Академический Документы
Профессиональный Документы
Культура Документы
Atomic Structure Notes
Загружено:
cgao30Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
Atomic Structure Notes
Загружено:
cgao30Авторское право:
Доступные форматы
IB Chemistry 2013
ATOMIC STRUCTURE
(Topics 2 & 12)
2.1 The atom (SL)
2.1.1 State the position of protons, neutrons and electrons in the atom
Protons: In the nucleus
Neutrons: In the nucleus
Electrons: Orbiting around the nucleus at different energy levels, in a negatively charged cloud
2.1.2 State the relative masses and relative charges of protons, neutrons and electrons
Subatomic Particle type Relative Mass Relative Charge
Proton 1 +1
Neutron 1 0
Electron 1/1836 -1
2.1.3 Define the terms mass number (A), atomic number (Z) and isotopes of an element
Mass number (A) Determined by the number of protons + neutrons inside the nucleus
Atomic number (Z) Determined by the number of protons in the nucleus
Isotopes Atoms of the same element, with the same number of protons but difference number of
neutrons
2.1.4 Deduce the symbol for an isotope given its mass number and atomic number
Notation:
AZX (A = mass number, Z = atomic number)
IB Chemistry 2013
2.1.5 Calculate the number of protons,
No. of protons never changes proton number defines an element
Electrons remain constant unless its an ion
Neutrons depends on the isotope
E.g. Na
+
ion
Atomic Number: 11
Number of protons: 11
Neutrons: 12
2.1.6 Compare the properties of the isotopes of an element
Properties of isotopes almost identical
Slight differences in physical properties, e.g.:
1. Density
2. Rate of diffusion
3. Whether or not an isotope is radioactive (can emit alpha, beta and gamma rays)
4. Melting and boiling points
5. Atomic mass
Uranium has 2 main isotopes 235 Uranium and 238 Uranium
235 isotope is required for nuclear power and explosives, but usually only occurs at low
percentages and must be separated from other isotope
Carried out by reacting uranium with fluorine, making uranium hexafluoride UF6 a volatile
gas
Uranium hexafluoride then passed through many centrifuges where the heaver UF6 made
from 238 uranium does not pass through as quickly
Eventually, after passing through many centrifuges, the final product is pure UF6, which can
be processed and reduced back to pure 235 uranium
2.1.7 Discuss the uses of radioisotopes
14
C is used in radiocarbon dating update stopped after death, with half-life of approx.
5,600 years
By calculating %
14
C left, can determine how many years ago an organism died
IB Chemistry 2013
Medicine
Cobalt 60
Iodine 131
Iodine 125
Cobalt 60 is a powerful gamma emitter and has been used for over 50 years to treat different types
of cancer.
Additionally, Cobalt 60 can also:
Sterilize medical equipment
Food Irradiation
Tracer for Cobalt in Chemical reactions.
Iodine 131 is used in the treatment of thyroid cancer.
Iodine 125 is used to treat prostate cancer and brain tumours.
2.2 The mass spectrometer
2.2.1 Describe and explain the operation of a mass spectrometer
A mass spectrometer is an instrument which separates particles according to their masses
and records the relative proportions of these.
In the first phase, the substance is converted into atoms or molecules in the vapour phase.
They are then turned into positive ions and then accelerated, as you can see from the
diagram.
The fast moving particles that have just been accelerated are then deflectedthe lighter
and the more positively charged the particle, the greater the deflection. Finally, particles of a
particular mass will be detected.
IB Chemistry 2013
1. Vaporisation
Substance is converted into atoms of molecules in the vapour phase
2. Ionisation
Particles are converted into positive ions
Done by bombarding them with accelerated electrons
These electrons collide with the electrons in the particle, knocking them off and leaving a
positive ion
3. Acceleration
Positive ions are accelerated by high potential difference between two parallel electrodes
with holes in their centres
4. Deflection
by an electromagnet, which causes fast moving ions to deflect
Particles of a certain mass will continue round the tube and strike the detector plate
Those with greater mass will not be deflected as much and will strike the wall of the
instrument
Only ions of a certain mass are detected, usually by means of the current flow required to
neutralise the positive charge
2.2.2 Describe how the mass spectrometer may be used to determine relative atomic mass
using the
12
C scale
Mass spectrometer gives lines which correspond to the mass/charge ratio of each particle
(ion)
Height of each line is proportional to the abundance of the specific particle
Hence, using knowledge of mass and % abundance of each, the relative mass of the element
can be calculated in relation to the
12
C scale
2.2.3 Calculate non-integer relative atomic masses and abundance of isotopes from given data
Calculating average mass of chlorine atom
Example:
Chlorine has two common isotopes - chlorine 35 and chlorine 37 with relative percentage
abundances of 77.5% and 22.5% respectively.
This means that in any naturally occurring sample of chlorine for every 100 atoms there
are 77-78 atoms with a mass of 35 units and 22-23 atoms with a mass of 37 units.
To find the average mass of one atom we must add up the masses of all 100 atoms and
then divide by 100.
IB Chemistry 2013
Mass of 100 chlorine atoms = (77.5 x 35) + 22.5 x 37) = 3545
therefore average mass of one chlorine atom = 3545/100 = 35.45
Clearly, it is not possible to have an atom with a mass of 35.45 units, but this represents
the average relative mass of a chlorine atom.
Calculating % abundance of isotope
Weight of isotope
1
x % abundance of isotope
1
+ Weight of isotope
2
x % abundance of isotope
2
=
average atomic weight of the element
weight * X + weight * (1 X) = average atomic weight
2.3 Electron arrangement
2.3.1 Describe the electromagnetic spectrum
Electromagnetic waves can travel through space and depending on the wavelength, also
through matter.
Electromagnetic radiation is a form of energy. Smaller wavelengths result in a higher
frequency, and the more energy the wave possesses.
Electromagnetic Radiation form part of the EM spectrum, which separates EM waves in
progressively increasing wavelengths (decreasing frequency).
IB Chemistry 2013
2.3.2 Distinguish between a continuous spectrum and a line spectrum
Continuous spectrum when the spectrum passes through all of the colours in the EM spectrum,
starting with red and ending with violet
Results when gas pressures are higher, so that lines are broadened by collisions between the
atoms until they are smeared into the continuum
An emission spectrum in which lines overlap with each other
Line spectrum spectrum where discrete lines are emitted and do not go through all of the 7
colours of the rainbow
Occurs when light passes through a cold, dilute gas and atoms in the gas absorb at
characteristic frequencies
Since re-emitted light is unlikely to be emitted in the same direction as the absorbed photon,
this causes dark lines (absence of light)
2.3.3 Explain how the lines in the emission spectrum of hydrogen are related to electron energy
levels
Electrons in their shells can receive energy in the form of heat or electricity and jump to
higher energy levels (promotion)
They cannot remain at these higher levels (in an excited state) for very long because it is
unstable, and soon fall back to their original shell (or other shells)
When they fall back (relax) they low the energy difference between two shells
This loss of energy is performed by releasing electromagnetic energy in the form of infrared,
visible light or UV radiation.
Movement of electrons between the shells is called electron transitions
When electron transitions take place, the energy emitted can be detected and the
wavelength measured
Provides information about the relative energies of the shells
In the H atom, energy emitted appears in several series of lines corresponding to electrons
falling back to different levels
IB Chemistry 2013
n = 1: UV section of the EMS; gives the value of the Ionisation energy (Lyman series)
n = 2: Visible spectrum Balmer series
n = 3,4,5 produces spectra in the Infrared spectrum
Spectral lines get closer (converge) with increasing frequency electronic energy levels get
closer the further away they are from the nucleus
2.3.4 Deduce the electron arrangement for atoms and ions up to Z = 20
E.g. 2, 8, 7 for Z = 17
12.1 Electron configuration (HL)
12.1.1 Explain how evidence from first ionization energies across periods accounts for the
existence of main energy levels and sub-levels in atoms
First ionisation energy varies moving from element to element
Outermost electron is being removed in each case
As electrons move further away from the nucleus, ionisation energy decreases as there is
less attraction between nucleus and electrons
An element at higher energy level will have lower ionisation energy and vice versa
Therefore, difference in first ionisation energy can be attributed to different energy levels
IB Chemistry 2013
12.1.2 Explain how successive ionisation energy data is related to the electron configuration of
an atom
Sudden increase in ionisation energy = moved up one energy level (s, p, d to f)
Highest ionisation energy in a table most likely groups 5 8 (highly electronegative)
12.1.3 State the relative energies of s, p, d and f orbitals in a single energy level
The relative energy of s, p, d and f orbitals in a single energy level is as follows:
s < p < d < f
12.1.4 State the maximum number of orbitals in a given energy level
Energy level Max no. of orbitals Max no. of electrons
s 1 2
p 3 6
d 5 10
f 7 14
12.1.5 Draw the structure of an s orbital and the shapes of the P
x,
P
y
and P
z
orbitals
1s orbital
2s orbital
3s orbital
Px orbital
Py orbital
Pz orbital
IB Chemistry 2013
12.1.6 Apply the Aufbau principle, Hunds rule and the Pauli exclusion principle to write electron
configurations for atoms and ions up to Z = 54
Hunds rule: every orbital in a subshell is singly occupied before any orbital is doubly occupied
by 2 electrons
Aufbaus principle: Electrons start filling up from the lowest possible energy level before
moving to higher energy levels
Paulis Exclusion principle: No two electrons can occupy the exact same space
E.g. Z = 23:
1s
2
2s
2
2p
6
3s
2
3p
6
4s
2
3d
3
OR [Ar]3d
3
4s
2
EXCEPTIONS:
Chromium: [Ar] 4s
2
3d
5
opper: [Ar] 4s
1
3d
10
Due to extra stability of half full and full sets of d orbitals respectively
Вам также может понравиться
- IBO Chemistry Syllabus Coverage in BoardworksДокумент28 страницIBO Chemistry Syllabus Coverage in BoardworksMary MannuОценок пока нет
- Dynamic Equilibrium Reactions Reach Constant ConcentrationsДокумент22 страницыDynamic Equilibrium Reactions Reach Constant ConcentrationsAN NGUYENОценок пока нет
- Pre-IB Chemistry Mid-Term Review List (Nagel)Документ3 страницыPre-IB Chemistry Mid-Term Review List (Nagel)Helie100% (1)
- U3 Oxidation and Reduction PPT WatermarkДокумент45 страницU3 Oxidation and Reduction PPT Watermarkapi-125934329Оценок пока нет
- CHem IA Oxalyic AcidДокумент5 страницCHem IA Oxalyic AcidKennard ChiaОценок пока нет
- Ib Chemistry SL SyllabusДокумент3 страницыIb Chemistry SL Syllabusapi-235378008Оценок пока нет
- Redox WKSHTДокумент4 страницыRedox WKSHTMarco ConopioОценок пока нет
- IB Chemistry Internal Assessment 2Документ18 страницIB Chemistry Internal Assessment 2beslisevvalОценок пока нет
- Edexcel A-Level Chemistry Redox I NotesДокумент6 страницEdexcel A-Level Chemistry Redox I NotesttjjjОценок пока нет
- Chapter: 1 Stoichiometric Relationships: SubtopicsДокумент108 страницChapter: 1 Stoichiometric Relationships: SubtopicsBОценок пока нет
- Moles Equations AtomsДокумент44 страницыMoles Equations AtomsRamesh IyerОценок пока нет
- Born-Haber CycleДокумент5 страницBorn-Haber CycleShahnaz AhmedОценок пока нет
- IGCSE Chemistry DefinitionsДокумент5 страницIGCSE Chemistry DefinitionsTanmay Karur100% (1)
- Structural Isomers But-1-ene & But-2-eneДокумент3 страницыStructural Isomers But-1-ene & But-2-enedanielmahsaОценок пока нет
- Edexcel IAS Bonding 1Документ14 страницEdexcel IAS Bonding 1mostafa barakatОценок пока нет
- Photochemical ReactionДокумент16 страницPhotochemical ReactionChandra ReddyОценок пока нет
- Organic Chemistry NomenclatureДокумент8 страницOrganic Chemistry NomenclaturetasneemОценок пока нет
- Chap 8 Reaction Kinetics 1415FARRAДокумент129 страницChap 8 Reaction Kinetics 1415FARRA黄麒安Оценок пока нет
- IB Chemistry Periodicity NotesДокумент5 страницIB Chemistry Periodicity Notescgao30Оценок пока нет
- H2 Chem Summary of Chemical PeriodicityДокумент7 страницH2 Chem Summary of Chemical Periodicityonnoez100% (2)
- Topic 4 Chemistry IA IBДокумент3 страницыTopic 4 Chemistry IA IBDanisa IriantoОценок пока нет
- IB Chemistry Objectives - KineticsДокумент1 страницаIB Chemistry Objectives - KineticslizarrdoОценок пока нет
- Brown Chemistry PracticalsДокумент26 страницBrown Chemistry PracticalsSadiaMaryamОценок пока нет
- Crystal Violet KineticsДокумент9 страницCrystal Violet KineticsMario VaОценок пока нет
- Things To Learn in 0654 Syllabus 2023 & 2024Документ53 страницыThings To Learn in 0654 Syllabus 2023 & 2024akdEp dkОценок пока нет
- Chemistry A LevelДокумент104 страницыChemistry A Levelrockykj100% (1)
- I A Extended Essay Ideas For Ib ChemistryДокумент3 страницыI A Extended Essay Ideas For Ib ChemistryAaliyaОценок пока нет
- Prescribed Practicals Lab Manual 2016Документ28 страницPrescribed Practicals Lab Manual 2016rbgrossОценок пока нет
- Electroplating of Chromium Coatings From CR (III) - Based Electrolytes Containing Water Soluble PolymerДокумент10 страницElectroplating of Chromium Coatings From CR (III) - Based Electrolytes Containing Water Soluble Polymertonny356Оценок пока нет
- Section 3 EnergeticsДокумент47 страницSection 3 Energeticsapi-3734333Оценок пока нет
- Assessment IB Chemistry PracticalsДокумент7 страницAssessment IB Chemistry Practicalsنور هدايو احمدОценок пока нет
- A-Level-Chemistry Edexcel FACER Sample-Chapter PDFДокумент36 страницA-Level-Chemistry Edexcel FACER Sample-Chapter PDFahamedОценок пока нет
- IB Acids and BasesДокумент45 страницIB Acids and BasesAhmad Hajj AliОценок пока нет
- Chemical Equilibria & Ionic Equilibria CIE AS Level Questions SolvedДокумент16 страницChemical Equilibria & Ionic Equilibria CIE AS Level Questions Solveddanielmahsa50% (2)
- Chem IA 3 Hess' LawДокумент8 страницChem IA 3 Hess' LawSimone Lund SøegaardОценок пока нет
- Chemistry Internal Assessment IBДокумент41 страницаChemistry Internal Assessment IBJuan VillanuevaОценок пока нет
- Ib Chemistry Syllabus 2016-2017Документ5 страницIb Chemistry Syllabus 2016-2017api-325581554Оценок пока нет
- Edexcel Chemistry A-level Organic Chemistry I NotesДокумент29 страницEdexcel Chemistry A-level Organic Chemistry I NotesttjjjОценок пока нет
- Sum of coefficients in balanced chemical equationДокумент34 страницыSum of coefficients in balanced chemical equationAruba Dhaduk100% (2)
- Voltaic Cell Design Lab - How Temperature Affects VoltageДокумент2 страницыVoltaic Cell Design Lab - How Temperature Affects VoltageTheVioletFrost83% (6)
- Chemistry Unit 4 Part 3 ReallyacademicsДокумент35 страницChemistry Unit 4 Part 3 ReallyacademicsWill AndyОценок пока нет
- 9647 H2 Chemistry PlanningДокумент3 страницы9647 H2 Chemistry PlanningNicholas Ow100% (1)
- Reaction Kinetics WSДокумент44 страницыReaction Kinetics WSMustufa FerozОценок пока нет
- A Level Notes On Redox ReactionДокумент32 страницыA Level Notes On Redox Reactionkmoiz427Оценок пока нет
- Chemistry Topic One QuestionsДокумент30 страницChemistry Topic One QuestionsAruba Dhaduk100% (1)
- Balancing Chemical EquationsДокумент2 страницыBalancing Chemical Equationsirfan_ali_balochОценок пока нет
- Chemistry Edexcel As Keywords Unit 1Документ4 страницыChemistry Edexcel As Keywords Unit 1Ashan BopitiyaОценок пока нет
- CIE Chemistry Revision Guide For A2 LevelДокумент15 страницCIE Chemistry Revision Guide For A2 LevelBakhita MaryamОценок пока нет
- Chemistry Periodic Trends ActivityДокумент6 страницChemistry Periodic Trends ActivityocОценок пока нет
- Diagram Economics Undergraduate ProgrammeДокумент1 страницаDiagram Economics Undergraduate ProgrammeLouisDiMariaОценок пока нет
- Molecular Shapes WorksheetДокумент5 страницMolecular Shapes WorksheetAbdur RehmanОценок пока нет
- Definitions of Standard Enthalpy ChangesДокумент9 страницDefinitions of Standard Enthalpy ChangesWang RuyiОценок пока нет
- A1 Chem Book 1 PDFДокумент292 страницыA1 Chem Book 1 PDFsheuli rahman100% (1)
- Practice Makes Perfect in Chemistry: Compounds, Reactions and MolesОт EverandPractice Makes Perfect in Chemistry: Compounds, Reactions and MolesОценок пока нет
- CD30 Draft CollationДокумент8 страницCD30 Draft Collationcgao30Оценок пока нет
- Commonwealth Statutory Declaration Form (May 2011) PDFДокумент2 страницыCommonwealth Statutory Declaration Form (May 2011) PDFcgao30Оценок пока нет
- Commonwealth Statutory Declaration Form (May 2011) PDFДокумент2 страницыCommonwealth Statutory Declaration Form (May 2011) PDFcgao30Оценок пока нет
- Pythagoras WorksheetДокумент2 страницыPythagoras Worksheetcgao30Оценок пока нет
- Theme IV Yr 5d Content Guide - MatrixДокумент9 страницTheme IV Yr 5d Content Guide - Matrixcgao30Оценок пока нет
- CD30 Literature Review PlanДокумент1 страницаCD30 Literature Review Plancgao30Оценок пока нет
- CD marker panel guide for flow cytometry cell identificationДокумент2 страницыCD marker panel guide for flow cytometry cell identificationcgao30Оценок пока нет
- CPPREP4002 - Annotated Unit GuideДокумент8 страницCPPREP4002 - Annotated Unit Guidecgao30Оценок пока нет
- Student Elective Rotation GuideДокумент3 страницыStudent Elective Rotation Guidecgao30Оценок пока нет
- Pelvic Organ ProlapseДокумент5 страницPelvic Organ Prolapsecgao30Оценок пока нет
- CD30 Literature Review PlanДокумент1 страницаCD30 Literature Review Plancgao30Оценок пока нет
- CD30 Literature Review PlanДокумент1 страницаCD30 Literature Review Plancgao30Оценок пока нет
- Lit Reviews For RX Students v7Документ20 страницLit Reviews For RX Students v7Joshua McdonaldОценок пока нет
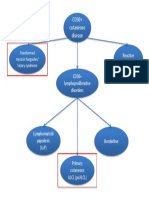
- CD30+ cutaneous lymphoproliferative disordersДокумент1 страницаCD30+ cutaneous lymphoproliferative disorderscgao30Оценок пока нет
- Paediatrics: Acyanotic Heart DiseaseДокумент5 страницPaediatrics: Acyanotic Heart Diseasecgao30Оценок пока нет
- Benign Lesions of The Vulva & Vagina (Table)Документ3 страницыBenign Lesions of The Vulva & Vagina (Table)cgao30Оценок пока нет
- Preterm LabourДокумент3 страницыPreterm Labourcgao30Оценок пока нет
- Pathology Lecture 2 - NeoplasiaДокумент15 страницPathology Lecture 2 - Neoplasiacgao30Оценок пока нет
- Fibroids: 1. Red DegenerationДокумент2 страницыFibroids: 1. Red Degenerationcgao30Оценок пока нет
- Matrix TopicsДокумент3 страницыMatrix Topicscgao30Оценок пока нет
- O&G GlossaryДокумент3 страницыO&G Glossarycgao30Оценок пока нет
- EndometriosisДокумент1 страницаEndometriosiscgao30Оценок пока нет
- Pathology Lecture 7 - LiverДокумент11 страницPathology Lecture 7 - Livercgao30Оценок пока нет
- Skin Terms: DescriptorsДокумент2 страницыSkin Terms: Descriptorscgao30Оценок пока нет
- Pathology Lecture 7 - LiverДокумент11 страницPathology Lecture 7 - Livercgao30Оценок пока нет
- Intro To Motor Systems: (Why Else Would You Go To The Gym, Right?)Документ13 страницIntro To Motor Systems: (Why Else Would You Go To The Gym, Right?)cgao30Оценок пока нет
- Pathology Lecture 5 - Upper GITДокумент10 страницPathology Lecture 5 - Upper GITcgao30Оценок пока нет
- Inflammatory Conditions (Disease Mechanisms)Документ3 страницыInflammatory Conditions (Disease Mechanisms)cgao30Оценок пока нет
- Cell Respiration Notes (3&8)Документ9 страницCell Respiration Notes (3&8)cgao30Оценок пока нет
- Dna Notes (3&7) - CompleteДокумент11 страницDna Notes (3&7) - Completecgao30100% (1)
- Beryllium and Beryllium Compounds: 2005 Wiley-Vch Verlag GMBH & Co. Kgaa, WeinheimДокумент28 страницBeryllium and Beryllium Compounds: 2005 Wiley-Vch Verlag GMBH & Co. Kgaa, WeinheimjaimeОценок пока нет
- Physics Questions Part 3Документ8 страницPhysics Questions Part 3Muhammad HuzaifaОценок пока нет
- Gay-Lussac's Law Problems and SolutionsДокумент1 страницаGay-Lussac's Law Problems and SolutionsBasic PhysicsОценок пока нет
- PNA Chemistry Expedite 8900 User's GuideДокумент114 страницPNA Chemistry Expedite 8900 User's GuideJohnОценок пока нет
- Solar System and Astronomy FactsДокумент535 страницSolar System and Astronomy FactsKavita KrishnamorthiОценок пока нет
- BEYOND SYLLABUS: CURVED, COMPOSITE, UNSYMMETRICAL BEAMSДокумент11 страницBEYOND SYLLABUS: CURVED, COMPOSITE, UNSYMMETRICAL BEAMSVignesh VickyОценок пока нет
- Jar TestДокумент1 страницаJar TestEduardo Tonino Chavez GaytanОценок пока нет
- Basic Hazen Williams FormulaДокумент28 страницBasic Hazen Williams FormulaDhimas IriantoОценок пока нет
- Chapter 22 Thermal Expansion: EXERCISE 122, Page 266Документ9 страницChapter 22 Thermal Expansion: EXERCISE 122, Page 266NurulAinMatAron0% (1)
- The Law of OneДокумент745 страницThe Law of OneSuprakash100% (18)
- Heat of Hydration StressesДокумент7 страницHeat of Hydration StressesAnkur BarsainyaОценок пока нет
- Chapter - 3.2 - Finale Internal Forced ConvectionДокумент18 страницChapter - 3.2 - Finale Internal Forced ConvectioneirinaОценок пока нет
- Laser Beam Energy Distribution Affects Weld DimensionsДокумент8 страницLaser Beam Energy Distribution Affects Weld Dimensionskppsiva87Оценок пока нет
- API 510 Pressure Vessel Inspector Certification Preparation CourseДокумент4 страницыAPI 510 Pressure Vessel Inspector Certification Preparation CoursejbsantoОценок пока нет
- Filter Vessel Calculations Per As Me Viii 1Документ40 страницFilter Vessel Calculations Per As Me Viii 1Anonymous J1vjrU2Оценок пока нет
- L-3/T-2/CE Date: 07/08/2016Документ30 страницL-3/T-2/CE Date: 07/08/2016নীল জোছনা0% (1)
- IOE, TU Questions and Solutions: Engineering Physics (for BE first yearДокумент235 страницIOE, TU Questions and Solutions: Engineering Physics (for BE first yearRajeev PaudelОценок пока нет
- Elzaki Transform For Two Tank Mixing Problems PDFДокумент15 страницElzaki Transform For Two Tank Mixing Problems PDFMarvin LabajoОценок пока нет
- Wiki Unified Soil Classification SystemДокумент2 страницыWiki Unified Soil Classification SystemGIRISHA001Оценок пока нет
- Cluster ExpansionДокумент4 страницыCluster ExpansionflytrapsolОценок пока нет
- NextFEM Designer Users Manual v1.10 p3Документ96 страницNextFEM Designer Users Manual v1.10 p3Anonymous kBodCGQ79Оценок пока нет
- Calculation of Electrical Induction Near Power LinesДокумент22 страницыCalculation of Electrical Induction Near Power LinesalpcruzОценок пока нет
- Radar Systems Range Equation PDFДокумент6 страницRadar Systems Range Equation PDFSanjid ElahiОценок пока нет
- 03 Modern Photoelectric Effect LabДокумент3 страницы03 Modern Photoelectric Effect LabJuan David ParraОценок пока нет
- Learning Plan in Grade 8 and Grade 7Документ14 страницLearning Plan in Grade 8 and Grade 7marilyncomia73100% (3)
- Worksheet On Quantum NumbersДокумент2 страницыWorksheet On Quantum NumbersJannah Mae IsioОценок пока нет
- Judo Bio MechanicsДокумент11 страницJudo Bio MechanicsAttilio Sacripanti100% (6)
- Stainless Steel PropertiesДокумент3 страницыStainless Steel Propertieskiwanis_lamОценок пока нет
- The Planets Comparative Superlative - 87358Документ2 страницыThe Planets Comparative Superlative - 87358Maria AdamОценок пока нет
- PFlow ScriptДокумент6 страницPFlow Scripttohu777Оценок пока нет