant ovarian neoplasms [ 1-3 ].
Ovarian germ cell neoplasms arise primarily in young women between 10 and 30 years of age and represent
70 percent of ovarian neoplasms in this age group [ 4 ].
The pathology, clinical manifestations, and diagnosis of ovarian germ cell neoplasms are reviewed here.
Treatment of malignant germ cell neoplasms of the ovary as well epithelial ovarian carcinoma is discussed
separately. (See "Treatment of malignant germ cell tumors of the ovary" and "Epithelial carcinoma of the
ovary, fallopian tube, and peritoneum: Clinical features and diagnosis" .)
OVERVIEW OF OVARIAN GERM CELL TUMORS
Histopathology overview The histological types of ovarian germ cell neoplasms (OGCNs) that arise from
the ovary are similar to those developing in the testes of men ( table 1 ) [ 2,5 ]. (See "Anatomy and pathology
of testicular tumors" .)
Ovarian germ cell neoplasms can be broadly divided into those that differentiate towards embryo-like
neoplasms (teratomas and their subtypes and dysgerminomas) and those that differentiate primarily toward
extraembryonic fetal-derived (placenta-like) cell populations or a mixture of both. Categories include:
Teratomas
Benign cystic mature teratomas (dermoid cysts) are the most common OGCNs. Some malignant
OGCNs develop when components of dermoid cysts develop into a somatic malignant neoplasm
(termed mature cystic teratoma with malignant degeneration).
Immature teratomas
Dysgerminomas These are the female version of the male seminoma and are essentially comprised
of immature germ cells. (See "Anatomy and pathology of testicular tumors" .)
Endodermal sinus (yolk sac) tumors These are carcinomas (epithelial neoplasms) that differentiate
toward yolk sac/primitive placenta forms.
Mixed germ cell tumors These are typically combinations of a teratoma with yolk sac,
dysgerminoma, and/or embryonal carcinoma.
Rare OGCNs pure embryonal carcinomas, nongestational choriocarcinomas, and pure
polyembryoma
Among malignant OGCNs, dysgerminoma, immature teratoma, yolk sac tumors, and mixed germ cell
neoplasms account for 90 percent of cases [ 2,3 ]. Pure embryonal carcinomas and nongestational
choriocarcinomas are rare, and pure polyembryomas are very rare.
A study of findings from a United States national cancer database from 1973 to 2002 reported 1262
28/5/2014 Ovarian germ cell neoplasms: Pathology, clinical manifestations, and diagnosis
file:///C:/Users/Ralt/Medicina%20Humana/Libros/Cl%EDnicas/Internal%20Medicine%20-%20Medicina%20Interna/Libros%20de%20Referencia%20de% 4/12
malignant OCGNs [ 6 ]. Incidence by histology was: pure dysgerminomas (39 percent); teratomas, immature
plus mature with malignant transformation (39 percent); and nondysgerminoma or mixed cell types (29
percent) ( table 2 ).
OGCNs grow rapidly, unlike the more common epithelial ovarian neoplasms, yet most patients present with
stage IA disease (limited to one ovary). Evidence of bilateral ovarian involvement suggests the presence of a
tumor with a propensity for involvement of the contralateral ovary, including benign cystic teratoma,
dysgerminoma, or a tumor with components of dysgerminoma (mixed germ cell tumor). These conditions are
bilateral in 10 to 12 percent of cases, while the majority of other histologies present as unilateral ovarian
masses [ 7 ].
Epidemiology OGCNs arise primarily in young women between 10 and 30 years of age; they represent
70 percent of ovarian neoplasms in this age group [ 4 ]. For unclear reasons, malignant OGCNs occur more
frequently among Asian/Pacific Islander and Hispanic women than Caucasians [ 6 ].
Clinical manifestations overview OGCNs often produce hormones, particularly the beta subunit of human
chorionic gonadotropin (hCG). Patients typically present with one or more of the following signs and
symptoms:
Abdominal enlargement from the mass itself, ascites, or both
Abdominal pain from rupture or torsion
Precocious puberty, abnormal vaginal bleeding presumably from hCG production
Symptoms of pregnancy from hCG production
Eighty-five percent of women with an OGCN have both abdominal pain and an abdominal mass; fever or
vaginal bleeding occurs in 10 percent. OGCNs tend to be large (median size 16 cm). Ascites, rupture (pre-
or intraoperative), and torsion are reported in 20, 20, and 5 percent of cases, respectively.
Diagnosis overview The diagnosis is made by histology at time of surgical excision. The diagnosis is
strongly suggested preoperatively by the presence of an adnexal mass on pelvic imaging and an elevated level
of an associated tumor marker (eg, hCG, alpha fetoprotein [AFP]).
For benign cystic mature teratomas, the diagnosis be made with reasonable confidence using pelvic
ultrasonography; however, removal of the cyst is still advised (see 'Mature cystic teratoma (dermoid cyst)'
below).
Tumor markers OGCNs are often associated with hormonal or enzymatic activity. Some of these
proteins can be measured in the serum, providing a highly sensitive and variably specific marker for the
presence of certain histologic components ( table 3 ). Some tumor markers are present in some, but not all
tumors of a specific histology. Tumor markers produced by tumors types are as follows:
hCG embryonal cell carcinomas, and ovarian choriocarcinomas, mixed germ cell tumors, and some
dysgerminomas
AFP endodermal sinus tumors, embryonal cell carcinomas and polyembryoma carcinomas, mixed
germ cell tumors, and some immature teratomas [ 8,9 ]; most dysgerminomas are associated with a
normal AFP.
Lactate dehydrogenase (LDH) dysgerminomas
Staging and surgical treatment Malignant germ cell neoplasms are staged according to the International
Federation of Gynecology and Obstetrics (FIGO) staging system for epithelial ovarian cancer ( table 4 ) [ 10
]. In brief, stage I disease is confined to the ovaries; stage II includes extension into other pelvic tissues; stage
28/5/2014 Ovarian germ cell neoplasms: Pathology, clinical manifestations, and diagnosis
file:///C:/Users/Ralt/Medicina%20Humana/Libros/Cl%EDnicas/Internal%20Medicine%20-%20Medicina%20Interna/Libros%20de%20Referencia%20de% 5/12
III refers to disease that has spread beyond the pelvis or to retroperitoneal lymph nodes but remains in the
abdomen; and stage IV refers to the presence of distant metastasis or involvement of liver parenchyma.
In virtually all cases, surgery is required for definitive histological diagnosis, treatment, and staging (if
malignant) of OGCNs. Oophorectomy, ovarian cystectomy, or resection of the ovarian mass can be
performed, depending on the clinical situation, and tissue sent for frozen section. Confirmation of the diagnosis
should be obtained prior to definitive surgical treatment.
The following sections review the pathology and clinical manifestations of the individual types of OGCNs.
Treatment of benign OGCNs is also described below, while management of malignant OGCNs is discussed
separately. (See "Treatment of malignant germ cell tumors of the ovary" .)
TERATOMAS Teratomas are the most common type of germ cell neoplasm. Most, but not all,
teratomas are benign. The designation teratoma refers to a neoplasm that differentiates toward somatic-type
cell populations (typically including cell populations that would normally derive from ectoderm, endoderm,
and mesoderm) that can be typical of either adult or embryonic development. The component tissues in a
teratoma range from immature to well differentiated, and are foreign to the anatomic site in which they are
found.
Teratomas are divided into four categories: mature (cystic or solid, benign), immature (malignant), malignant
due to a component of another somatic malignant neoplasm, and monodermal or highly specialized.
Mature cystic teratoma (dermoid cyst) Most teratomas are cystic and composed of mature differentiated
(adult); they are better known as dermoid cysts. The mature cystic teratoma accounts for more than 95
percent of all ovarian teratomas and is almost invariably benign [ 11 ]. Dermoid cysts are the most common
ovarian tumor in women in the second and third decade of life.
Histopathology Mature cystic teratomas contain mature tissue of ectodermal (eg, skin, hair follicles,
sebaceous glands), mesodermal (eg, muscle, urinary), and endodermal origin (eg, lung, gastrointestinal) [ 12 ].
The mechanism by which these cysts develop is possibly by failure of meiosis II or from a premeiotic cell in
which meiosis I has failed [ 13 ]. They are bilateral in 10 to 17 percent of cases [ 14 ].
The characteristic macroscopic appearance of benign cystic teratomas is a multicystic mass that contains hair,
teeth, and/or skin that is mixed into sebaceous, thick, sticky, and often foul-smelling material ( picture 1 ). A
solid prominence (Rokitansky's protuberance) is located at the junction between the teratoma and normal
ovarian tissue [ 2 ]. The greatest cellular variety is found in the area of this junction, which should therefore be
examined carefully by the pathologist to exclude immature/malignant components.
Clinical manifestations Most women with dermoid cysts are asymptomatic. If present, symptoms depend
upon the size of the mass. Torsion is not uncommon. Rupture of dermoid cysts with spillage of sebaceous
material into the abdominal cavity can occur, but is uncommon. Shock and hemorrhage are the immediate
sequelae of rupture; a marked granulomatous reaction (chemical peritonitis) may subsequently develop and
lead to formation of dense adhesions.
A rare condition associated with either mature or immature teratomas is Anti-N-methyl-D-aspartate
(NMDA) receptor encephalitis [ 15 ]. (See "Paraneoplastic and autoimmune encephalitis", section on 'Anti-
NMDA receptor encephalitis' .)
Diagnosis These tumors have a characteristic ultrasound appearance, which allows reasonably accurate
noninvasive diagnosis in many cases [ 16 ]. The reported specificity is 98 to 100 percent [ 16,17 ]. Definitive
diagnosis is made at the time of surgical excision. (See "Sonographic differentiation of benign versus malignant
28/5/2014 Ovarian germ cell neoplasms: Pathology, clinical manifestations, and diagnosis
file:///C:/Users/Ralt/Medicina%20Humana/Libros/Cl%EDnicas/Internal%20Medicine%20-%20Medicina%20Interna/Libros%20de%20Referencia%20de% 6/12
adnexal masses" .)
Treatment Ovarian cystectomy is suggested in order to make a definitive diagnosis, preserve ovarian
tissue, and avoid potential problems such as torsion, rupture, or development of malignant components. For
women who have completed childbearing, salpingo-oophorectomy is also acceptable treatment. Benign cystic
teratomas do not recur if surgically resected.
Dermoid cysts may be removed via either laparoscopy or laparotomy. With either approach, the abdomen
should be copiously irrigated to avoid a chemical peritonitis from spillage of the sebaceous cyst fluid. (See
"Oophorectomy and ovarian cystectomy", section on 'Laparoscopic cystectomy' .)
Malignant transformation Malignant transformation occurs in 0.2 to 2 percent of mature cystic teratomas [
18-20 ]. Mature teratomas with malignant transformation comprise 2.9 percent of all malignant OGCNs (
table 2 ) [ 6 ]. Although any of the components of a mature cystic teratoma may undergo malignant
degeneration, squamous cell carcinoma arising from the ectoderm is the most common secondary neoplasm [
14,21 ].
Risk factors for malignant neoplasm in a mature cystic teratoma include age over 45 years (mean age 50
years versus 33 years for benign teratomas), tumor diameter greater than 10 cm, rapid growth, and findings
on imaging (eg, low resistance intra-tumor flow on Doppler) [ 14,21 ].
Other possible malignant neoplasms include (but are not limited to) basal cell carcinoma, melanoma,
adenocarcinoma, sarcoma, and thyroid carcinoma. When malignant transformation has occurred within a
teratoma, treatment must be tailored to the transformed histology.
Monodermal highly specialized teratomas The specialized or monodermal teratomas are a rare and
remarkable subset of teratomas that consist of a predominant mature histologic cell type, the most common of
which are struma ovarii and carcinoid, a well-differentiated neuroendocrine neoplasm. They are usually
unilateral, although a contralateral teratoma may be present.
Struma ovarii Struma ovarii is a benign teratoma predominantly composed of mature thyroid tissue [
12 ]. The secretion of thyroid hormones results in clinical hyperthyroidism in 25 to 35 percent of
patients. (See "Struma ovarii" .)
Carcinoid neoplasms Ovarian carcinoid neoplasms are rare [ 22 ]. Primary ovarian carcinoid
neoplasms are usually unilateral, localized to the ovary, and indistinguishable histologically from
metastasis. They have similar appearances to those that arise in any other site (eg, gastrointestinal or
respiratory). They are comprised of nests and cords of relatively bland cells with endocrine features
and a fine vascular network. Some carcinoid neoplasms secrete bioactive polypeptides and amines,
producing a constellation of symptoms, predominantly flushing and diarrhea ( table 5 ). Carcinoid
syndrome develops in about one-third of cases, and it can develop without hepatic metastases due to
direct venous drainage from the ovary into the systemic circulation. (See "Clinical characteristics of
carcinoid tumors", section on 'Ovary' and "Clinical features of the carcinoid syndrome", section on
'Clinical features' .)
5-hydroxyindoleacetic acid, a metabolite of serotonin ( figure 2 ), is excreted in the urine and can be
used to confirm the diagnosis of carcinoid syndrome, and as a marker of disease activity in patients
with advanced disease or the carcinoid syndrome. (See "Diagnosis of the carcinoid syndrome and
tumor localization", section on 'Biochemical testing for the carcinoid syndrome' .)
Carcinoid tumors metastatic to the ovary are even more rare; they tend to be bilateral and arise from
28/5/2014 Ovarian germ cell neoplasms: Pathology, clinical manifestations, and diagnosis
file:///C:/Users/Ralt/Medicina%20Humana/Libros/Cl%EDnicas/Internal%20Medicine%20-%20Medicina%20Interna/Libros%20de%20Referencia%20de% 7/12
primary ileal carcinoid tumors [ 23 ]. In such cases, disseminated abdominal disease is common.
Mixed struma ovarii and carcinoid The presence of a mixed struma ovarii and carcinoid is even
more rare. These lesions usually follow a benign course.
Mature solid teratoma In rare instances, a teratoma is solid but is composed entirely of benign-appearing
heterogeneous collections of tissue and organized structures derived from all three cell layers. Most mature
solid teratomas are unilateral and benign, although peritoneal implants have been described. Grossly, it may
be difficult/impossible to differentiate these neoplasms from malignant solid immature teratomas, which are
almost always solid and they therefore require liberal sampling (see 'Immature teratoma' below). Management
is as described above for mature cystic teratomas (see 'Treatment' above).
Immature teratoma Immature teratomas are also called malignant teratoma, teratoblastoma, or embryonal
teratoma [ 12 ]. They comprise less than 1 percent of ovarian teratomas and are most common in the first
two decades of life. They comprise 35.6 percent of all malignant OGCNs ( table 2 ) [ 6 ].
Histopathology These neoplasms are typically composed of tissue from the three germ cell layers:
ectoderm, mesoderm, and endoderm, arranged in a haphazard manner. Histologically, there are varying
amounts of immature tissue, most frequently with neural differentiation, although immature stromal elements
can also be present.
Immature teratomas are the only OGCN that are histologically graded. The grade of differentiation (ranging
from I [well differentiated] to III [poorly differentiated]) is based upon the proportion of tissue in histologic
sections containing immature neural elements [ 24 ]. Grade is an important indicator of the risk for
extraovarian spread. The presence of foci of yolk sac tumor in immature teratomas generally reflects more
aggressive behavior and a worse outcome [ 25 ].
Clinical manifestations The clinical presentation is similar to that of other OGCNs (incidentally discovered
adnexal mass, abdominal enlargement or pain). In some cases, AFP or LDH may be elevated.
A rare condition associated with either mature or immature teratomas is anti-N-methyl-D-aspartate (NMDA)
receptor encephalitis. (See "Paraneoplastic and autoimmune encephalitis", section on 'Anti-NMDA receptor
encephalitis' .)
Treatment is discussed in detail separately. (See "Treatment of malignant germ cell tumors of the ovary" .)
DYSGERMINOMA Although dysgerminomas are relatively uncommon among all ovarian neoplasms
(accounting for only about 2 percent), they account for 32.8 percent of malignant OGCNs ( table 2 ) [ 6 ].
The majority of cases (75 percent) arise in adolescents and young adults, in whom they account for about
one-third of all ovarian malignant neoplasms [ 26 ]. Because of their predilection for young women, they are
one of the more common ovarian malignant neoplasms detected during pregnancy. Nevertheless,
dysgerminoma can occur at any age; case reports have described patients with dysgerminoma between 7
months and 70 years of age.
Histopathology Although dysgerminomas are considered to be malignant, the degree of histologic atypia
is variable, and only about one-third behave aggressively. The neoplasm is composed of undifferentiated germ
cells, large vesicular cells with clear cytoplasm, well-defined cell boundaries, and centrally placed regular
nuclei; the overall appearance is sometimes described as resembling "fried eggs" ( picture 2 ). The stroma is
infiltrated by clusters of small lymphocytes and frequently contains granulomas. Dysgerminoma is the ovarian
counterpart of testicular seminoma; histologically it has a similar appearance. (See "Anatomy and pathology of
testicular tumors" .)
28/5/2014 Ovarian germ cell neoplasms: Pathology, clinical manifestations, and diagnosis
file:///C:/Users/Ralt/Medicina%20Humana/Libros/Cl%EDnicas/Internal%20Medicine%20-%20Medicina%20Interna/Libros%20de%20Referencia%20de% 8/12
Grossly, dysgerminoma appears as a lobulated mass that is firm and cream colored or pale tan ( picture 3 ) [
2 ].
Dysgerminomas may develop within a gonadoblastoma (a benign or in situ germ cell ovarian neoplasm
composed of germ cells and sex cord stroma) in phenotypic females who have a Y chromosome. Included in
this group are patients with pure gonadal dysgenesis 46XY, mixed gonadal dysgenesis 45X/46XY, or
complete androgen insensitivity (formerly called testicular feminization) 46XY. Occasional patients may have
stigmata of Turner syndrome. These latter patients may have a 45X, 45X/46XX, or 45X/46XY karyotype.
(See "Clinical manifestations and diagnosis of Turner syndrome (gonadal dysgenesis)", section on 'Risk of
malignancy' .)
The first two patients with unique gonadal tumors were described in 1953, at which time the term
gonadoblastoma was introduced [ 27 ]. These tumors may produce either testosterone or estrogens. Clinical
presentation may include developmental abnormalities of the genitalia, primary amenorrhea, or virilization.
Although gonadoblastoma may be overgrown by dysgerminoma, as occurs in approximately 50 percent of
cases, other malignant germ cell components may predominate, including yolk sac tumor, immature teratoma,
embryonal carcinoma, or choriocarcinoma. Thus, karyotyping is recommended for all patients with the
operative finding of gonadoblastoma, with or without a coexistent malignant germ cell tumor, or young
patients who present with an ovarian mass or masses and either primary amenorrhea or abnormalities of the
genitalia.
It is important to determine whether a gonadoblastoma is present, since oophorectomy should be performed
in these patients to prevent the development of gonadal neoplasia, although the age at which the procedure is
performed depends upon the underlying etiology [ 28,29 ]. Women at risk for having dysgenetic gonads
ideally should be identified preoperatively; frozen section is not reliable.
Clinical manifestations The growth of dysgerminomas is usually rapid; as a result, patients often present
with abdominal enlargement and pain due to rupture with hemoperitoneum or torsion. Menstrual abnormalities
may occur if the tumor is hormonally active.
Dysgerminomas can contain syncytiotrophoblastic giant cells that produce placental alkaline phosphatase, and
lactate dehydrogenase (LDH) [ 30,31 ]. Serial measurements of these markers can be useful for monitoring
disease ( table 3 ). In addition, 3 to 5 percent of dysgerminomas produce human chorionic gonadotropin
(hCG). In general, dysgerminomas do not produce alpha-fetoprotein (AFP), although borderline elevations
(<16 ng/mL) are described in case series, but most often in the setting of mixed germ cell tumors that contain
a yolk sac element.
Seventy-five percent of women with dysgerminomas present with stage I disease ( table 4 ); the contralateral
ovary is involved in 10 to 15 percent [ 32,33 ]. Bilateral ovarian disease is more common with dysgerminoma
than with any other malignant OGCN.
Surgery is performed for definitive diagnosis, staging, and initial treatment. For a unilateral neoplasm confined
to the ovary without capsular involvement or rupture (stage IC), simple salpingo-oophorectomy is curative in
over 95 percent. Treatment is discussed in detail separately. (See "Treatment of malignant germ cell tumors of
the ovary" .)
ENDODERMAL SINUS (YOLK SAC) TUMOR Endodermal sinus tumors (also called yolk sac
tumors) make up 14 to 20 percent of all malignant OGCNs ( table 2 ) [ 6,34 ]. The name was chosen
because the tumor structure is similar to that of the endodermal sinuses of the rat yolk sac and is derived from
the primitive yolk sac. These neoplasms usually occur in young girls and women; the median age at
28/5/2014 Ovarian germ cell neoplasms: Pathology, clinical manifestations, and diagnosis
file:///C:/Users/Ralt/Medicina%20Humana/Libros/Cl%EDnicas/Internal%20Medicine%20-%20Medicina%20Interna/Libros%20de%20Referencia%20de% 9/12
presentation is 23 years and one-third of patients are premenarchal [ 35,36 ].
Histopathology Histologically, these epithelial neoplasms consist of tubules or spaces lined by single layers
of flattened cuboidal cells, reticular stroma, and scattered globules ( picture 4 ) [ 37 ]. Invaginated papillary
structures with a central vessel (Schiller-Duval bodies) are found within some of the spaces ( picture 5 ) [ 2 ].
Clinical manifestations Patients with endodermal sinus tumors often present with abdominal pain and a
pelvic mass, similar to dysgerminomas. The pain may be acute and is commonly misdiagnosed as
appendicitis.
Tumor growth can be very rapid and aggressive with extensive intraperitoneal dissemination. Serum AFP
levels are elevated in a significant number of patients and, if elevated, are useful for monitoring the response to
treatment and for post-treatment surveillance [ 38,39 ]. Serum LDH may also be elevated [ 40 ].
Treatment of endodermal sinus tumors is discussed in detail elsewhere.
EMBRYONAL CARCINOMA Embryonal carcinoma accounts for 4 percent of malignant OGCNs (
table 2 ) [ 6,41 ]. It resembles the more common embryonal carcinoma of the testis and is one of the most
aggressive ovarian malignant neoplasms. The average age at diagnosis is 15 years. (See "Anatomy and
pathology of testicular tumors" .)
Histopathology Histologically, this neoplasm is epithelial and therefore forms nests and may form papillary
or gland-like structures. Many atypical mitotic figures are usually present, reflecting the high proliferative
activity of the neoplastic cells. Multinucleated giant cells resembling syncytial cells may be present; these are
the cells that produce hCG [ 12 ].
Clinical manifestations Patients with embryonal carcinoma usually present with an abdominal or pelvic
mass and abdominal pain [ 42 ]. Most of these neoplasms produce hCG, while some also make AFP ( table
3 ).
Treatment is discussed separately. (See "Treatment of malignant germ cell tumors of the ovary" .)
MIXED GERM CELL NEOPLASMS Mixed germ cell neoplasms consist of two or more admixed
types of ovarian germ cell neoplasms. They account for 5.3 percent of all malignant OGCNs ( table 2 ) [ 6 ].
Components of dysgerminoma mixed with an endodermal sinus tumor are found most commonly. In cases in
which a dysgerminoma component is present, the contralateral ovary is involved 10 percent of the time. The
neoplasms may secrete tumor markers, such as LDH, AFP, or hCG, depending upon the type of tissue
present.
Treatment is discussed separately. (See "Treatment of malignant germ cell tumors of the ovary" .)
POLYEMBRYOMA Polyembryoma is composed of embryoid bodies that morphologically resemble
normal embryos [ 2 ]. This malignant germ cell neoplasms is very rare and, in most instances, is associated
with other germ cell elements such as immature teratoma. It usually occurs in young girls and may present with
signs of pseudopuberty.
Polyembryoma is a very aggressive tumor with extensive local infiltration and distant metastasis [ 43 ]. Serum
hCG and AFP concentrations may be elevated.
Treatment is discussed separately. (See "Treatment of malignant germ cell tumors of the ovary" .)
28/5/2014 Ovarian germ cell neoplasms: Pathology, clinical manifestations, and diagnosis
file:///C:/Users/Ralt/Medicina%20Humana/Libros/Cl%EDnicas/Internal%20Medicine%20-%20Medicina%20Interna/Libros%20de%20Referencia%20de 10/12
CHORIOCARCINOMA Non-gestational choriocarcinoma is a rare and highly malignant type of
OGCN [ 44 ]. Choriocarcinomas are more commonly of placental than ovarian origin; the estimated
incidence of a primary ovarian choriocarcinoma is 1 in 369,000,000. They comprise 2.1 percent of all
malignant OGCNs ( table 2 ) [ 6 ]. A choriocarcinoma of ovarian origin derives from an extraembryonic
differentiation of malignant germ cells. This highly malignant germ cell epithelial neoplasm differentiates
towards trophoblastic structures and often contains other malignant germ cell elements.
Non-gestational ovarian choriocarcinoma is histologically identical to primary gestational choriocarcinoma
associated with pregnancy [ 45-47 ]. The two entities can be distinguished by DNA analysis; the presence of
paternal DNA within the tumor indicates a gestational (placental) origin [ 48 ]. (See "Gestational trophoblastic
disease: Pathology", section on 'Choriocarcinoma' .)
All choriocarcinomas produce hCG, which may cause isosexual precocity in young girls and irregular vaginal
bleeding of uterine origin. Serum levels of hCG are useful for monitoring response to treatment.
Like gestational choriocarcinomas, those arising in the ovary tend to develop early hematogenous metastasis
to several different sites, including lung, liver, brain, bone, vagina, and other viscera. In contrast to gestational
choriocarcinomas, those arising in the ovary are relatively chemoresistant.
Treatment is discussed separately.
SUMMARY AND RECOMMENDATIONS
Ovarian germ cell neoplasms are derived from primordial germ cells of the ovary ( figure 1 ). They may
be benign or malignant. These neoplasms comprise approximately 20 to 25 percent of ovarian
neoplasms overall, but account for only about 5 percent of all malignant ovarian neoplasms. (See
'Introduction' above.)
Ovarian germ cell neoplasms arise primarily in young women between 10 and 30 years of age; they
represent 70 percent of ovarian neoplasms in this age group. (See 'Introduction' above.)
Ovarian germ cell neoplasms often produce tumor markers ( table 3 ). (See 'Tumor markers' above.)
The most common ovarian germ cell neoplasm is the benign mature cystic teratoma (dermoid cyst),
which can be bilateral. Approximately 1 percent contain a secondary malignancy arising from one of
the components, usually a squamous cell cancer. Ovarian cystectomy or oophorectomy provides
definitive diagnosis and treatment (see 'Mature cystic teratoma (dermoid cyst)' above).
Dysgerminoma is the most common malignant ovarian germ cell neoplasm. Bilateral ovarian disease is
more common than with any other ovarian germ cell neoplasm. These neoplasms are less likely to
produce tumor markers than other malignant germ cell neoplasms ( table 3 ), but lactic dehydrogenase
is often elevated (see 'Dysgerminoma' above).
Use of UpToDate is subject to the Subscription and License Agreement .
REFERENCES
1. Sagae S, Kudo R. Surgery for germ cell tumors. Semin Surg Oncol 2000; 19:76.
2. Talerman A. Germ cell tumours of the ovary. In: Blaustein's Pathology of the Female Genital Tract,
Kurman RJ (Ed), Springer Verlag, New York 1994. p.849.
3. Tewari K, Cappuccini F, Disaia PJ, et al. Malignant germ cell tumors of the ovary. Obstet Gynecol
2000; 95:128.
4. Zalel Y, Piura B, Elchalal U, et al. Diagnosis and management of malignant germ cell ovarian tumors in
28/5/2014 Ovarian germ cell neoplasms: Pathology, clinical manifestations, and diagnosis
file:///C:/Users/Ralt/Medicina%20Humana/Libros/Cl%EDnicas/Internal%20Medicine%20-%20Medicina%20Interna/Libros%20de%20Referencia%20de 11/12
young females. Int J Gynaecol Obstet 1996; 55:1.
5. Serov SF, Scully RE, Sobin LJ. Histological typing of ovarian tumors. In: International Histological
Classification of Tumors, World Health Organization, Geneva 1973.
6. Smith HO, Berwick M, Verschraegen CF, et al. Incidence and survival rates for female malignant
germ cell tumors. Obstet Gynecol 2006; 107:1075.
7. Pectasides D, Pectasides E, Kassanos D. Germ cell tumors of the ovary. Cancer Treat Rev 2008;
34:427.
8. Ihara T, Ohama K, Satoh H, et al. Histologic grade and karyotype of immature teratoma of the ovary.
Cancer 1984; 54:2988.
9. Mann JR, Raafat F, Robinson K, et al. The United Kingdom Children's Cancer Study Group's second
germ cell tumor study: carboplatin, etoposide, and bleomycin are effective treatment for children with
malignant extracranial germ cell tumors, with acceptable toxicity. J Clin Oncol 2000; 18:3809.
10. Berek JS. Epithelial ovarian cancer. In: Practical Gynecologic Oncology, 4th, Berek JS, Hacker NF
(Eds), Lippincott Williams & Wilkins, Philadelphia 2005. p.443.
11. Ayhan A, Bukulmez O, Genc C, et al. Mature cystic teratomas of the ovary: case series from one
institution over 34 years. Eur J Obstet Gynecol Reprod Biol 2000; 88:153.
12. DiSaia PJ, Creasman WT. Germ cell, stromal and other ovarian tumors. In: Clinical Gynecologic
Oncology, 7th, Mosby-Elsevier, 2007. p.381.
13. Caspi B, Lerner-Geva L, Dahan M, et al. A possible genetic factor in the pathogenesis of ovarian
dermoid cysts. Gynecol Obstet Invest 2003; 56:203.
14. Hackethal A, Brueggmann D, Bohlmann MK, et al. Squamous-cell carcinoma in mature cystic
teratoma of the ovary: systematic review and analysis of published data. Lancet Oncol 2008; 9:1173.
15. Dalmau J, Gleichman AJ, Hughes EG, et al. Anti-NMDA-receptor encephalitis: case series and
analysis of the effects of antibodies. Lancet Neurol 2008; 7:1091.
16. Patel MD, Feldstein VA, Lipson SD, et al. Cystic teratomas of the ovary: diagnostic value of
sonography. AJR Am J Roentgenol 1998; 171:1061.
17. Tongsong T, Luewan S, Phadungkiatwattana P, et al. Pattern recognition using transabdominal
ultrasound to diagnose ovarian mature cystic teratoma. Int J Gynaecol Obstet 2008; 103:99.
18. Westhoff C, Pike M, Vessey M. Benign ovarian teratomas: a population-based case-control study. Br
J Cancer 1988; 58:93.
19. Comerci JT Jr, Licciardi F, Bergh PA, et al. Mature cystic teratoma: a clinicopathologic evaluation of
517 cases and review of the literature. Obstet Gynecol 1994; 84:22.
20. Singh P, Yordan EL, Wilbanks GD, et al. Malignancy associated with benign cystic teratomas
(dermoid cysts) of the ovary. Singapore Med J 1988; 29:30.
21. Dos Santos L, Mok E, Iasonos A, et al. Squamous cell carcinoma arising in mature cystic teratoma of
the ovary: a case series and review of the literature. Gynecol Oncol 2007; 105:321.
22. Davis KP, Hartmann LK, Keeney GL, Shapiro H. Primary ovarian carcinoid tumors. Gynecol Oncol
1996; 61:259.
23. Strosberg J, Nasir A, Cragun J, et al. Metastatic carcinoid tumor to the ovary: a clinicopathologic
analysis of seventeen cases. Gynecol Oncol 2007; 106:65.
24. Norris HJ, Zirkin HJ, Benson WL. Immature (malignant) teratoma of the ovary: a clinical and
pathologic study of 58 cases. Cancer 1976; 37:2359.
25. Woodruff JD, Protos P, Peterson WF. Ovarian teratomas. Relationship of histologic and ontogenic
factors to prognosis. Am J Obstet Gynecol 1968; 102:702.
26. La Vecchia C, Morris HB, Draper GJ. Malignant ovarian tumours in childhood in Britain, 1962-78. Br
J Cancer 1983; 48:363.
27. SCULLY RE. Gonadoblastoma; a gonadal tumor related to the dysgerminoma (seminoma) and
capable of sex-hormone production. Cancer 1953; 6:455.
28. Krasna IH, Lee ML, Smilow P, et al. Risk of malignancy in bilateral streak gonads: the role of the Y
28/5/2014 Ovarian germ cell neoplasms: Pathology, clinical manifestations, and diagnosis
file:///C:/Users/Ralt/Medicina%20Humana/Libros/Cl%EDnicas/Internal%20Medicine%20-%20Medicina%20Interna/Libros%20de%20Referencia%20de 12/12
chromosome. J Pediatr Surg 1992; 27:1376.
29. De Arce MA, Costigan C, Gosden JR, et al. Further evidence consistent with Yqh as an indicator of
risk of gonadal blastoma in Y-bearing mosaic Turner syndrome. Clin Genet 1992; 41:28.
30. Levato F, Martinello R, Campobasso C, Porto S. LDH and LDH isoenzymes in ovarian
dysgerminoma. Eur J Gynaecol Oncol 1995; 16:212.
31. Schwartz PE, Morris JM. Serum lactic dehydrogenase: a tumor marker for dysgerminoma. Obstet
Gynecol 1988; 72:511.
32. Gordon A, Lipton D, Woodruff JD. Dysgerminoma: a review of 158 cases from the Emil Novak
Ovarian Tumor Registry. Obstet Gynecol 1981; 58:497.
33. Boran N, Tulunay G, Caliskan E, et al. Pregnancy outcomes and menstrual function after fertility
sparing surgery for pure ovarian dysgerminomas. Arch Gynecol Obstet 2005; 271:104.
34. Fujita M, Inoue M, Tanizawa O, et al. Retrospective review of 41 patients with endodermal sinus
tumor of the ovary. Int J Gynecol Cancer 1993; 3:329.
35. Berek JS, Hacker NF. Nonepithelial ovarian and fallopian tube cancers. In: Practical Gynecologic
Oncology, 4th, Berek JS, Hacker NF (Eds), Lippincott Williams & Wilkins, Philadelphia 2005.
p.511.
36. Shah JP, Kumar S, Bryant CS, et al. A population-based analysis of 788 cases of yolk sac tumors: A
comparison of males and females. Int J Cancer 2008; 123:2671.
37. Kurman RJ, Norris HJ. Endodermal sinus tumor of the ovary: a clinical and pathologic analysis of 71
cases. Cancer 1976; 38:2404.
38. Talerman A, Haije WG, Baggerman L. Serum alphafetoprotein (AFP) in diagnosis and management of
endodermal sinus (yolk sac) tumor and mixed germ cell tumor of the ovary. Cancer 1978; 41:272.
39. Gershenson DM, Del Junco G, Herson J, Rutledge FN. Endodermal sinus tumor of the ovary: the M.
D. Anderson experience. Obstet Gynecol 1983; 61:194.
40. Kawai M, Kano T, Kikkawa F, et al. Seven tumor markers in benign and malignant germ cell tumors
of the ovary. Gynecol Oncol 1992; 45:248.
41. Abu-Rustum NR, Aghajanian C. Management of malignant germ cell tumors of the ovary. Semin
Oncol 1998; 25:235.
42. Ueda G, Abe Y, Yoshida M, Fujiwara T. Embryonal carcinoma of the ovary: a six-year survival. Int J
Gynaecol Obstet 1990; 31:287.
43. Chapman DC, Grover R, Schwartz PE. Conservative management of an ovarian polyembryoma.
Obstet Gynecol 1994; 83:879.
44. DiSaia PJ, Creasman WT. Germ cell, stromal and other ovarian tumors. In: Clinical Gynecologic
Oncology, 7th, Mosby-Elsevier, 2007. p.379.
45. Wheeler CA, Davis S, Degefu S, et al. Ovarian choriocarcinoma: a difficult diagnosis of an unusual
tumor and a review of the hook effect. Obstet Gynecol 1990; 75:547.
46. Steigrad SJ, Cheung AP, Osborn RA. Choriocarcinoma co-existent with an intact pregnancy: case
report and review of the literature. J Obstet Gynaecol Res 1999; 25:197.
47. Cunanan RG Jr, Lippes J, Tancinco PA. Choriocarcinoma of the ovary with coexisting normal
pregnancy. Obstet Gynecol 1980; 55:669.
48. Fisher RA, Newlands ES, Jeffreys AJ, et al. Gestational and nongestational trophoblastic tumors
distinguished by DNA analysis. Cancer 1992; 69:839.
Topic 3236 Version 18.0
2013 UpToDate, Inc. All rights reserved. | Subscription and License Agreement | Release: 21.3 - C21.34
Licensed to: Morehouse School of Medicine | Support Tag: [1104-118.195.65.244-B2F8B938AB-
S473950.14]
Вам также может понравиться
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeОт EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeРейтинг: 4 из 5 звезд4/5 (5795)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreОт EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreРейтинг: 4 из 5 звезд4/5 (1090)
- Never Split the Difference: Negotiating As If Your Life Depended On ItОт EverandNever Split the Difference: Negotiating As If Your Life Depended On ItРейтинг: 4.5 из 5 звезд4.5/5 (838)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceОт EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceРейтинг: 4 из 5 звезд4/5 (895)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersОт EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersРейтинг: 4.5 из 5 звезд4.5/5 (345)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureОт EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureРейтинг: 4.5 из 5 звезд4.5/5 (474)
- The Emperor of All Maladies: A Biography of CancerОт EverandThe Emperor of All Maladies: A Biography of CancerРейтинг: 4.5 из 5 звезд4.5/5 (271)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)От EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Рейтинг: 4.5 из 5 звезд4.5/5 (121)
- The Little Book of Hygge: Danish Secrets to Happy LivingОт EverandThe Little Book of Hygge: Danish Secrets to Happy LivingРейтинг: 3.5 из 5 звезд3.5/5 (400)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyОт EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyРейтинг: 3.5 из 5 звезд3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)От EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Рейтинг: 4 из 5 звезд4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaОт EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaРейтинг: 4.5 из 5 звезд4.5/5 (266)
- Akita SavvyДокумент170 страницAkita Savvylinbillion99386% (7)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryОт EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryРейтинг: 3.5 из 5 звезд3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnОт EverandTeam of Rivals: The Political Genius of Abraham LincolnРейтинг: 4.5 из 5 звезд4.5/5 (234)
- The Unwinding: An Inner History of the New AmericaОт EverandThe Unwinding: An Inner History of the New AmericaРейтинг: 4 из 5 звезд4/5 (45)
- Dna ActivationДокумент34 страницыDna ActivationLiviu Angelescu50% (6)
- Microbial Interactions With The Host in Periodontal DiseasesДокумент74 страницыMicrobial Interactions With The Host in Periodontal Diseasesrupali100% (2)
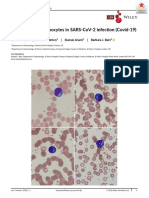
- Foldes, D., Et Al. (2020) - Plasmacytoid Lymphocytes in SARS CoV 2 Infection (Covid 19) - American Journal of Hematology PDFДокумент2 страницыFoldes, D., Et Al. (2020) - Plasmacytoid Lymphocytes in SARS CoV 2 Infection (Covid 19) - American Journal of Hematology PDFRalt MedОценок пока нет
- HHS Public Access: Understanding The Biology of CRLF2-Overexpressing Acute Lymphoblastic LeukemiaДокумент15 страницHHS Public Access: Understanding The Biology of CRLF2-Overexpressing Acute Lymphoblastic LeukemiaRalt MedОценок пока нет
- XN Series TechnologyДокумент23 страницыXN Series TechnologyRalt MedОценок пока нет
- Staphylococcus LectureДокумент27 страницStaphylococcus LectureRalt MedОценок пока нет
- Equilibrium: Application To Drug Design: Nature BiotechnologyДокумент5 страницEquilibrium: Application To Drug Design: Nature BiotechnologysgybleeОценок пока нет
- Physical Aging Process: When Does Old Age Begin?Документ32 страницыPhysical Aging Process: When Does Old Age Begin?ZeusKimОценок пока нет
- Robbins Ch. 27 Peripheral Nerve and Skeletal Muscle Review QuestionsДокумент2 страницыRobbins Ch. 27 Peripheral Nerve and Skeletal Muscle Review QuestionsPA2014100% (1)
- Rescue AsdДокумент17 страницRescue Asdaloisiaa8Оценок пока нет
- Patterns of InheritanceДокумент13 страницPatterns of Inheritancesadam 10Оценок пока нет
- Abnormal Cervical CytologyДокумент21 страницаAbnormal Cervical CytologyNatalia HaikaliОценок пока нет
- Neutrophil EbookДокумент468 страницNeutrophil EbookNathália LuísaОценок пока нет
- Bacterial Diseases Rice 2015Документ13 страницBacterial Diseases Rice 2015Teguh PratamaОценок пока нет
- Joseph MerrickДокумент12 страницJoseph Merrickyairherrera100% (1)
- Bone DensitometryДокумент42 страницыBone DensitometryWira Dat100% (1)
- TASK 1.6 MonologueДокумент5 страницTASK 1.6 MonologueLore HernandezОценок пока нет
- Abdi Et Al. 2008Документ6 страницAbdi Et Al. 2008argos1301Оценок пока нет
- Cell and Organs of The Immune SystemДокумент43 страницыCell and Organs of The Immune Systemcyrhenmie100% (1)
- Inflammatory Bowel DiseaseДокумент27 страницInflammatory Bowel DiseaseMihai VladescuОценок пока нет
- Acute Kidney Injury in Patients With CancerДокумент13 страницAcute Kidney Injury in Patients With CancerzikryauliaОценок пока нет
- Gavrila Catalin 1Документ1 страницаGavrila Catalin 1Doina RusuОценок пока нет
- Nucleus Arthroplasty Volume IДокумент40 страницNucleus Arthroplasty Volume IHelifunoОценок пока нет
- Prenatal DevelopmentДокумент33 страницыPrenatal DevelopmentDexter Nario0% (1)
- OAT Practice Biology QuestionsДокумент10 страницOAT Practice Biology QuestionsioatprepОценок пока нет
- Datasheet of IU1 - CAS 314245-33-5Документ2 страницыDatasheet of IU1 - CAS 314245-33-5LouisОценок пока нет
- Bei Resources - Highlights Link To MutationsДокумент1 страницаBei Resources - Highlights Link To MutationslllОценок пока нет
- Bio OssДокумент4 страницыBio OssVizi AdrianОценок пока нет
- Biotechnology and The Human GoodДокумент225 страницBiotechnology and The Human GoodIvan Castillo Dcv100% (1)
- Pus Poppin FrogsДокумент4 страницыPus Poppin FrogsDalia DeebОценок пока нет
- Campylobacter Spp. and Related Organisms in PoultryДокумент212 страницCampylobacter Spp. and Related Organisms in PoultryNicku MalanceaОценок пока нет
- Body Cavities PPT & Anatomical SystemДокумент10 страницBody Cavities PPT & Anatomical Systemnandhini raguОценок пока нет
- Field Trial Evaluating The Efficacy of Porcine Epidemic Diarrhea Vaccine, RNA (Harris Vaccine) in The PhilippinesДокумент1 страницаField Trial Evaluating The Efficacy of Porcine Epidemic Diarrhea Vaccine, RNA (Harris Vaccine) in The PhilippinesJona MayОценок пока нет