Академический Документы
Профессиональный Документы
Культура Документы
Crystal Geometry 6up
Загружено:
Tan Phung NguyenИсходное описание:
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
Crystal Geometry 6up
Загружено:
Tan Phung NguyenАвторское право:
Доступные форматы
1
Geometry of Crystals Chapter 2
Outline
The lattice and unit cells
Describing lines and planes in crystals
The reciprocal lattice
Symmetry
Crystal Systems
Lattice centering and Bravais lattices
Indexing in the hexagonal crystal system
Crystal structures and fractional coordinates
Crystal habit (shape)
Stacking faults and twins
Introduction to the stereographic projection
Describing condensed phase structures
Describing the structure of an isolated small
molecule is easy to do
Just specify the bond distances and angles
How do we describe the structure of a condensed
phase ?
we have ~ Avogadros number of atoms to locate
we should either give up on specifying the position of
every atom or find a trick to help us out
The structure of liquids and glasses
We can use pair distribution functions to describe
the structure of such systems
Crystals
Crystals are materials with a regular internal
structure
They have internal translational symmetry
Some structural motif is repeated at regular intervals
throughout the solid
Just because a material has nice flat faces it is not
necessarily a crystal
Cut crystal glass is not a crystal!
Not all crystals have well developed faces
The structure of crystalline materials
We can use the symmetry of a crystal to reduce the
number of unique atom positions we have to specify
The most important type of symmetry is translational
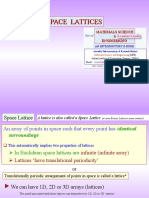
this can be described by a lattice
A lattice is just a series of points describing the
translational symmetry of solid
The lattice points do not represent individual atoms!!
The structure associated with the lattice can be carved
up into boxes (unit cells) that pack together to
reproduce the whole crystal structure
2
The lattice and unit cell in 1D
The lattice
Lattices and unit cells 2 D
Identify a lattice and unit cell
Lattice and unit cell in 3D
We specify the lattice using the vectors a, b and c.
The vector between any two lattice points (r) satisfies the
relationship, r = na + pb + qc, where n, p and q are integers
There is no fixed relationship between the positions of
a unit cell and the lattice that is associated with it
The lattice only places constraints on the size and shape of
the unit cell, not position
Lattice and unit cell in 3D
3
The unit cell
Always use a right handed axis system
Axis lengths are specified by a, b and c
Interaxial angles are specified by , and
Picking a unit cell for NaCl
Specifying points in crystals
Frequently need to specify the position of a
point, the direction of a line or the orientation
of a plane in a crystal
Can specify a point using
r = (n+u)a + (p+v)b + (q+w)c n,p,q integers
r = (na + pb +qc) + (ua + vb +wc)
u, v, w are fractional coordinates specifying a position
within a unit cell. All positions with the same u, v and w
are symmetry equivalent
Specifying the direction of a line
The direction of any line can be specified by drawing a line
parallel to the one of interest so that it goes through the unit cell
origin and picking any point u, v, w on the line
Conventionally, u,v,w are multiplied by the smallest number that
produces integers u,v,w
Denote direction of line using the symbol [uvw]
[uvw] are the indices of the lines direction
The use of a square bracket implies that we are talking about a direction
The notation <uvw> implies that we are talking about all
directions in a crystal that are symmetry equivalent to [uvw]
For example, in a cubic material all the body diagonals of the cube are
symmetry equivalent. So all the directions [111], [-111], [1-11], [11-1], [-
1-11], [-11-1], [1-1-1] and [-1-1-1] are symmetry equivalent. All of them
are directions of the form <111>.
NOTE typically negative indices are specified using a bar over the number
not by a negative sign
The orientation of lattice planes
It is possible to describe certain directions and planes
with respect to the crystal lattice using a set of three
integers referred to as Miller Indices
Miller indices (hkl)
Miller Indices are the
reciprocal intercepts of the
plane on the unit cell axes
Identify plane adjacent to
origin
can not determine for plane
passing through origin
Find intersection of plane on
all three axes
Take reciprocal of intercepts
If plane runs parallel to axis,
intercept is at , so Miller
index is 0
4
Examples of Miller indices
Families of planes
Miller indices describe the orientation and
spacing of a family of planes
The spacing between adjacent planes in a
family is referred to as the d-spacing
Three different
families of planes
d-spacing between
(300) planes is one
third of the (100)
spacing
Note all
(100) planes
are members
of the (300)
family
Planes of a form
The symbol {hkl} refers to all planes that are symmetry
equivalent to (hkl). This group of equivalent planes are
referred to as planes of a form.
For the cubic system all the planes (100), (010), (001),
(-100), (0-10) and (00-1) belong to the form {100}
For a tetragonal material a=b c the form {100} would
only include (100), (010), (-100), and (0-10)
Planes of a zone
Planes of a zone are
planes that are all parallel
to one axis, the zone axis
Miller indices for all
planes in a zone obey the
relationship hu +kv + lw
= 0, where [uvw] is the
zone axis
Shaded planes belong to the
[001] zone
d-spacing formulae
For a unit cell with orthogonal axes
(1 / d
2
hkl
) = (h
2
/a
2
) + (k
2
/b
2
) + (l
2
/c
2
)
Hexagonal unit cells
(1 / d
2
hkl
) = (4/3)([h
2
+ k
2
+ hk]/ a
2
) + (l
2
/c
2
)
Unit cells and d
hkl
5
Lattice point density
Low index lattice planes contain a high density of
lattice points
(010)
(110)
(120)
(210)
(210)
The reciprocal lattice
It is convenient when talking about diffraction to use
the concept of a reciprocal lattice
The reciprocal lattice is related to the real space lattice
by:
a
1
, a
2
, a
3
are the vectors of the real space lattice
(alternatively a,b,c) and b
1
,b
2
,b
3
are the vectors of the
reciprocal lattice (alternatively a*,b*,c*).
Note a
1
.a
2
xa
3
is the unit cell volume
3 2 1
3 2
1
a a a
a a
b
=
3 2 1
1 3
2
a a a
a a
b
=
3 2 1
2 1
3
a a a
a a
b
=
Properties of the reciprocal lattice
Note a
i
.b
j
=
ij
So a
1
.b
1
= 1, but a
1
.b
2
= 0 and a
1
.b
3
= 0 etc.
This is the origin of the term reciprocal lattice.
The reciprocal lattice and real space lattice are orthonormal
Any point on the reciprocal lattice can be specified by
a vector H
hkl
= hb
1
+ kb
2
+ lb
3
(hkl are integers)
This vector is perpendicular to the plane in real space with
Miller indices (hkl)
The length of this vector H
hkl
= 1/d
hkl
where d
hkl
is the
interplanar spacing in real space
We get to represent a whole family of planes in real space by
a single point in reciprocal space
Geometrical relationship between real and
reciprocal space
Note reciprocal lattice
vector is always
perpendicular to the
corresponding real space plane
Only in orthogonal axis systems
are the real and reciprocal lattice
vectors parallel
Point symmetry elements
In addition to the translational symmetry
associated with a lattice, most materials have
addition point symmetry
Point symmetry elements operate to change the
orientation of structural motifs
A point symmetry operation does not alter at least
one point that it operates on
Point symmetry elements include
rotation axes
mirror planes
rotation-inversion axes
A two fold rotation
6
A mirror plane An inversion center
A rotation inversion axis The symmetry elements of a cube
Identify the point symmetry elements
All combinations of point
symmetry elements are not possible
A three fold axis can not just have one two
fold axis perpendicular to it
In three dimensions the existence of two
perpendicular two folds implies the
existence of a third perpendicular two fold
The allowed combinations of point
symmetry elements are called point groups
7
Point symmetry elements
compatible with 3D translations
1 Center of symmetry
n (= 1,2,3,4,6) Inversion axis
n = 2,3,4,6 Rotation axis
m Mirror plane
Symbol Symmetry element
Point symmetry and packing
The 32 point groups
Only 32 point groups
are consistent with
periodicity in 3D
Schnflies and Hermann-
Maugin symbols for
crystallographic point
groups
Symbols for symmetry elements 1
Symbols for symmetry elements 2
Unit cell choice
There is always more than possible choice of unit cell
By convention the unit cell is chosen so that it is as
small as possible while reflecting the full symmetry
of the lattice
If the unit cell contains only one lattice point is said
to be primitive. If it contains more than one lattice
point it is centered
There are seven distinct shapes of unit cell, that are
referred to as the seven crystal systems
8
Unit cell choice in 2D
Unit cell choice in 2D
The seven crystal systems
Four three-fold axes at 109 23 to
each other
= = = 90
a = b = c
Cubic
One four-fold axis or one four-
fold improper-axis
= = = 90
a = b ! c
Tetragonal
One six-fold axis or one six-fold
improper axis
= = 90
= 120
a = b ! c
Hexagonal
One three-fold axis = = ! 90
a = b = c
Trigonal
Any combination of three
mutually perpendicular two-fold
axes or planes of symmetry
= = = 90
a ! b ! c
Orthorhombic
One two-fold axis or one
symmetry plane
= = 90
! 90
a ! b ! c
Monoclinic
None ! ! ! 90
a ! b ! c
Triclinic
Minimum Symmetry Unit Cell System
Unit cells in 3D
Centering
Centering operators
The location of the additional lattice points
within the unit cell is described by a set of
centering operators
Body centered (I) has additional lattice point at
Face centered (F) has additional lattice points at
0, 0, and 0
Side centered (C) has an additional lattice point at
0
9
Centering 3
Not all centering possibilities occur for each of
the seven crystal systems
Only 14 unique combinations (Bravais lattices)
Some centering types are not allowed because they
would lower the symmetry of the unit cell
E.g side centered cubic is not possible as this would
destroy the three fold symmetry that is an essential
component of cubic symmetry
Some centering types are redundant
C-centered tetragonal can always be described using a
smaller primitive tetragonal cell
Bravais Lattices
Indexing in the hexagonal system
In hexagonal unit cells it is
common to refer the
orientation of planes and
lines to four coordinate
axes
The fourth axis a
3
is just
= -a
2
-a
1
. This approach
reflects the three fold
symmetry associated with
the unit cell
Properties of hexagonal indices
Indices are expressed as
(hkil)
h + k = -i
All cyclic permutations of
h, k and i are symmetry
equivalent
So (10-10), (-1100),
(0-110) are equivalent
Describing crystal structures
The location of all the atoms in a crystalline solid can
be specified by a combination of all the symmetry
elements that are appropriate and the fractional
coordinates for a unique set of atoms (asymmetric unit)
Full symmetry of a crystal is described by its space
group
We specify atomic coordinates for a small number of
atoms. We then apply all the symmetry elements
including the lattice symmetry to build up the full 3D
structure.
Note each lattice point may be associated with many atoms
Combining symmetry elements
For three dimensions
32 point groups
14 Bravais lattices
but only 230 space groups
For two dimensions
5 lattices
10 point groups
but only 17 plane groups
10
Hexagonal close packing
Each lattice point is
associated with two atoms.
One at the corner of the unit
cell and one inside the unit
cell
Note this structure is an
ABABAB. repeat of
closepacked layers
Atomic positions in FCC structure
Can represent atoms on unit cell projection drawing
with heights marked
Can also give atomic coordinates
0,0,0 0,, ,,0 ,0,
Only need to specify these four atoms as others a produced
by unit cell translational symmetry
The NaCl (rock salt) structure
Need to specify only one Cl and
Na position. The others are
produced by the lattice centering
operators 0,0,0 0,, ,,0
and ,0,
Na at 0,0,0 and Cl at 0.5,0,0
The ZnS (Zinc Blende) structure
S at 0,0,0 and Zn at 0.25,0.25,0.75
Need to specify only one Zn and S position. The
others are produced by the lattice centering
operators 0,0,0 0,, ,,0 and ,0,
AuBe structure
Structure based on primitive cubic
lattice
Au and Be atoms displaced along
the cubic the fold axes of the
material
No atoms at corners of unit cell!
No four fold symmetry!
Au at uuu, (+u)(-u)-u,
-u(+u)(-u), (-u)-u(+u)
Be at www, (+w)(-w)-w,
-w(+w)(-w), (-w)-w(+w)
u = 0.100 and w = 0.406
Substitutional solid solutions
The perfect long range order in a crystal can be lost be
replacing some of the atoms with others. This happens
when solid solutions are formed.
Describe the average structure using lattice coordinates
etc rather than the actual structure
11
Crystal shape
The external shape of a crystal is referred to as its
Habit
Not all crystals have well defined external faces
Typically see faces on crystals grown from solution
Natural faces always have low indices (orientation
can be described by Miller indices that are small
integers)
- Law of rational indices
The faces that you see are the lowest energy faces
- Surface energy is minimized during growth
Defects in crystals
No crystal of significant size is perfect
Most crystals contain defects
Point defects missing atoms, substituted atoms,
displaced atoms etc.
Line defects dislocations
Planar defects Stacking faults, twins,
Crystallographic shear planes
Stacking faults
Stacking faults occur in wide variety of materials not
just simple metals
Consider a structure to be built up from successive
layers of atoms or other units, if the regular stacking of
these units is interrupted we have a stacking fault
Close packed metals provide simple examples
Perfect FCC has a ABCABCABCABCABC sequence
A, B, C represent different close packed (111) layers
The sequence ABCABCBCABCABC has a stacking fault
Perfect HCP is ABABABABABABAB
ABABABCABABABABAB has a stacking fault
Faults that put two of the same layers together AA BB or CC
are unlikely due to their very high energy
Twins
Sometime the regular internal structure of a crystal will
be interrupted in such a way that two pieces of the
crystal are related to one another by rotation about an
axis or reflection in a mirror plane
We then have a rotation or a reflection twin
Twin because the two components of the twin are related to each other
by a symmetry operation and have a plane of atoms in common
(Composition plane)
Reflection plane is twin plane
Rotation axis is twin axis
In one piece of material this twinning process can occur
multiple times leading so several components all with a well
defined relative orientation
Twins in close packed metals
Annealing twins often form in FCC metals such as
Cu, Ni, -brass, Al that have been cold worked an
annealed
As the crystal regrows a stacking fault leads to the formation
of twins
Components are related by 180 rotation around <111>
Deformation twins can occur in deformed HCP metals
such as Zn, Mg, Be and BCC metals (-Fe)
They are reflection twins
Appearance and structure of annealing twins
In (a) only two components are
present in a crystal in (b) there are
three components. B is twin band
sandwiched between components
A
1
and A
2
with same orientation as
each other. The atomic structure of
such as twin band in an FCC metal
is shown on the right.
Вам также может понравиться
- Crystal Physics: T.Y.Bsc Solid State Physics Unit 1Документ39 страницCrystal Physics: T.Y.Bsc Solid State Physics Unit 1Monika KadavОценок пока нет
- Crystal StructureДокумент123 страницыCrystal StructuresupyyyyОценок пока нет
- MO 201: Materials Science: DR Bratindranath Mukherjee Dept. of Metallurgical Engineering Bratindra - Met@itbhu - Ac.inДокумент109 страницMO 201: Materials Science: DR Bratindranath Mukherjee Dept. of Metallurgical Engineering Bratindra - Met@itbhu - Ac.inRashmiОценок пока нет
- Chapter 1: Crystal Structure: Types of SolidsДокумент10 страницChapter 1: Crystal Structure: Types of Solidsnahom teferaОценок пока нет
- Structure of Crystalline SolidsДокумент20 страницStructure of Crystalline SolidsAnwesha SamaddarОценок пока нет
- Introduction 2Документ30 страницIntroduction 2niteshОценок пока нет
- Crystal - 1Документ60 страницCrystal - 1Shivam SahuОценок пока нет
- Structure of Metals and MaterialsДокумент67 страницStructure of Metals and MaterialsAshutosh kumarОценок пока нет
- Chapter 3: Fundamentals of CrystallographyДокумент51 страницаChapter 3: Fundamentals of CrystallographyAli ZbayelОценок пока нет
- Introduction Crystal PhysicsДокумент118 страницIntroduction Crystal PhysicsSilambarasan Physics60% (5)
- Smith CH 03Документ75 страницSmith CH 03KevinSaldañaОценок пока нет
- Smart Materials PDFДокумент285 страницSmart Materials PDFChidu KОценок пока нет
- SSP - Solid State Physics (I)Документ48 страницSSP - Solid State Physics (I)Edward HallОценок пока нет
- CrystalДокумент56 страницCrystalPrinceОценок пока нет
- 2 Crystals Symmetry and Space Groups - Andrew LeslieДокумент42 страницы2 Crystals Symmetry and Space Groups - Andrew LeslieAna AvilaОценок пока нет
- 4 - Crystal Structure PDFДокумент66 страниц4 - Crystal Structure PDFManoj SelvamОценок пока нет
- Lecture 2a Solid State PhysicsДокумент17 страницLecture 2a Solid State PhysicsShehnila KarimОценок пока нет
- Symmetry and Crystallography - Lectures PDFДокумент190 страницSymmetry and Crystallography - Lectures PDFJanhavi NistaneОценок пока нет
- SYMMETRY AND GROUP THEORYДокумент27 страницSYMMETRY AND GROUP THEORYAnonymous Tph9x741Оценок пока нет
- Metallurgy MMS Module 1 and 2Документ245 страницMetallurgy MMS Module 1 and 2Sahal T YousephОценок пока нет
- Lecture 1 (9th Jan, 2024)Документ24 страницыLecture 1 (9th Jan, 2024)Harsh JainОценок пока нет
- 4-Crystallography Mod A 10-20Документ37 страниц4-Crystallography Mod A 10-20Tsuki NakamiОценок пока нет
- Crystallography Space Lattice StructureДокумент9 страницCrystallography Space Lattice Structuresatyendra singhОценок пока нет
- 211 3Документ57 страниц211 3Mada ChohОценок пока нет
- CrystalДокумент34 страницыCrystalArslan Zulfiqar AhmedОценок пока нет
- Section-A: Some Basic DefinitionsДокумент81 страницаSection-A: Some Basic DefinitionsappliedphyОценок пока нет
- Curs Solid StateДокумент121 страницаCurs Solid StateAna Maria TrandafirОценок пока нет
- Condensed Matter Physics ConceptsДокумент37 страницCondensed Matter Physics ConceptslingarajugowdaОценок пока нет
- Introduction To Group Theory PDFДокумент111 страницIntroduction To Group Theory PDFSumanta PadhiОценок пока нет
- 3 The Structure of Crystalline SolidsДокумент51 страница3 The Structure of Crystalline SolidsNeko-neko CasugaОценок пока нет
- Chap 3Документ32 страницыChap 3محمود عليОценок пока нет
- Crystal StructuresДокумент33 страницыCrystal StructuresrmeenakshiОценок пока нет
- Crystal Symmetry LectureДокумент47 страницCrystal Symmetry LectureZul FadliОценок пока нет
- Solid State ChemistryДокумент61 страницаSolid State ChemistrydhananjaylandgeОценок пока нет
- LatticeДокумент56 страницLatticeCarlos UrregoОценок пока нет
- Determine Crystal Symmetry and Point GroupДокумент63 страницыDetermine Crystal Symmetry and Point GroupMaisha MujibОценок пока нет
- Classification of Solids: Solid MaterialsДокумент99 страницClassification of Solids: Solid MaterialsHarsha SathvikОценок пока нет
- Crystallography and Structure of MaterialsДокумент96 страницCrystallography and Structure of MaterialsNafis AhmedОценок пока нет
- Basic CrystallographyДокумент81 страницаBasic CrystallographyVasudevan SubramaniyanОценок пока нет
- Lecture1 Chapter 1 New (Hamda)Документ58 страницLecture1 Chapter 1 New (Hamda)sales zfОценок пока нет
- Crystal Structure FundamentalsДокумент20 страницCrystal Structure FundamentalsReddyvari VenugopalОценок пока нет
- CrystallographyДокумент39 страницCrystallographypoornachandhu022Оценок пока нет
- Simple Crystal Structures-2 PDFДокумент135 страницSimple Crystal Structures-2 PDFRoslina ShariffОценок пока нет
- Slides Part-1 MO201Документ36 страницSlides Part-1 MO201Rahul kumar KeshriОценок пока нет
- Crystal Structure LatticesДокумент39 страницCrystal Structure Latticeskaran sahniОценок пока нет
- Classification of Solids: Crystalline, Polycrystalline and AmorphousДокумент24 страницыClassification of Solids: Crystalline, Polycrystalline and AmorphousETHJDTJRYJ2Оценок пока нет
- Crystal Structure FundamentalsДокумент24 страницыCrystal Structure FundamentalsSyed ShaОценок пока нет
- Internal Order in Crystals1Документ35 страницInternal Order in Crystals1Kripa KattelОценок пока нет
- CrystallographyДокумент25 страницCrystallographyAkhil Sai KrishnaОценок пока нет
- XRD NotesДокумент54 страницыXRD NotesBME62Thejeswar SeggamОценок пока нет
- Structure of Crystals WebexДокумент22 страницыStructure of Crystals WebexmareasanthaОценок пока нет
- Mineralogy and CrystallographyДокумент20 страницMineralogy and CrystallographySachin FfОценок пока нет
- 256 MAT ShortNotesJan26-Feb7 06Документ5 страниц256 MAT ShortNotesJan26-Feb7 06gombossandorОценок пока нет
- Crystal Structures and SymmetriesДокумент18 страницCrystal Structures and SymmetriesZrusОценок пока нет
- Ewald SphereДокумент57 страницEwald SphereMohammad Rameez0% (1)
- Crystallography 2Документ3 страницыCrystallography 2Akshay KumarОценок пока нет
- Robot Manipulators: Modeling, Performance Analysis and ControlОт EverandRobot Manipulators: Modeling, Performance Analysis and ControlОценок пока нет
- Cartesian Tensors in Engineering Science: The Commonwealth and International Library: Structures and Solid Body Mechanics DivisionОт EverandCartesian Tensors in Engineering Science: The Commonwealth and International Library: Structures and Solid Body Mechanics DivisionОценок пока нет
- Miller IndicesДокумент19 страницMiller IndicesNizar Achmad NizarОценок пока нет
- XRD Theory PresentationДокумент47 страницXRD Theory Presentationsimongerardgerona50% (2)
- Mec-Appl 2011-56-3 A6 DonescuДокумент13 страницMec-Appl 2011-56-3 A6 DonescuTan Phung NguyenОценок пока нет
- MillerДокумент8 страницMillerhulkhellboyОценок пока нет
- sẽ có ích cho những ai đang và sẽ tìm việcДокумент10 страницsẽ có ích cho những ai đang và sẽ tìm việcTan Phung NguyenОценок пока нет
- EPMA Introduction To Powder MetallurgyДокумент36 страницEPMA Introduction To Powder MetallurgyPranjal SinghОценок пока нет
- XXXXXXX XXXXXXX: Pour Exemple: Pour Exemple: ArteorДокумент5 страницXXXXXXX XXXXXXX: Pour Exemple: Pour Exemple: ArteorGilbert MartinezОценок пока нет
- GameBoy Programming ManualДокумент298 страницGameBoy Programming Manualdiceman2037100% (4)
- Digital Logic DesignДокумент4 страницыDigital Logic DesignkiranОценок пока нет
- MEITRACK MVT100 User Guide V2.4Документ19 страницMEITRACK MVT100 User Guide V2.4MeitrackОценок пока нет
- Oral and Practical Tests: MechanicДокумент19 страницOral and Practical Tests: MechanicHugo AlmeidaОценок пока нет
- GuidewireClaimCenter Performance TestPlanДокумент18 страницGuidewireClaimCenter Performance TestPlanshanthan117Оценок пока нет
- Development and Evaluation - KocabaДокумент263 страницыDevelopment and Evaluation - KocabaWRLSОценок пока нет
- YaskawaДокумент375 страницYaskawaCristian IozsaОценок пока нет
- Address Book in JAVAДокумент18 страницAddress Book in JAVAmelyfony100% (1)
- Engineering Structures: SciencedirectДокумент8 страницEngineering Structures: SciencedirectFeleki AttilaОценок пока нет
- BPO2-Module 9 PROJECT PLANДокумент16 страницBPO2-Module 9 PROJECT PLANJudame Charo ZozobradoОценок пока нет
- Describe The Physical Properties of Propylene Glycols.: PrintДокумент4 страницыDescribe The Physical Properties of Propylene Glycols.: PrintKaarthicNatarajanОценок пока нет
- The Weka Guard and Protector - Weka MarineДокумент2 страницыThe Weka Guard and Protector - Weka MarineJoko SusiloОценок пока нет
- Shop Manual WA380-3LE SN A50001Документ758 страницShop Manual WA380-3LE SN A50001Eliecer godoy100% (2)
- Tu 1-5Документ8 страницTu 1-5Made easy classes0% (2)
- Usage of Regular Expressions in NLPДокумент7 страницUsage of Regular Expressions in NLPInternational Journal of Research in Engineering and TechnologyОценок пока нет
- Mechanical Power Transmission ReviewДокумент17 страницMechanical Power Transmission ReviewRoshan TiwariОценок пока нет
- Supplier Deviation Request FormДокумент2 страницыSupplier Deviation Request Formjainik shahОценок пока нет
- Software Test ReportДокумент4 страницыSoftware Test ReportSabahat HussainОценок пока нет
- Emergency CallДокумент6 страницEmergency CallNugrohoОценок пока нет
- Instruction Manual STR 155 RL-1Документ24 страницыInstruction Manual STR 155 RL-1VENDI100% (3)
- Android Tutorial - Broadcast ReceiversДокумент15 страницAndroid Tutorial - Broadcast ReceiversTrieu Ngo HuyОценок пока нет
- Torque Specifications: Service Specifications - Ra60F Manual TransmissionДокумент1 страницаTorque Specifications: Service Specifications - Ra60F Manual TransmissionPedro Javier Castro SanchezОценок пока нет
- 1998 CAT 3126 Oper & Maint ManualДокумент93 страницы1998 CAT 3126 Oper & Maint Manualbatuhan kılıç100% (2)
- Elective-II: Pavement Analysis & Design: B.E. (Civil Engineering) Eighth Semester (C.B.S.)Документ6 страницElective-II: Pavement Analysis & Design: B.E. (Civil Engineering) Eighth Semester (C.B.S.)Adesh DeshbhratarОценок пока нет
- fEA CourseДокумент3 страницыfEA CourseAnant KumbhojkarОценок пока нет
- 4.failure Theories and Stress ConcentrationsДокумент21 страница4.failure Theories and Stress ConcentrationsAmr El SaeedОценок пока нет
- Green Aviation SeminarДокумент19 страницGreen Aviation SeminarAromalSPillaiОценок пока нет
- NM Group Plumbing WorkДокумент33 страницыNM Group Plumbing WorkNM GROUPОценок пока нет