Академический Документы
Профессиональный Документы
Культура Документы
Quantum Mechanics of NMR
Загружено:
mishs14Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
Quantum Mechanics of NMR
Загружено:
mishs14Авторское право:
Доступные форматы
The Quantum Mechanics of MRI
Part 1: Basic concepts
David Milstead
Stockholm University
Background reading
Fundamentals of Physics, Halliday,
Resnick and Walker (Wiley)
The Basics of NMR, J. Hornak
(http://www.cis.rit.edu/htbooks/nmr/inside.
htm )
Outline
What is quantum mechanics
Wave-particle duality
Schrdingers equation
Bizarreness
uncertainty principle
energy and momentum quantisation
precession of angular momentum
What is quantum mechanics ?
Basic concepts
What is a wave ?
What is a particle ?
What is electromagnetic radiation ?
What happens to a magnet in a magnetic
field ?
What is a wave ?
Double slit diffraction
Properties of waves:
Superposition, no localisation (where is a wave??)
What is electromagnetic radiation ?
EM radiation is made up of electromagnetic waves
of various wavelengths and frequencies
Radio frequency is of most interest to us
Circularly polarised light
Radiation can be produced and filtered to produce a rotating magnetic
field.
Rotating magnetic field
Light is a wave!
Photon
Photons
34
6.626 10
Quanta of light:
Energy
=Planck's constant= Js
=frequency
= =wavelength, speed
E hf
h
f
v
v
f
=
Wave-particle Duality
34
10
Particles also show wave-like properties
Wave-length = Energy=
=frequency, =momentum, =6.6 Js Planck's constant.
h
hf
p
f p h
Weve just learned a basic result of quantum mechanics.
Now we move onto some maths.
Fundamental equation of quantum
mechanics
( )
wave function
=particle energy
=potential energy of particle
x
E
U
=
( )
Re( ) x
x
Free particle
0
Well defined wavelength and momentum but no preferential
position.
"Uncertainty on position":
"Uncertainty on momentum":
x
p
=
=
( )
2 2
2
2
2
0
2
cos sin
2
2
; Fixed
-
constant - particle can be anywhere!
; ;
fixed wavelength and momentum
U E
E
m x
A kx i kx
h p
p k E
m
=
=
= +
=
= = =
A particle not confined or subject to forces.
Wave function of a confined particle
p
x
p
Eg particle trapped in a tiny region of space. How do we model the
wave function ?
Solution to Schrdingers equation would be
sum lots of sine waves with different wavelengths/momenta.
x
x
Similarly:
y z
p y h p z h > >
Question
A 12-g bullet leaves a rifle at a speed of 180m/s. a) What is the wavelength of this
bullet? b) If the position of the bullet is known to an accuracy of 0.60 cm (radius of
barrel), what is the minimum uncertainty in its momentum? c) If the accuracy of
the bullet were determined only by the uncertainty principle (an unreasonable
assumption), by how much might the bullet miss a pinpoint target 200m away?.
34
34
34
31 1
31
31
6.6 10
3.06 10
0.012 180
6.6 10
10
0.006
10
200 200 9 10
0.12 180
(a) Wavelength m
(b) kgms
(c) Displacement m Tiny!
Because is so small, quantum effects are nev
h
p
h
p
x
p
p
h
= = =
er seen for macroscopic
objects.
Hydrogen atom
Solve Schrdingers equation for an electron around a
proton in a hydrogen atom.
The electron is confined due to a Coulomb potential.
2
0
4
proton charge
e
U e
r
= =
( )
- , - 1 ,...., 1,
(2 1)
Component along one direction:
different states.
z l l
L m m l l l l
l
= =
+
2
where
h
=
Where is the electron ?
Wave functions
Current loops and magnetic dipoles
-
+
N
S
Current loop
Bar magnet
Orbiting electron as a current loop
2
1
2 2 2 2
2
Tiny current loop.
charge
current
period
Magnetic moment
area of loop
Angular momentum:
(parallel to angular momentum)
Gyrom
e
l
e
dq e
I
dt T
IAn
A
v ev ev evR
IA A R
T R R R
L m vr
e
L
m
= = =
=
=
= = = = =
=
=
2
agnetic ratio: =-
l
e
e
L m
=
e
R
into the page. n
Recall that the electron has fixed values of
Z
L
2 2
l z
z z
em eL
U B B
m m
= =
An atom in a magnetic field
l=1 and therefore 2l+1 states
E
- -
2
(1) 1
2 2
(2) 0 0 ; 0
(3) 1
2 2
; ( antiparallel with )
; ( parallel with )
Two states with
l
l lz z lz
e
l lz z l
e e
l lz
l lz z z l
e e
em
U B B
m
e e
m U B B
m m
m U
e e
m B U B B
m m
= = =
= = =
= = =
= = =
shifted energy one state with no energy shift. +
2
z z
B
(1)
(2)
(3)
l=0
l=1
( ) ( )
2
2 2 2 2 2 2
,
1
What happens when a circulating electron is placed
in a magnetic field.
We know that are fixed and known.
constant
By symmetry, same spread of results of
or when mea
Z
x y Z l
x y
L L
L L L L l l m
L L
+ = = + =
sin
1 1
sin
sin sin
2
2
surements of made.
i.e. just as likely to find angular momentum and .
Magnetic field introduces a torque
=
i.e
l l
l
l
e
l
e
X Y
dL
B B
dt
B d dL
B
dt L dt L L
m eB
L
e m
= = =
= = =
= =
.
2
. the angular momentum vector precesses around the magnetic
field ( -axis) with frequency This is Larmor precession.
Gyromagnetic ratio :
e
z
e
B m
= =
L
Z
U
X
x
y
z
Angular momentum precession
L
Z
constant and L precesses around z-axis
Question
.
Is it possible for an electron in a hydrogen atom to have ?
Show that this value can be approached for large values of
Would be possible using classical physics ?
No - it can never happen o
Z
Z
L L
l
L L
=
=
( )
2 2 2 2 2
,
0
1
therwise we would "know" values of
i.e.
For large
Max
(comes close)
In classical physics there is no restriction on what we can and ca
x y
x y
Z l Z
Z
L L
L L
L l l l L l L l
L m L l
L L
= =
= + = =
= =
n't know.
The mathematics of spin angular momentum is identical to orbital
angular momentum.
F
N
F
S
F
N
F
S
Magnetic field
Magnetic field
Ignore force not parallel to North-South axis.
Magnetic field
Magnetic field
Magnetic field
Zeeman effect with orbital and spin
angular momentum
In the presence of
a magnetic field,
multiplicities of
spectral lines
appear
1
0
2
2 1 2
Eg for ,
number of lines:
l s
n s
= =
= + =
2
B
B
2
2
Identical to orbital case.
;
spin magnetic moment
Electron:
electron g-factor 2, electron mass.
Nucleus:
nuclear g-factor, proton mass.
(ne
s
s
e e
e
e e
N N p
p
S B
e
g
m
g m
e
g g m
m
= =
=
=
= =
= = =
xt lecture)
S
Z
x
y
z
Larmor precession for spin
S sin
S
Summary
Established basic quantum mechanics
theory needed for NMR
wave-particle duality
Light is either photons or electromagnetic waves
Schrdingers equation and the wave function
at the heart of QM predictions
Energy and angular momentum are quantised
Larmor precession
Angular momentum comes in two varieties
(orbital and spin)
The Quantum Mechanics of MRI
Part 2: Understanding MRI
David Milstead
Stockholm University
Outline
Spin - reminder
Fermions and bosons
Nuclear energy levels
2 2
( 1)
2
2
Identical in form to orbital case.
spin magnetic moment
Electron:
electron g-factor 2, electron mass.
Nucleus:
nuclear g-factor,
z s
s
s
e e
e
e e
N N
p
S s s S m
B
S
e
g
m
g m
e
g g
m
= + =
=
=
=
=
= =
= =
proton mass.
(next lecture)
p
m =
S
Z
x
y
z
Spin
S sin
S
Gyromagnetic ratio
Why do they have different values ?
Fermions and bosons
Fermions
Spin 1/2, 3/2, 5/2 objects
Electrons, protons and neutrons have spin 1/2
Tricky bit comes when combining their spins
to form the spin of, eg, an atom or a nucleus
Bosons
Integer spin objects
Similar shell structure for nuclear
physics as for atomic physics
Need to fill up, shell by shell
Pairs of protons and neutrons cancel
each others spins.
Paulis exclusion principle ensures
that many shells are filled.
Nuclei with uneven (even) atomic
number have half-integer (integer)
spin
Nuclei with even atomic and mass
numbers have zero spin.
Unpaired neutrons/protons provide
the spin for MRI.
Question
Nature would prefer all electrons to be in
the lowest shell and all nucleons
protons/neutrons) in the lowest shell ?
Why doesnt this happen ?
Usefulness for MRI
Need isotopes with unpaired protons (to
produce signal for MRI)
Most elements have isotopes with non-
zero nuclear spin
Natural abundance must be high enough
for MRI to be performed.
Spins of various nuclei
Now we can understand MRI
0
1
1 0
?
We know the basics:
A uniform magnetic field B
A short pulse of a rf field B
A system out of equilibrium
measurement of return to equilibrium.
Why is
(a)
(b) The rf pulse shorter than recovery
B B
<<
time ?
No magnetic field
Apply an external magnetic field
.
, 0
1
2
Spins precess at Larmor
frequency
Gyromagnetic ratio:
Precessions incoherent:
Total spin
Z x y
Z
B
S S S S
S
=
= = =
=
B
0
In fact, there are continual transitions
and interactions from thermal energy.
Another look at the system
Split into spin-zones.
For uniform system we can regard the macroscopic system as giving
a single magnetisation.
Conventional to talk about magnetisation :
; no. dipoles, volume
s
M
N
M N V
V
= = =
Putting together what weve learned
0
0 0 0
,
2
1
2 2 4
2
2
Need to know its energy splitting.
=spin angular momentum nuclear g-factor proton mass.
;
Frequency of light need
s
N p
p
sZ sZ z N N
p p
p
sZ
p
e
g S g m
S m
e e
E B S g g
m m
g e
E B B
m
= = = =
= = = =
= = =
0 0
0
0 0
2
2
ed for excitation
Larmor frequency:
How many nuclei can be excited ?
p
p
N
p
g e
B
m
eB
B g
m
=
= = =
0
0
Energy level population
Nature has a preference for the lowest energy states.
In thermal equilibrium the lowest states are a bit more
populated than the higher energy states.
2
23
0 0 0
6 26
0
1 ...
2!
1
2
1.5 42.576 10 2 4.2 10
Taylor expansion:
Boltzmann's constant = 1.38 10 J/K
=room temperature 310 K
T J
x
B B
B
p
p
x
e x
N E E
e
N k T k T
k
T
g e
E B B
m
B E
N
N
= + + +
= +
=
= = =
= = =
0
1.000009
1
2 2
p
p B
g e N
N
N N N N B
N m k T
=
= =
Changing the spin populations
15
Tiny differences in, eg, 0.02ml of water
expect 610 more in parallel state.
Question
Chemists can excite certain samples using UV light. Would you expect
to be substantially different in this case ? What does this imply for
the relative size of the sample of a MRI scan and a test
N
N
8
8 16 1
8
34 16 17
17
23
3 10
10 3 10
10
6.6 10 3 10 2 10
2 10
1 1 1 5000
1.4 10 300
made by
chemists with infrared light ?
UV-light m s
J
In fact, approximation no longer valid since it
B
c
f
E
N E
N k T
= = =
=
+ + +
is no longer a
small difference!!
A chemist can use far smaller samples since the energy gap
is larger and there are far more in the lower energy state.
Exciting the nuclei - Rf pulse
Rotating magnetic field B
1
Typical pulse duration ~1ms.
Two ways to think about the pulse. Both are needed to understand MRI.
Excitation
Rotating magnetic field B
1
B
0
Pulse of rf-
1
0.
The rf pulse acts in two ways:
(1) The photons are absorbed, saturating the system and reducing
(2) The rotating magnetic field acts on the magnetisation vector
to rotate into the complex plan
Z
M
B
1 1
1
1
1
e.
Individual dipole: ;
Use magnetisation: - simple form of the Bloch equations.
s
s s
d dS
B B
dt dt
dM
M B
dt
= = = =
=
B
1
Rotating frame of reference
, ', '
'
Easier to understand if a rotating frame of reference
(with Larmor frequency) is used.
Rotate co-ordinates
Magnetic field constant on axis.
x y x y
x
B
0
B
1
X
y
Different types of pulses
90
o
180
o
1
1
1
5
1
3 6
0
2
2 42.58
1 1
10
10 42.58 10
1
Duration of pulse and size of B determine angle of rotation of magnetisation:
Spins precess around
Consider a full rotation of in 1ms
MHz/T
T
B
B
t
B
B T
= =
=
=
=
1 0
. B B <<
Question
1
1 0
.
When applying a new magnetic field is obtained:
Why don't the protons just align with respect to that field ?
n
B
B B B = +
0
B
n
B
What happens next ?
-
.
Consider a 90 rotation.
The magnetisation vector is in the plane.
The -pulse is turned off.
The system must return to equilibrium.
We have two components: and
o
Z xy
x y
rf
M M
Longitudinal relaxation
Spin-lattice effect:
higher energy state interacts with lower energy state due and lose
energy through rotation and vibration .
Relaxation times for different materials
Transverse relaxation
In the laboratory frame
Dipole moments are initially in phase.
M precesses and decays.
As it precesses phase decoherence occurs.
Complicated process: contributing factor non-uniform magnetic
field ove
xy
r sample different precession rates for different regions.
Free induction decay
*
2
.
As the transverse magnetisation decays, a changing magnetic
field is produced:
=- an emf is produced in a receiver.
The signal is a decaying sinusoidal wave with lifetime
Measurement of gives
t
T
information on composition of sample.
Free induction decay
Easier to interpret a single line on a "frequency" spectrum.
Use a Fourier transform to move from damped exponential to
signal.
Summary
Basic quantum mechanics at the heart of
nuclear magnetic resonance
Angular momentum quantisation
Energy quantisation
Features of a MRI experiment
investigated.
Вам также может понравиться
- Feynman Lectures Simplified 2C: Electromagnetism: in Relativity & in Dense MatterОт EverandFeynman Lectures Simplified 2C: Electromagnetism: in Relativity & in Dense MatterОценок пока нет
- Classical Mechanics - 20%Документ9 страницClassical Mechanics - 20%bekozagreОценок пока нет
- Drude Model For Dielectric Constant of MetalsДокумент24 страницыDrude Model For Dielectric Constant of MetalsRupesh ChaudhariОценок пока нет
- Atomic SpectrosДокумент36 страницAtomic SpectrosAswin AlexОценок пока нет
- Chapter 5. Periodicity & Atomic Structure Wave PhenomenaДокумент4 страницыChapter 5. Periodicity & Atomic Structure Wave PhenomenaVictoria MooreОценок пока нет
- Lecture 12Документ26 страницLecture 12Taylor JammiesonОценок пока нет
- Quantum Model of The Hydrogen Atom - Clase Martes - 15!10!2013Документ44 страницыQuantum Model of The Hydrogen Atom - Clase Martes - 15!10!2013cperezScribdОценок пока нет
- Studysheet For GREДокумент15 страницStudysheet For GREMuqarraulAhmadОценок пока нет
- General SB12 2005Документ14 страницGeneral SB12 2005Asif HameedОценок пока нет
- Rydberg's Spectrum AnalysisДокумент50 страницRydberg's Spectrum AnalysisUday Prakash SahuОценок пока нет
- Optical Spectra and X-RaysДокумент19 страницOptical Spectra and X-Rayssanjay sОценок пока нет
- Charles Kittel Intro Solid State PhysicsДокумент27 страницCharles Kittel Intro Solid State PhysicsAndrés Escárraga CuéllarОценок пока нет
- Atomic StructureДокумент28 страницAtomic StructurePavan GoudОценок пока нет
- Quantum TheoryДокумент20 страницQuantum TheorynathansolaiОценок пока нет
- Chem Notes Chapter 7 - AlkanesДокумент66 страницChem Notes Chapter 7 - AlkanesjohnОценок пока нет
- Determining n and μ: The Hall Effect V, E I, J B r = qE r + qv r In steady state, = −ev, the Hall Field Since v =-J /en,Документ14 страницDetermining n and μ: The Hall Effect V, E I, J B r = qE r + qv r In steady state, = −ev, the Hall Field Since v =-J /en,Bestha HarishОценок пока нет
- Lecture 2Документ55 страницLecture 2Zahid SaleemОценок пока нет
- Physical Chemistry Study GuideДокумент9 страницPhysical Chemistry Study Guidekrymxen100% (2)
- ZeemanДокумент26 страницZeemanscrusnОценок пока нет
- Chapter 8 Atomic StructureДокумент68 страницChapter 8 Atomic StructureHaqnawaz100% (1)
- Atomic and Laser Physics: PH-102 (Physics) : B. Tech. - I Year Spring Semester: 2006-07 Tutorial Sheet No. 3Документ2 страницыAtomic and Laser Physics: PH-102 (Physics) : B. Tech. - I Year Spring Semester: 2006-07 Tutorial Sheet No. 3Tegar Wicaksana AdiansyachОценок пока нет
- Structure of AtomДокумент29 страницStructure of AtomAbhisek DehuriОценок пока нет
- GCL 08Документ19 страницGCL 08sahanishubham317Оценок пока нет
- Chapter 4-Structure of AtomДокумент49 страницChapter 4-Structure of AtomDn Zack0% (1)
- Instrukcja PS1 cw4Документ22 страницыInstrukcja PS1 cw4verenichdanielfiОценок пока нет
- 4 Unit-2Документ21 страница4 Unit-2hopefulantonelliОценок пока нет
- Magnetism and Magnetic MaterialsДокумент56 страницMagnetism and Magnetic Materialsbhuneshwar paswanОценок пока нет
- XI-Chemistry-DOE Support Material 2019-20 - 2Документ22 страницыXI-Chemistry-DOE Support Material 2019-20 - 2MeersОценок пока нет
- Nuclear ModelsДокумент15 страницNuclear ModelsLouis FortunatoОценок пока нет
- CH 7Документ60 страницCH 7Paul ArcillaОценок пока нет
- Inorganic Chemistry by Shriver & AtkinsДокумент81 страницаInorganic Chemistry by Shriver & AtkinsSalma KhoirunnisaОценок пока нет
- Microwave Infrared: SpectrosДокумент66 страницMicrowave Infrared: SpectrosPrathamesh Dash100% (2)
- Chapter 1 - Atomic Structure CHEMISTRYДокумент16 страницChapter 1 - Atomic Structure CHEMISTRYMaite Aícua UbiernaОценок пока нет
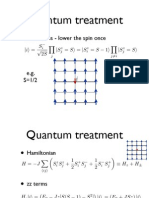
- Quantum Treatment: Excitations - Lower The Spin OnceДокумент21 страницаQuantum Treatment: Excitations - Lower The Spin OnceAllie IelanОценок пока нет
- Lecture 1 - Quantum & Atomic StructureДокумент41 страницаLecture 1 - Quantum & Atomic Structurejasumin91Оценок пока нет
- Lecture 4 Wavefunction NewДокумент53 страницыLecture 4 Wavefunction NewkedirОценок пока нет
- Inorganic Chem. I Ch. 1Документ98 страницInorganic Chem. I Ch. 1Shifa GhannamОценок пока нет
- A HidrogenatomДокумент33 страницыA HidrogenatomRévész CsabiОценок пока нет
- IOSR 2019 YanvarДокумент6 страницIOSR 2019 YanvarShahla Ganbar IlkinОценок пока нет
- Elements of Physical Chemistry Atkin & DepaulaДокумент40 страницElements of Physical Chemistry Atkin & DepaulaKaushikОценок пока нет
- Atomic Term SymbolДокумент13 страницAtomic Term SymbolUmendra KhokharОценок пока нет
- DR TMMP (Quantum)Документ50 страницDR TMMP (Quantum)Tmmp SmileОценок пока нет
- Physics of Magnetic Resonance: Suetens Chapter 4Документ38 страницPhysics of Magnetic Resonance: Suetens Chapter 4Leo DingОценок пока нет
- NMR and ESR NotesДокумент38 страницNMR and ESR NotesJasonLopez100% (1)
- Motion of The Atoms: Lattice VibrationsДокумент11 страницMotion of The Atoms: Lattice VibrationsBadri DadiОценок пока нет
- 1997-Linear Modes by MofizДокумент7 страниц1997-Linear Modes by MofizTehsin ChowdhuryОценок пока нет
- Electronic Theory of ChemistryДокумент43 страницыElectronic Theory of ChemistryMaheshОценок пока нет
- CHEM 111 UNIT 02 - Atomic Structure & PeriodicityДокумент48 страницCHEM 111 UNIT 02 - Atomic Structure & PeriodicitycarlОценок пока нет
- PlasmonsДокумент42 страницыPlasmonsNguyễn Thanh TiềnОценок пока нет
- Lecture 6 - Jan 24 PDFДокумент22 страницыLecture 6 - Jan 24 PDFhirankrОценок пока нет
- Rotational Motion: Rotation in Two Dimensions: A Particle On A RingДокумент41 страницаRotational Motion: Rotation in Two Dimensions: A Particle On A RingAdelia Ayu WandiraОценок пока нет
- General Chemistry: CHEM F111Документ30 страницGeneral Chemistry: CHEM F111Harsh TiwariОценок пока нет
- Slides EC574 Part I F2013Документ175 страницSlides EC574 Part I F2013Lauren StevensonОценок пока нет
- MIT6 007S11 Lec37 PDFДокумент33 страницыMIT6 007S11 Lec37 PDFSidharth AryaОценок пока нет
- Concept Map of Solid State PhysicsДокумент5 страницConcept Map of Solid State PhysicsSusan LingОценок пока нет
- Curie-Weiss Law Wiess Theory of ParamagnetismДокумент11 страницCurie-Weiss Law Wiess Theory of ParamagnetismShashank SharmaОценок пока нет
- ACFrOgC xiuXYskoUCylB1F9L1IEJcj Ta KoLk6CTvaGqltDFWA2qAIKTeqUvP 0 7U6kDWb0J0D4WA8tzfPGbl3oDF5E2WbfWJwsRiEHl3syXtQRSt2h85SwNwBgДокумент71 страницаACFrOgC xiuXYskoUCylB1F9L1IEJcj Ta KoLk6CTvaGqltDFWA2qAIKTeqUvP 0 7U6kDWb0J0D4WA8tzfPGbl3oDF5E2WbfWJwsRiEHl3syXtQRSt2h85SwNwBgJihanaisyОценок пока нет
- Interactions between Electromagnetic Fields and Matter: Vieweg Tracts in Pure and Applied PhysicsОт EverandInteractions between Electromagnetic Fields and Matter: Vieweg Tracts in Pure and Applied PhysicsОценок пока нет
- Pulsed NMRДокумент26 страницPulsed NMRmishs14Оценок пока нет
- Physics of MRIДокумент83 страницыPhysics of MRImishs14100% (1)
- Primer in QM NMRДокумент21 страницаPrimer in QM NMRmishs14Оценок пока нет
- MRI NotesДокумент33 страницыMRI Notesmishs14Оценок пока нет
- Chapter 5 of MRIДокумент16 страницChapter 5 of MRImishs14Оценок пока нет
- Chapter 2 of MRI NotesДокумент42 страницыChapter 2 of MRI Notesmishs14Оценок пока нет
- Chapt-4 of MRI NotesДокумент11 страницChapt-4 of MRI Notesmishs14Оценок пока нет
- Quantum Mechanical Principles in NMRДокумент54 страницыQuantum Mechanical Principles in NMRmishs14Оценок пока нет
- Magnetic Nanoparticles Synthesis, Protection, Functionalization, and ApplicationДокумент23 страницыMagnetic Nanoparticles Synthesis, Protection, Functionalization, and Applicationl0ngfellowОценок пока нет
- Excel Slab ProgramsДокумент18 страницExcel Slab ProgramsSanjithRNair100% (1)
- Sierra Megonnell and Kyle Lovisone ExpДокумент1 страницаSierra Megonnell and Kyle Lovisone Expapi-528179516Оценок пока нет
- ISA - 75.01.01.2012 - Flow Capacity - Sizing Equations For Fluid FlowДокумент70 страницISA - 75.01.01.2012 - Flow Capacity - Sizing Equations For Fluid FlowCarlos Ramos100% (1)
- Stress Strain Curve ExplanationДокумент8 страницStress Strain Curve ExplanationGptc Chekkanurani0% (1)
- Kde Pa KDF Pa KDG Pa KDH PaДокумент1 страницаKde Pa KDF Pa KDG Pa KDH PafalanksОценок пока нет
- BetaДокумент3 страницыBetacrg1234Оценок пока нет
- Cutting FluidДокумент8 страницCutting FluidDevarakonda KondayyaОценок пока нет
- Dioscorea HispidaДокумент9 страницDioscorea HispidaHorcruxesОценок пока нет
- Amali 3Документ9 страницAmali 3Aiman FarhanОценок пока нет
- Chapter 1-DMC 101-Basic ConceptsДокумент11 страницChapter 1-DMC 101-Basic ConceptsArivalagan RevichandranОценок пока нет
- Eurovent Energy Efficiency CalculatorДокумент1 страницаEurovent Energy Efficiency CalculatorPradeep Sukumaran100% (1)
- Confined Space Identification and Hazard Documentation: Executive DevelopmentДокумент31 страницаConfined Space Identification and Hazard Documentation: Executive Developmentequipaeng0% (1)
- Phenolics TableДокумент2 страницыPhenolics TableKULDEEP THAKUR100% (1)
- Robustness of The QAL2 Calibration EN14181 UncertaДокумент10 страницRobustness of The QAL2 Calibration EN14181 UncertaAnaibar TarikОценок пока нет
- Ismael AnabalonДокумент35 страницIsmael Anabalondnavarrete01Оценок пока нет
- Wave Induced AccelerationДокумент6 страницWave Induced AccelerationAnonymous g5FCwMRОценок пока нет
- Section 9 Introduction To Welding ProcessesДокумент16 страницSection 9 Introduction To Welding ProcessesS GoudaОценок пока нет
- ADVANTAGES. of Polymer Insulator PDFДокумент1 страницаADVANTAGES. of Polymer Insulator PDFviksoniОценок пока нет
- Eurocode 8-1-3Документ18 страницEurocode 8-1-3joaoОценок пока нет
- Dapus SuspensiДокумент6 страницDapus SuspensiRachmiОценок пока нет
- Welded Connections PDFДокумент16 страницWelded Connections PDFAnkit SinghОценок пока нет
- Check Valve (Swagelok) MS-01-176 PDFДокумент16 страницCheck Valve (Swagelok) MS-01-176 PDFIsmailIbrahimОценок пока нет
- Nuclear Magnetic Resonance (NMR)Документ49 страницNuclear Magnetic Resonance (NMR)hanifghaziruОценок пока нет
- Pak Ketut 1 PDFДокумент32 страницыPak Ketut 1 PDFArini Aprilliani LanriОценок пока нет
- Assignment 8 9Документ2 страницыAssignment 8 9Nuwan BandaraОценок пока нет
- Sample CylinderДокумент9 страницSample CylindercanakyuzОценок пока нет
- Implementing Claim, Evidence, ReasoningДокумент12 страницImplementing Claim, Evidence, ReasoningGreeshma ColumbusОценок пока нет
- Assigment of NDTДокумент3 страницыAssigment of NDTHamid AliОценок пока нет
- Comparative Investigation of Organic CompundsДокумент6 страницComparative Investigation of Organic CompundsKizer Dela Cruz100% (1)