Академический Документы
Профессиональный Документы
Культура Документы
Breast
Загружено:
candiddreamsИсходное описание:
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
Breast
Загружено:
candiddreamsАвторское право:
Доступные форматы
LETTER TO THE EDITOR
Myxoid myofibroblastoma of the breast with atypical cells:
a potential diagnostic pitfall
Gaetano Magro & Paolo Amico & Alessandra Gurrera
Received: 16 December 2006 / Revised: 11 January 2007 / Accepted: 18 January 2007 / Published online: 15 February 2007
# Springer-Verlag 2007
Keywords Myofibroblastoma
.
Myxoid variant
.
Atypical cells
.
Breast
Sir,
Myofibroblastoma of the breast is an unusual benign
mesenchymal tumour that belongs to the family of the so-
called benign spindle cell tumours of the mammary
stroma [5, 6]. In its classic type, it is typically composed of
bland-looking spindle cells exhibiting a variable fibro
myofibroblastic differentiation [5, 6]. In recent years, the
morphological spectrum of this tumour has been broadened
by the recognition of several variants, including epithelioid,
lipomatous, fibromatosis-like, fibrous/collagenized, myxoid
ones [46].
Recently, we have encountered two cases of mammary
myofibroblastoma with atypical cells embedded within a
prominent myxoid stroma. To the best of our knowledge,
this unusual morphological variant has not hitherto been
reported. Two male patients (63 and 56 years) presented
with two nodular breast masses, measuring 3 and 3.5 cm in
greatest dimension, respectively, which were surgically
excised.
Histologically, both surgical samples were represented
by unencapsulated mesenchymal tumours with pushing
borders, composed of spindle to epithelioid cells, embedded
in an abundant myxoid stroma containing isolated thick
eosinophilic collagen bands (Figs. 1a and 2a). Myxoid
matrix stained positively with Alcian blue at pH 2.5 and
negative for periodic acid-Schiff. One tumour was hypo-
cellular (Fig. 1a), while the other one showed alternating
hypocellular and moderately cellular areas (Fig. 2a). Neo-
plastic cells had pale to deeply eosinophilic cytoplasm and
round to oval nuclei containing one or two visible nucleoli.
In both cases, a significant number of neoplastic cells (50
60%) exhibited a moderate to severe degree of nuclear
pleomorphism (Figs. 1a,b and 2a,b). Some cells were bi- or
multinucleated (Fig. 2b). A minor mature fatty component
was scattered throughout both tumours. Mitoses were
absent in the hypocellular tumour, while one mitosis per
ten high power fields was observed in the other neoplasm.
Necrosis was absent. No epithelial component was identi-
fied within both tumours. Immunohistochemically, the cells
of both cases had a similar profile: diffuse and strong
immunoreactivity to vimentin, -smooth muscle actin and
desmin (Fig. 1c), and a variable staining to CD34, bcl-2
protein, CD99, estrogen (ER), progesterone (PR), and
androgen (AR) receptors. No immunoreactivity was
obtained for pancytokeratin, epithelial membrane antigen,
h-caldesmon, calponin, S-100 protein, and human mela-
noma black-45 (HMB-45). This immunophenotype was
consistent with a fibro-myofibroblastic nature of the neo-
plastic cells.
We believe that the tumours herein presented fit within
the spectrum of breast myofibroblastoma [5, 6], represent-
ing an uncommon morphological variant, for which the
descriptive term myxoid myofibroblastoma with atypical
cells is proposed. The following morphological and
immunohistochemical features, typically seen in myofibro-
blastoma [5, 6], support our opinion: (1) Tumours were
pure mesenchymal lesions lacking any epithelial compo-
nent; (2) Tumours had pushing borders; (3) The myxoid
extracellular matrix contained thick eosinophilic collagen
bands; (4) Neoplastic cells resulted to have a fibro-
Virchows Arch (2007) 450:483485
DOI 10.1007/s00428-007-0373-z
G. Magro (*)
:
P. Amico
:
A. Gurrera
Dipartimento G.F. Ingrassia, Anatomia Patologica,
Universit di Catania,
Via S. Sofia 87,
95123 Catania, Italy
e-mail: g.magro@unict.it
myofibroblastic profile by means of immunohistochemistry
(diffuse immunoreactivity to vimentin, -smooth muscle
actin and desmin); and (5) Neoplastic cells variably
expressed CD34, bcl-2, CD99, ER, PR, and AR receptors.
Although the presence of myxoid areas or atypical cells
has been documented in some breast myofibroblastomas [1,
3, 5, 6], their association in the same tumour could pose
diagnostic problems with some malignant myxoid neo-
plasms. However, the absence of infiltrative margins, high
mitotic activity, atypical mitoses, and necrosis argues
against malignancy. In our opinion, cellular pleomorphism
in breast myofibroblastoma should be interpreted as a
degenerative phenomenon, similarly to what is observed
in the so-called atypical bizarre leiomyomas, ancient
schwannoma, which does not adversely affect the bio-
logical behaviour of tumour, accordingly. Differential
diagnosis mainly revolved around the myxoid type of
spindle cell lipoma and solitary fibrous tumour. Discrim-
ination between myofibroblastoma and spindle cell lipoma/
solitary fibrous tumour may be difficult, as they are closely
related neoplasms, likely arising from a common mammary
stromal stem cell capable to differentiate along several
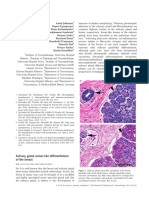
Fig. 1 a At low magnification, a hypocellular myxoid tumour with
pushing borders can be appreciated. b Myxoid hypocellular tumour
with atypical spindle-shaped cells. Thick eosinophilic collagen bands
are seen. Severe nuclear pleomorphism is evident. c Neoplastic cells
are immunoreactive to desmin
Fig. 2 a Myxoid tumour with
moderate cellularity. b At
higher magnification, neoplastic
spindle, epithelioid cells with
severe nuclear pleomorphism
are seen. Some cells are
multinucleated
484 Virchows Arch (2007) 450:483485
mesenchymal lines, including the fibroblastic, myofibro-
blastic, and lipomatous ones [46]. This hypothesis is
supported by the fact that all these tumours share some
morphological and immunohistochemical features and by
the recognition of hybrid tumours that simultaneously
exhibit features of MFB and solitary fibrous tumour or
MFB and spindle cell/pleomorphic lipoma [3, 5, 6].
Cytogenetic analyses, revealing that spindle cell lipoma
and myofibroblastoma have similar chromosome 13 rear-
rangements associated with the loss of the 13q14 chromo-
somal region [2, 7], seem to confirm what we have
previously postulated. We admit that immunohistochemis-
try was crucial for classification of our tumours as
myofibroblastomas, in consonance with the ability of
neoplastic cells to differentiate along a myofibroblastic line,
as demonstrated by their immunophenotype (-smooth
muscle actin
+
, desmin
+
, calponin
, h-caldesmon
).
In conclusion, the present paper, describing two cases of
myxoid myofibroblastoma with atypical cells, contributes
to widen the morphological spectrum of this unusual
mammary tumour. Although this rare variant exhibits some
worrisome morphological features that could alarm a
pathologist, the correct diagnosis is confidentially achieved
if the well-established morphological and immunohisto-
chemical criteria of myofibroblastoma are applied [5].
References
1. Lazaro-Santander R, Garcia-Prats MD, Nieto S, Andres-Gozalvo C,
Cortes, Vizcaino V, Vargas-Holguin S, Vera-Roman JM (1999)
Myofibroblastoma of the breast with diverse histological features.
Virchows Arch 434:547550
2. Maggiani F, Debiec-Rychter M, Verbeeck G, Sciot R (2006)
Extramammary myofibroblastoma is genetically related to spindle
cell lipoma. Virchows Arch 449:244247
3. Magro G, Fraggetta F, Torrisi A, Emmanuele C, Lanzafame S
(1999) Myofibroblastoma of the breast with hemangiopericytoma-
like pattern and pleomorphic lipoma-like areas. Report of a case
with diagnostic and histogenetic considerations. Pathol Res Pract
195:257262
4. Magro G, Michal M, Bisceglia M (2000) Lipomatous myofi-
broblastoma: a potential diagnostic pitfall in the spectrum of
the spindle cell lesions of the breast. Virchows Arch 437:540
547
5. Magro G, Michal M, Bisceglia M (2001) Benign spindle cell
tumors of the mammary stroma: diagnostic criteria, classi-
fication and histogenesis. Review. Pathol Res Pract 197:453
466
6. Magro G, Bisceglia M, Michal M, Eusebi V (2002) Spindle cell
lipoma-like tumor, solitary fibrous tumor and myofibroblastoma
of the breast: a clinico-pathological analysis of 13 cases in
favor of a unifying histogenetic concept. Virchows Arch
440:249260
7. Pauwels P, Sciot R, Croiset F, Rutten H, Van den Berghe H, Dal
Cin P (2000) Myofibroblastoma of the breast: genetic link with
spindle cell lipoma. J Pathol 191:282285
Virchows Arch (2007) 450:483485 485
Вам также может понравиться
- Case Report Epithelial-Myoepithelial Carcinoma of The Breast With Rhabdoid FeaturesДокумент4 страницыCase Report Epithelial-Myoepithelial Carcinoma of The Breast With Rhabdoid FeaturesGabriela Izabela BaltatescuОценок пока нет
- Breast Immuno PaperДокумент16 страницBreast Immuno PaperArlen ElisaОценок пока нет
- Imagenes Citologicas, Benignas, de MamaДокумент19 страницImagenes Citologicas, Benignas, de MamaPauloRosalesОценок пока нет
- Acino in BreastДокумент2 страницыAcino in BreastDima PathОценок пока нет
- Complex Adnexal TumorДокумент2 страницыComplex Adnexal TumorFaduahSalazarОценок пока нет
- IHC of Salivary DifferentiationДокумент12 страницIHC of Salivary DifferentiationMohamed ArafaОценок пока нет
- Stem CellsДокумент16 страницStem CellsdregopokeОценок пока нет
- Management Fam 1998Документ5 страницManagement Fam 1998Envhy WinaОценок пока нет
- Alonso Et Al. 1996Документ10 страницAlonso Et Al. 1996Priyanka Vaswani HareshОценок пока нет
- Articulo de Serie de CasoДокумент8 страницArticulo de Serie de CasoJairo Lino BОценок пока нет
- Fibromatosis-Like Metaplastic CarcinomaДокумент8 страницFibromatosis-Like Metaplastic CarcinomaKata TölgyesiОценок пока нет
- Myxofibrosarcoma: Christina L. Roland,, Wei-Lien Wang,, Alexander J. Lazar,, Keila E. TorresДокумент14 страницMyxofibrosarcoma: Christina L. Roland,, Wei-Lien Wang,, Alexander J. Lazar,, Keila E. TorresMarina GorelikОценок пока нет
- (12204749 - Romanian Journal of Internal Medicine) Multiple Histological Subtypes of Dermatofibrosarcoma Protuberans Occurring in The Same TumorДокумент10 страниц(12204749 - Romanian Journal of Internal Medicine) Multiple Histological Subtypes of Dermatofibrosarcoma Protuberans Occurring in The Same TumordrelvОценок пока нет
- Liebmann 1998Документ11 страницLiebmann 1998Lucy ReyesОценок пока нет
- Adenomyoepithelioma of The Breast: A Brief Diagnostic ReviewДокумент5 страницAdenomyoepithelioma of The Breast: A Brief Diagnostic ReviewSFCHIKIОценок пока нет
- Bilateral Giant Juvenile Fibroadenoma of Breasts: Ase EportДокумент2 страницыBilateral Giant Juvenile Fibroadenoma of Breasts: Ase EportCata santaОценок пока нет
- 2011-01 Goldblum Common Morphologic PatternsДокумент35 страниц2011-01 Goldblum Common Morphologic PatternsDrRobin SabharwalОценок пока нет
- Pathologic Quiz Case: Residents' PageДокумент3 страницыPathologic Quiz Case: Residents' Pagejeka222Оценок пока нет
- Komatsu 2020Документ5 страницKomatsu 2020Felipe RohamОценок пока нет
- Neurofibroma, Schwannoma or A Hybrid Tumor of The Peripheral Nerve Sheath?Документ4 страницыNeurofibroma, Schwannoma or A Hybrid Tumor of The Peripheral Nerve Sheath?As AsОценок пока нет
- Molecular Diagnosis in Breast Cancer: Mini-Symposium: Breast PathologyДокумент12 страницMolecular Diagnosis in Breast Cancer: Mini-Symposium: Breast PathologyOber Van Gómez LópezОценок пока нет
- Malignant Phyllodes Tumor of The Breast: A Practice Review: AbstractДокумент11 страницMalignant Phyllodes Tumor of The Breast: A Practice Review: AbstractFrancis BillanesОценок пока нет
- Density of Tumor Associated MacrophДокумент8 страницDensity of Tumor Associated MacrophPalloma PortoОценок пока нет
- IHC - 2014 0057 RaДокумент16 страницIHC - 2014 0057 Raparisa rezaieОценок пока нет
- Pediatric Surgery Update Volume 33, 2009Документ12 страницPediatric Surgery Update Volume 33, 2009rajarshikОценок пока нет
- Histological Types of Breast Cancer How Special Are TheyДокумент17 страницHistological Types of Breast Cancer How Special Are TheysilviailieОценок пока нет
- Stem Cells and Cancer Stem Cells, Volume 3 - Stem Cells and Cancer Stem Cells, Therapeutic Applications in Disease and Injury - Volume 3Документ426 страницStem Cells and Cancer Stem Cells, Volume 3 - Stem Cells and Cancer Stem Cells, Therapeutic Applications in Disease and Injury - Volume 3Artan100% (1)
- Tumor Phyloides JurnalДокумент5 страницTumor Phyloides JurnalSuzika DewiОценок пока нет
- Oncovet 2008Документ18 страницOncovet 2008Frederico VelascoОценок пока нет
- Oligodendroglioma in The Cervical Spinal Cord of A Dog: T. M, A. M - L, M. H - T, W. BДокумент3 страницыOligodendroglioma in The Cervical Spinal Cord of A Dog: T. M, A. M - L, M. H - T, W. BSamir BazanОценок пока нет
- Sfasciotti GL., 2021Документ3 страницыSfasciotti GL., 2021Bruna FerreiraОценок пока нет
- Arpa - 2012 0443 RaДокумент12 страницArpa - 2012 0443 RaAdriana Gabriela Ugarte MacíasОценок пока нет
- Abstract For Cytocon 2010Документ1 страницаAbstract For Cytocon 2010brijmkumarОценок пока нет
- Adenomatoid Tumors of The Uterus: An Analysis of 60 CasesДокумент7 страницAdenomatoid Tumors of The Uterus: An Analysis of 60 CasesGabriela OliveiraОценок пока нет
- Small Round Cells of Head and NeckДокумент10 страницSmall Round Cells of Head and NeckBlazxy EyreОценок пока нет
- Tan 2011Документ7 страницTan 2011Jairo Lino BОценок пока нет
- Jcad 2 8 30Документ3 страницыJcad 2 8 30Mariana ValerianoОценок пока нет
- Cancers: Drug Treatment of Cancer Cell Lines: A Way To Select For Cancer Stem Cells?Документ18 страницCancers: Drug Treatment of Cancer Cell Lines: A Way To Select For Cancer Stem Cells?AppleОценок пока нет
- Azurophilic Inclusions in Plasma CellДокумент2 страницыAzurophilic Inclusions in Plasma CellManjiri JoshiОценок пока нет
- Pathogenesis and Malignant Transformation of Adenomyosis (Review)Документ7 страницPathogenesis and Malignant Transformation of Adenomyosis (Review)miss betawiОценок пока нет
- MamosphereДокумент10 страницMamospheremuruganvvkОценок пока нет
- Rabdomyosarcoma Case ReportДокумент7 страницRabdomyosarcoma Case ReportAaslesha Jakkampudi100% (1)
- Stem Cells in Breast Tumours - Are They Ready For The Clinicď Ą, 2012Документ13 страницStem Cells in Breast Tumours - Are They Ready For The Clinicď Ą, 2012Daniela GologanОценок пока нет
- Article - Cytological Features of The Warthin-Like Variant of Salivary Mucoepidermoid CarcinomaДокумент5 страницArticle - Cytological Features of The Warthin-Like Variant of Salivary Mucoepidermoid CarcinomaCandeОценок пока нет
- A Comparative Review of Canine and Human Rhabdomyosarcoma Con Enfasis en Clasificacion y Patogenesis - Caserto2013Документ21 страницаA Comparative Review of Canine and Human Rhabdomyosarcoma Con Enfasis en Clasificacion y Patogenesis - Caserto2013Jhoel Sebastian Torres GaonaОценок пока нет
- What Are Markers For Breast Cancer Stem CellsДокумент8 страницWhat Are Markers For Breast Cancer Stem CellsTahir AliОценок пока нет
- Metaplastic Carcinoma Breast: A Case SeriesДокумент4 страницыMetaplastic Carcinoma Breast: A Case SeriesIJAR JOURNALОценок пока нет
- Pathology of Germ Cell Tumors of The TestisДокумент14 страницPathology of Germ Cell Tumors of The TestisAnonymous be1sWu6l6Оценок пока нет
- Saimura 1999Документ6 страницSaimura 1999Mariela Judith UgarteОценок пока нет
- Immunohistochemistry As A Diagnostic Aid in The Evaluation of Ovarian TumorsДокумент30 страницImmunohistochemistry As A Diagnostic Aid in The Evaluation of Ovarian TumorsFarah MutiaraОценок пока нет
- Prognostic Factors in Phyllodes Tumor of The Breast: Are Immunohistochemical Biomarkers Useful?Документ2 страницыPrognostic Factors in Phyllodes Tumor of The Breast: Are Immunohistochemical Biomarkers Useful?Harley Septian WilliОценок пока нет
- Clinical and Genetic Characterization of Basal Cell Carcinoma and Breast Cancer in A Single PatientДокумент7 страницClinical and Genetic Characterization of Basal Cell Carcinoma and Breast Cancer in A Single PatientMahmoud AlshahatОценок пока нет
- Lim 2016Документ11 страницLim 2016Chi NgôОценок пока нет
- X BibliДокумент7 страницX BibliBJ CarminatorОценок пока нет
- Subtypes of Breast CancerДокумент6 страницSubtypes of Breast CancerAcademic JournalОценок пока нет
- Cirugi A Espan Ola: Calcifying Cystic Fibrous Tumour. A Rare Form of Benign Peritoneal CarcinomatosisДокумент2 страницыCirugi A Espan Ola: Calcifying Cystic Fibrous Tumour. A Rare Form of Benign Peritoneal CarcinomatosisSani Widya FirnandaОценок пока нет
- p63 Expression3Документ15 страницp63 Expression3isela castroОценок пока нет
- Lipoblastoma Case ReportДокумент10 страницLipoblastoma Case ReportFaisal AshfaqueОценок пока нет
- Melanocytic Lesions: A Case Based ApproachОт EverandMelanocytic Lesions: A Case Based ApproachMai P. HoangОценок пока нет
- Best Television SeriesДокумент37 страницBest Television SeriescandiddreamsОценок пока нет
- NeuroendoДокумент8 страницNeuroendocandiddreamsОценок пока нет
- MesotheliomaДокумент7 страницMesotheliomacandiddreamsОценок пока нет
- Polymorphous Breast CA.Документ6 страницPolymorphous Breast CA.candiddreamsОценок пока нет
- Combined Set of Kaplan 900 and High Frequency Words PDFДокумент17 страницCombined Set of Kaplan 900 and High Frequency Words PDFcandiddreams100% (2)
- MGCTДокумент11 страницMGCTcandiddreamsОценок пока нет
- Lung OsteomaДокумент4 страницыLung OsteomacandiddreamsОценок пока нет
- Eye AstrocytomaДокумент5 страницEye AstrocytomacandiddreamsОценок пока нет
- Gastric CancerДокумент8 страницGastric CancercandiddreamsОценок пока нет
- Primary Intracranial Leiomyoma: A Case Report and Literature ReviewДокумент3 страницыPrimary Intracranial Leiomyoma: A Case Report and Literature ReviewcandiddreamsОценок пока нет
- MANI Quality Control in Hematology AnalysersДокумент65 страницMANI Quality Control in Hematology Analyserscandiddreams100% (1)
- Meningioma SДокумент10 страницMeningioma ScandiddreamsОценок пока нет
- LeiomyomaДокумент3 страницыLeiomyomacandiddreamsОценок пока нет
- Apocrine Breast LesionsДокумент7 страницApocrine Breast LesionscandiddreamsОценок пока нет
- Current Practice of Gleason Grading of Prostate Carcinoma: ReviewarticleДокумент8 страницCurrent Practice of Gleason Grading of Prostate Carcinoma: ReviewarticlecandiddreamsОценок пока нет
- Statistical Approach in HematologyДокумент33 страницыStatistical Approach in HematologycandiddreamsОценок пока нет
- Clonality Analysis in Hematolymphoid Malignancies: DR Jay MehtaДокумент65 страницClonality Analysis in Hematolymphoid Malignancies: DR Jay MehtacandiddreamsОценок пока нет
- AtherosclerosisДокумент8 страницAtherosclerosiscandiddreamsОценок пока нет
- Validation Cell AnalyzersДокумент45 страницValidation Cell AnalyzerscandiddreamsОценок пока нет
- Normal Hematolymphoid TissuesДокумент182 страницыNormal Hematolymphoid TissuescandiddreamsОценок пока нет
- Statistical Approach in HematologyДокумент33 страницыStatistical Approach in HematologycandiddreamsОценок пока нет
- TMH PBS PresentationДокумент61 страницаTMH PBS PresentationcandiddreamsОценок пока нет
- Basic IHC FinalДокумент81 страницаBasic IHC FinalcandiddreamsОценок пока нет
- Gujral FCMДокумент102 страницыGujral FCMcandiddreamsОценок пока нет
- Mean Normal Prothombin Time (MNPT)Документ10 страницMean Normal Prothombin Time (MNPT)candiddreamsОценок пока нет
- Gujral FCM 2Документ128 страницGujral FCM 2candiddreamsОценок пока нет
- A Borges AccreditationДокумент23 страницыA Borges AccreditationcandiddreamsОценок пока нет
- Bone Marrow Aspiration in HematooncologyДокумент60 страницBone Marrow Aspiration in HematooncologycandiddreamsОценок пока нет
- Final Flags WorkshopДокумент29 страницFinal Flags WorkshopcandiddreamsОценок пока нет
- 13 - L J Charts Wastgard RuleДокумент36 страниц13 - L J Charts Wastgard RulecandiddreamsОценок пока нет
- Cardiology Case 1Документ2 страницыCardiology Case 1vil62650% (2)
- Role of Trace Minerals in Reproduction of Dairy AnimalДокумент27 страницRole of Trace Minerals in Reproduction of Dairy AnimalDr. Umesh Sontakke83% (6)
- GBS Management, Medications and Nursing ConsiderationsДокумент4 страницыGBS Management, Medications and Nursing Considerationssouledg3100% (1)
- Peripheral Nerve InjuryДокумент28 страницPeripheral Nerve InjuryRoydenPTОценок пока нет
- Plant Structure and FunctionДокумент14 страницPlant Structure and FunctionPatrickNantesSalvani100% (1)
- ReceptionДокумент37 страницReceptionRyanОценок пока нет
- CH 11 Head, Hand, Face ExamДокумент29 страницCH 11 Head, Hand, Face ExamDyan Karla Cosare - BacayanaОценок пока нет
- Transfusion Reaction PDFДокумент1 страницаTransfusion Reaction PDFKah Man GohОценок пока нет
- Introduction To The Body As A WholeДокумент29 страницIntroduction To The Body As A Wholekhizer hayatОценок пока нет
- Reticulocyte - Wikipedia, The Free EncyclopediaДокумент2 страницыReticulocyte - Wikipedia, The Free EncyclopediaAniket MittalОценок пока нет
- NSG Process-Chitra MamДокумент44 страницыNSG Process-Chitra MamJalajarani AridassОценок пока нет
- Bu LesДокумент91 страницаBu LesAnonymous zQ72D9cPrCОценок пока нет
- Psychology StudiesДокумент9 страницPsychology StudiesaaravОценок пока нет
- Bio ACE Form 4Документ3 страницыBio ACE Form 4Myramel KlarisОценок пока нет
- Oxidative Phosphorylation V Inhibitors and UncouplersДокумент15 страницOxidative Phosphorylation V Inhibitors and UncouplersIffatnazОценок пока нет
- Antiasthamatic DrugsДокумент72 страницыAntiasthamatic DrugsDeepak kumarОценок пока нет
- EPA Air Quality Index School Activity ChartДокумент2 страницыEPA Air Quality Index School Activity ChartCourier JournalОценок пока нет
- Handbook of Pediatric Eye and Systemic Disease PDFДокумент650 страницHandbook of Pediatric Eye and Systemic Disease PDFBangun Said SantosoОценок пока нет
- Notes On Cell OrganellesДокумент11 страницNotes On Cell Organellesapi-241062194Оценок пока нет
- Compact: 3 EditionДокумент155 страницCompact: 3 EditionDr S n vermaОценок пока нет
- Biochemistry Quizzes by Ronnie BaticulonДокумент20 страницBiochemistry Quizzes by Ronnie BaticulonMhartin GarciaОценок пока нет
- Pharmacotherapeutics, Pharmacokinetics, and PharmacodynamicsДокумент40 страницPharmacotherapeutics, Pharmacokinetics, and PharmacodynamicsPerry BearОценок пока нет
- Ivan Pavlov Classical ConditioningДокумент6 страницIvan Pavlov Classical ConditioningJulie Ann NazОценок пока нет
- Conditions That Cause Interference On Most Hematology AnalyzersДокумент2 страницыConditions That Cause Interference On Most Hematology AnalyzersSamantha IsabelОценок пока нет
- Lecture 2 Tooth Eruption and SheddingДокумент35 страницLecture 2 Tooth Eruption and SheddingAMIT GUPTAОценок пока нет
- CHAMBER 9 GENETIC KISS (The Conclusion or Is It?)Документ18 страницCHAMBER 9 GENETIC KISS (The Conclusion or Is It?)Atyeb Ba Atum Re100% (3)
- NCM 112 Pre Test On EcgДокумент2 страницыNCM 112 Pre Test On EcgTrixia AlmendralОценок пока нет
- Eighth Grade (Grade 8) Respiration, Digestion, and Excretion Questions For Tests and WorksheetsДокумент1 страницаEighth Grade (Grade 8) Respiration, Digestion, and Excretion Questions For Tests and WorksheetsJam Abdul Razzak(Student)Оценок пока нет
- Instant Download Ebook PDF Essentials of Anatomy Physiology 2nd Edition 2 PDF ScribdДокумент41 страницаInstant Download Ebook PDF Essentials of Anatomy Physiology 2nd Edition 2 PDF Scribdandrew.harrell532100% (41)
- Features of The Muscular SystemДокумент34 страницыFeatures of The Muscular SystemQuỳnh NhưОценок пока нет