Академический Документы
Профессиональный Документы
Культура Документы
Heat and Temperature
Загружено:
WanMardziyyahАвторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
Heat and Temperature
Загружено:
WanMardziyyahАвторское право:
Доступные форматы
X INTRODUCTION
Do you know that the human body has a built in thermostat i.e. a temperature-
control mechanism called the hypothalamus? The hypothalamus region of the brain
helps keep our bodies at a constant temperature of about 37C. However,
sometimes it requires our intervention as well. Imagine sitting in a packed class
room where the air-conditioner does not function. As time goes on, you begin to
feel uncomfortable as the temperature in the room begins to soar. After a longer
period, the room may become too hot for your comfort! By this time, your
concentration starts to wander. An iced drink now or a short spell under a fan will
definitely help cool your body.
Nomads living in desert regions where the temperature can reach 50C have to
wear special clothing to prevent dehydration and heat stroke. By contrast, when
visiting temperate countries, we need to wear more clothes or stay indoors to keep
warm.
Thus, in this topic we will discuss heat and temperature. We often confuse the two,
but they are very different. We will also encounter the concept of heat that is
caused by temperature differences. Heat associated with temperature change in a
T
T
o
o
p
p
i
i
c
c
1
1
X
Heat and
Temperature
LEARNING OUTCOMES
By the end of this topic, you should be able to:
1. Identify various temperature scales and thermometers;
2. Explain the thermal expansion of a heated object;
3. Differentiate between specific and latent heat; and
4. Apply the above to related problems.
X TOPIC 1 HEAT AND TEMPERATURE
2
body is known as specific heat whereas heat associated with phase changes is
known as latent heat.
THE TEMPERATURE SCALE AND
THERMOMETERS
In this section, you will be introduced to temperature, temperature scales and the
units associated with these scales. The conversions between these different
temperature scales will be discussed. You will also be introduced to the various
types of thermometers that are available.
1.1.1 Heat and Temperature
Temperature T is a measure of how hot or cold an object is and is measured using
a thermometer. Temperature is measured in the SI units of Kelvin (K). Alternative
units that are in use are the Celsius or centigrade (C) and Fahrenheit (F).
Temperature does not depend on the amount of matter in a body.
Heat Q is a form of energy that is transferred from one body to another body when
there is a temperature difference between them. Heat applied to a body results in
molecular vibration. The kinetic energy of these vibrating molecules is known as
thermal energy. Heat depends on the amount of matter in a body. For example, a
bucket of water has more heat than a glass of water although both have the same
temperature.
1.1.2 Thermal Equilibrium and the Zeroth Law of
Thermodynamics
When two bodies are in contact with each other, heat will flow from one body to
another. The hotter body will transfer heat at a higher rate to the colder body while
the colder body will transfer heat as a lower rate to the hotter body. Thermal
equilibrium is reached when the rates of heat exchange are the same between the
two bodies. At this state, both bodies will have a physical quality of the same
value. This physical quality is called temperature.
The Zeroth Law of Thermodynamics states that if a body C is in thermal
equilibrium with body A and B, then body A and B are in thermal equilibrium with
respect to each other even in the absence of any thermal contact between them. If
T
A
= T
C
and T
B
= T
C
, therefore T
A
= T
B
. See the Figure 1.1 below:
1.1
TOPIC 1 HEAT AND TEMPERATURE W
3
Figure 1.1: A graphical representation of the Zeroth Law of Thermodynamics
The Zeroth Law of Thermodynamics is the basis of how a thermometer operates.
At thermal equilibrium, the temperature of the thermometer is equal to the
temperature of the environment (surroundings).
1.1.3 Temperature Scale
A thermometer is an instrument that measures temperature. It operates based on
how a thermometric quantity x changes with temperature T. The quantity x can
represent length (l), volume (V), pressure (p), electrical resistance (R) or the
electromotive force (emf).
A thermometer has two fixed points i.e.
(a) The Fixed Lower Point (ice point) is the temperature at which pure ice and
water exist in thermal equilibrium at a pressure of 1 atmosphere. The ice
point of water is 273.15 K which is equal to 0C or 32F.
(b) The Fixed Upper Point (steam point) is the temperature of water when it
exists in thermal equilibrium with steam at a pressure of 1 atmosphere. The
steam point of water is 373.15 K which is equal to 100C or 212F.
You place a cup of hot coffee on a table. Is it in thermal equilibrium
with the surroundings?
ACTIVITY 1.1
X TOPIC 1 HEAT AND TEMPERATURE
4
(c) In addition, the Triple Point is a unique point where ice, water and steam
exist in thermal equilibrium. The triple point of water is 273.16 K or 0.01C.
To calibrate the temperature scale on a thermometer, the upper point x
steam
and
lower point x
ice
which corresponds to the temperatures of steam T
steam
and ice T
ice
respectively are determined as shown in Figure 1.2. The temperature T which
corresponds to the thermometric value of x positioned between
x
ice
and x
steam
is given by:
ice
steam ice
x x
T n
x x
| |
|
\ .
(1.1)
where n is the number of equally spaced divisions between x
ice
and x
steam
.
Figure 1.2: Temperature scale
Thermometers calibrated in the Kelvin temperature scale have the ice point
and the steam point of 273.15 K and 373.15 K respectively. Therefore, the
temperature T in Kelvin is defined as:
100 273.15
ice
K
steam ice
x x
T
x x
| |
|
\ .
= +
(1.2)
Thermometers calibrated in the Celsius temperature scale have an upper point of
100C and lower point of 0C with 100 unit divisions between these two points.
TOPIC 1 HEAT AND TEMPERATURE W
5
From Equation 1.1, the temperature T in Celsius is defined as:
100
ice
C
steam ice
x x
T
x x
| |
|
\ .
(1.3)
By substituting T
C
from Equation 1.3 into Equation 1.2, we obtain the relation
between the Kelvin and Celsius temperature scales:
273.15
K C
T T = + (1.4)
For thermometers calibrated in the Fahrenheit temperature scale, the upper point
is 212F and lower point is 32F divided into 180 unit divisions between these two
points. Equation 1.1 can be written as:
180 32
ice
F
steam ice
x x
T
x x
| |
|
\ .
= +
(1.5)
With reference to Equations 1.3 and 1.5 above, the relation between the units
Fahrenheit and Celsius is:
9
5
32
F C
T T = + (1.6)
The absolute temperature scale is only applicable on special thermometers that are
calibrated in the absolute scale. This is because the zeroth point on this scale is the
temperature of absolute zero in the absolute temperature scale (Kelvin) at 0 K.
Example 1.1
Convert the temperature of 300 K to units of Celsius (C) and Fahrenheit (F).
Solution
With reference to Equation 1.4,
T
C
= T
K
273.15 K
T
C
= 300 K 273.15 K
= 26.85C
300 K is equal to 26.85C.
X TOPIC 1 HEAT AND TEMPERATURE
6
With reference to Equation 1.6,
9
32
5
9
26.85 32
5
80.33 F
F C
T T = +
| |
= +
|
\ .
=
Example 1.2
At what temperature is the Fahrenheit scale reading equal to the Celcius scale?
Solution
Let the unknown temperature be T and T = T
F
= T
C
. From Equation 1.6.
9
32
5
9
Then, 32
5
5 9 160
4 160
Therefore, 40 C 40 F
F C
T T
T T
T T
T
T
= +
= +
= +
=
= =
1.1.4 Thermometers
The various types of thermometers are explained below:
(a) Mercury Thermometer
Mercury thermometers are usually used in science laboratories. Mercury is
filled into a thick walled Pyrex glass capillary with a thin walled bulb at the
bottom. When temperature increases, the molecules in the mercury vibrate
with larger amplitudes. Consequently the average separation distance
between the molecules increase and mercury expands. The thermal expansion
of mercury is far more pronounced than that of glass for the equal amount of
heat absorbed. Hence the thermal expansion of the glass capillary is
negligible compared to the mercury column. The temperature T of a body
which corresponds to a length of l
T
in Celsius is:
TOPIC 1 HEAT AND TEMPERATURE W
7
0
100 0
( ) 100
T
l l
T C
l l
(1.7)
where l
100
and l
0
respectively are the lengths of the mercury column length at
steam and ice points.
Liquid mercury is used because it expands uniformly, does not adhere to the
glass capillary, is easily seen and rapidly achieves thermal equilibrium. The
operating temperature range of the mercury thermometer is from 39C to
357C only. The addition of inert gases into the capillary tube raises the
boiling point of mercury to 800C, hence raising the maximum operating
temperature.
(b) Constant Volume Gas Thermometer
These thermometers are rarely used and are only found in institutions of
higher learning. It consists of a glass bulb filled with a particular gas
connected to a glass column filled with mercury by a U-shaped rubber or
plastic tube. Figure 1.3 illustrates such a thermometer.
When the temperature increases, the pressure of the gas (P
T
) in the bulb
increases and is proportional to the difference h between the levels of
mercury in the arms of the U-shaped tube. The temperature T at pressure P
T
in units of Celsius is given by:
0
100 0
( ) 100
T
P P
T C
P P
(1.8)
where P
T
= P
A
+ gh, is the density of mercury, g is the gravitational
acceleration and P
A
is the atmospheric pressure which is equal to 1.1013
10
5
Pascal (Pa). P
100
is the pressure at steam point, while P
0
is the pressure at
ice point.
Expose a mercury thermometer in broad daylight. Does the reading on the
thermometers temperature scale indicate the temperature of the sun or
environment around it?
ACTIVITY 1.2
X TOPIC 1 HEAT AND TEMPERATURE
8
Figure 1.3: Constant volume gas thermometer
The constant volume thermometer has an operating range between 270C to
1500C. Note that since constant volume gas thermometers are extremely
sensitive to small temperature variations (or changes), it is usually used to
calibrate other thermometers.
(c) Thermocouple
Consider two wires of different materials connected at its ends. Each end or
junction is placed in separate mediums at different temperatures. It will be
noticed that an electromotive force (emf) will be induced between these two
junctions. This is known as the thermoelectric or the Seebeck effect.
Figure 1.4 shows a basic thermocouple which consists of copper and
constantan wires. The induced emf (in units of mV) varies with the
temperature difference. The magnitude of the emf will be greater if the
temperature difference is greater.
The temperature T which corresponds to the emf of
T
in units of Celsius is:
0
100 0
( ) 100
T
T C
(1.9)
where
0
is the emf at ice point and
100
is the emf at boiling point.
TOPIC 1 HEAT AND TEMPERATURE W
9
Figure 1.4: Thermocouple
Thermocouples are extremely sensitive and react promptly to temperature
changes. It is usually connected to a computer via an electronic device that
converts the analogue signals to digital data. Temperature changes can be
monitored accurately and recorded onto the computer. The operating range of
thermocouples is from 150C to 1150C.
(d) Resistance Thermometer
Resistance thermometers usually consist of a mica core wrapped by a
platinum coil. The ends of the coil are connected to a Wheatstone bridge by a
connecting wire A as in Figure 1.5. An identical connector B is connected to
another arm of the bridge and functions as a buffer. When the temperature of
the platinum coil changes, the resistance of the wire correspondingly
changes. The equilibrium point of the galvanometer G can be determined by
adjusting the rheostat S.
The resistance of the platinum coil R
T
with respect to temperature T of the
Wheatstone bridge is:
1
2
T
R
R S
R
= (1.10)
The temperature T at resistance of R
T
in units of Celsius is:
0
100 0
( ) 100
T
R R
T C
R R
(1.11)
X TOPIC 1 HEAT AND TEMPERATURE
10
Figure 1.5: Platinum resistance thermometer
The resistance thermometer measures temperatures accurately and has an
operating range between 180C to 1770C. It is unsuitable for measuring
rapid changes in temperature. This is because it takes time to adjust the
rheostat S to achieve the balance point on the galvanometer.
Example 1.3
The ice point of a resistance thermometer is 30.0O whereas its steam point is
62.0O. Calculate the temperature in units of Celsius when the thermometer
registers a resistance of 38.8O.
Solution
0
100 0
Room temperature 100
38.8 30.0
100
62.0 30.0
27.5 C
T
R R
T
R R
=
(e) Thermistor
Thermistors are semiconductor devices that respond to temperature changes.
The resistance of a thermistor decreases exponentially with respect to
temperature. Thermistors are approximately 20 times more sensitive than
resistance thermometers. Thermistors are extremely sensitive to minute
temperature changes and are very suitable for measuring rapid changes in
temperature. However thermistors are not as stable as resistance
thermometers and are not accurate. The operating range lies between 70C
to 300C.
TOPIC 1 HEAT AND TEMPERATURE W
11
(f) Optical Pyrometer
Extremely high temperatures cannot be measured by thermometers that
require direct physical contact. Such thermometers would be destroyed due to
the extreme heat involved. Pyrometers are optical thermometers that function
on the basis of the detection of thermal radiation emitted by all bodies with
temperatures above absolute zero. Direct physical contact is not required for
the application of a pyrometer. The colour of a heated metal changes as its
temperature increases. For example, molten steel changes colour from red to
yellow and finally white when it is heated further.
Figure 1.6: Optical pyrometer
Figure 1.6 illustrates an optical pyrometer that consists of a converging lens
that focuses thermal radiation emitted from a hot body onto a tungsten
filament connected to an electric circuit. The current to the filament is
adjusted until the colour of the heated filament cannot be differentiated from
the colour of the hot body. When this is achieved, the temperature of the
filament will be equal to the temperature of the body. An optical pyrometer
can measure temperature from ambient to almost 3000C.
How do you measure the surface temperature of the sun?
ACTIVITY 1.3
X TOPIC 1 HEAT AND TEMPERATURE
12
You should attempt the following questions before proceeding to the next section.
THERMAL EXPANSION
The increase (i.e. expansion) in length, area and volume due to temperature
increase is known as thermal expansion. When the temperature increases, the
kinetic energy of a bodys molecules correspondingly increases. The amplitude of
vibration and the average separation distance also increases. These result in linear,
area and volume expansions in solids and only a volume expansion in liquids. The
rate of expansion depends on the substance. For example, copper has a greater rate
of expansion than glass.
1.2.1 Linear Expansion
Let us consider linear expansion in one dimension (1-D). For an object of initial
length L
0
, the change in length L due to a change in temperature T is given by
0
L L T o A = A (1.12)
where o is the coefficient of linear expansion. Table 1.1 summarises the
coefficients of linear expansion of some substances.
1.2
1. What is the difference between temperature and heat?
2. What is meant by thermal equilibrium? State its importance.
3. Convert 300 K to units of Celsius and Fahrenheit and 100C into
units of Kelvin and Fahrenheit.
4. If the upper and lower points of a resistance thermometer are 75O
and 60O respectively, what is the temperature in units of Celsius
when the resistance is 68O? Assume the thermometer varies
linearly with temperature.
EXERCISE 1.1
TOPIC 1 HEAT AND TEMPERATURE W
13
Table 1.1: Coefficients of Linear Expansion for Some Substances
Substance o(K
1
)
Steel 1.2 10
5
Iron 1.2 10
5
Copper 1.7 10
5
Aluminium 2.4 10
5
Lead 2.9 10
5
Glass (0.4 0.9) 10
5
Ice 5.1 10
5
You can show that the new length of the rod L at temperature T = T
0
+ T is:
0
(1 ) L L T o = + A (1.13)
Example 1.4
During a hot day, the temperature of a 10 m steel rod increases from 25C to 75C.
Find the increase in its length. (o = 1.2 10
5
K
1
)
Solution
Apply the Equation 1.12
0
5
3
1.2 10 10 (75 25)
6 10 m
0.6 cm
L L T o
A = A
=
=
=
1.2.2 Area Expansion
For expansion in two-dimensions, the increase in surface area A is directly
proportional to the increase in temperature T and the original surface area A
0
.
Hence,
0
A A T | A = A (1.14)
X TOPIC 1 HEAT AND TEMPERATURE
14
where | is the coefficient of area expansion. The surface area at temperature
T = T
0
+ T is given by:
0
(1 ) A A T | A = + A (1.15)
where
2 | o ~ (1.16)
1.2.3 Volume Expansion
For expansion in three dimensions, the increase in volume of a substance V is
directly proportional to the change in temperature T and the original volume V
0
.
Hence,
0
V V T A = A (1.17)
where is the coefficient of volume expansion. The volume of the substance at
temperature T = T
0
+ AT is given by:
0
(1 ) V V T = + A (1.18)
where
3 o ~ (1.19)
Example 1.5
A glass beaker holds exactly 1 Litre at 0C.
(a) What is its volume at 100C?
(b) The beaker is now filled with mercury at 0C. What volume of mercury will
overflow when the temperature is raised to 100C?
Given
G
o
= Coefficient of expansion of glass = 8.3 10
6
K
1
m
= Coefficient of volume expansion of mercury = 1.82 10
4
K
1
TOPIC 1 HEAT AND TEMPERATURE W
15
Solution
(a) The volume of the glass beaker at 100C is
6
0
(1 3 ) (1L)(1 3(8.3 10 )100) 1.00249L
g G
V V T o
= + A = + =
(b) The volume of mercury at 100C is
4
0
(1 ) (1L)(1 (1.82 10 )100) 1.0182L
m m
V V T
= + A = + =
The volume that overflows is 0.0157L
m g
V V =
You should attempt the following question in the exercise to test your current level
of understanding of the topic.
The unusual behaviour of water
Most substances will expand when heated, but not water! Also, water expands
when It freezes, unlike other substances. Did you know that the volume of water
actually decreases when it is heated from 0C to 4C? Its density is greatest at
4C. However, above 4C, water will expand when heated. How does this
unusual behaviour of water help to sustain plant and animal life in lakes of cold
countries?
Why does pouring some hot water over a glass jar help loosen the metal
lid?
ACTIVITY 1.4
X TOPIC 1 HEAT AND TEMPERATURE
16
SPECIFIC HEAT AND LATENT HEAT
In this section, you will be introduced to specific heat and latent heat. What is the
definition and difference between these two quantities? This section will attempt to
answer these questions.
1.3.1 Specific Heat
The amount of heat Q needed to raise the temperature of a mass m of a substance
by an amount T depends on m and T. Mathematically, this can be written as:
T Q m A (1.20)
or
T Q mc A = A (1.21)
where c is the specific heat of the substance. From Equation 1.21, c can be defined
as:
1 Q
c
m T
A
=
A
(1.22)
The specific heat capacity of a substance is the amount of heat required to raise
1.3
1. The coefficient of linear expansion of a 1 m steel ruler at
20C is 1.12 10
5
K
1
. Determine the length of the ruler when it
is immersed in hot water of temperature 80C.
2. A piece of steel of area 10 m
2
is heated from 20C to 100C. What
is the increase in area of the piece of steel?
3. A Pyrex cup of 50 cm
3
capacity is completely filled to the brim
with a certain liquid at 25C. Calculate the amount of liquid that
will spill out of the cup if the temperature of both the cup and the
water is increased to 30C. Given: Coefficient of volume expansion
for the cup = 1 10
3
K
1
Coefficient of volume expansion for the
liquid = 5 10
3
K
1
EXERCISE 1.2
TOPIC 1 HEAT AND TEMPERATURE W
17
1 kg of that substance by 1 K. The SI unit for specific heat capacity is J kg
1
K
1
.
Example 1.6
A wooden stick of mass 16 g is heated in boiling water and subsequently placed in
a 70 g copper calorimeter at a temperature of 28C. The calorimeter contains 30 g
of water. Upon stirring, the temperature of the mixture is 39C. Determine the
specific heat of the wooden stick. [Specific heat of water is 4200 J kg
1
K
1
whereas the specific heat capacity of the copper calorimeter is 400 J kg
1
K
1
].
Assume no heat loss.
Solution
Heat supplied by the wooden stick, mc (100 39) = 0.016 61c = 0.976c J.
Heat absorbed by water, m
w
c
w
(39 28) = 0.03 4200 11 = 1386 J.
Heat absorbed by the calorimeter, m
c
c
c
(39 28) = 0.07 400 11 = 308 J. Since
there is no heat loss
Heat supplied by the wooden stick = Heat absorbed by water and calorimeter
1 1
0.976 308 1386
1694
1736 J kg K
0.976
c
c
= +
= =
Example 1.7
A thermos bottle contains 250 g of coffee at 100C. To this we add 250g of
milk at 10C. What is the final temperature of the system if there is no heat loss?
Take the specific heat capacity of coffee and milk to be the same as water, i.e.
4200 J kg
1
K
1
.
Solution
Let us call the final temperature of the system T.
Heat loss by coffee as it cools down from 100C to temperature T is
1
0.25 4200 (100 ) Q mc T T = A =
Heat gained by the milk as its temperature rises from 10C to T is
2
0.25 4200 ( 10) Q mc T T = A =
X TOPIC 1 HEAT AND TEMPERATURE
18
Since there is no heat loss, the heat lost by the coffee must equal the heat gained by
the milk. In other words,
Q
1
= Q
2
.
Thus,
0.25 4200 (100 T) = 0.25 4200 (T 10)
2T = 110C
T = 55C
1.3.2 Molar Specific Heat
If the quantity of matter is measured in moles and not in kilograms, then molar
specific heat must be used. It is defined as:
1 dQ
c
n dT
= (1.23)
where n is the number of moles of the substance. The SI unit of molar heat
capacity is J mol
1
K
1
. In gases, specific heat depends on pressure and volume.
This will be discussed in the following topic.
The molar heat capacity of all metals is almost the same i.e. approximately 25 J
mol
1
K
1
. This is due to the fact that the number of atoms in 1 mole of a particular
metal is approximately equal. The amount of heat required to heat up a substance
depends on the number of atoms in the substance and not on the individual masses
of the atoms in the substance.
TOPIC 1 HEAT AND TEMPERATURE W
19
Table 1.2 displays the specific heats and molar specific heats of several selected
substances.
Table 1.2: Specific Heat Capacities and Molar Specific
Heat Capacities of Several Selected Substances
Substance
Specific Heat Capacity,
c
(J kg
1
K
1
)
Molar Heat Capacity,
c molar
(J mol
1
K
1
)
Aluminium 910 24.6
Iron 470 25.0
Silver 234 25.4
Mercury 138 27.7
Water 4190 75.4
Ethanol 2430 112.0
Ice 2000 36.5
Wood 1700
Glass 840
1.3.3 Phase Transitions
When a substance in the solid phase changes to the liquid phase or when the liquid
phase changes to gaseous phase, heat is absorbed with no change in temperature.
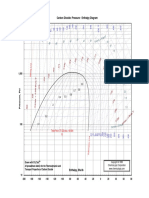
Figure 1.7 is a graph of temperature T against heat Q which illustrates the temperature
changes for the phase transitions from solid to liquid and from liquid to gas.
Figure 1.7: Phase transitions of matter
X TOPIC 1 HEAT AND TEMPERATURE
20
Source http://www.beginnerphysics.net/GCSEPhysicsSpecificHeat.html
Discuss this problem with your friends and tutor in the next tutorial
session.
1. Figure 1.8 shows the heating curve of a substance.
Figure 1.8: The heating curve of a subtance
(a) What is the melting point and boiling point of the substance?
(b) Which part of the graph represents:
(i) Melting.
(ii) Boiling.
(c) What is/are the physical state of the substance between:
(i) AB.
(ii) BC.
(iii) CD.
(iv) DE.
(d) During melting or boiling, the temperature remains constant.
What happens to the heat supplied during this period?
ACTIVITY 1.5
TOPIC 1 HEAT AND TEMPERATURE W
21
1.3.4 Latent Heat
Latent heat (L) is the quantity of heat required to change the phase of 1 kg of a
substance without a change in temperature.
Q = mL (1.24)
1.3.5 Latent Heat of Fusion and Vaporisation
The latent heat of fusion (L
f
) is the quantity of heat required for the phase change
from the solid phase to the liquid phase or vice versa, without temperature change.
The latent heat of vaporisation (L
V
) is the quantity of heat required for the phase
change from the liquid phase to the gas phase or vice versa, without temperature
change.
For comparison, the latent heat of fusion and vaporisation for water at a pressure of
1 atmosphere is 3.34 10
5
J kg
1
and 2.26 10
6
J kg
1
respectively.
Example 1.7
2.0 kg of hot water at a temperature of 100C is poured into a container which has
0.5 kg ice at 0C. What is the final temperature of the ice-water mixture? Assume
no heat is lost to the surroundings.
Solution
Heat required to melt all of the ice,
Q
1
= m
1
L = 0.5 3.34 10
5
= 1.67 10
5
J
Heat loss from the hot water,
Q
2
= m
2
c (100 0) = 2 4200 100 = 8.40 10
5
J
X TOPIC 1 HEAT AND TEMPERATURE
22
Since Q
2
> Q
1
all the ice melts and the temperature increases to a final temperature
T. Since there is no heat loss,
Heat gain by ice = Heat loss from hot water
1 1
5
5 5
( 0) (100 )
1.67 10 0.5 4200 2.0 4200 100 2.0 4200
8.4 10 1.67 10
64.1 C
2.4 4200
m L mc T mC T
T T
T
+ =
+ =
= =
Example 1.8
How many grams of ice at 0C must be added to a 500 g cup of tea at 80C
to cool it to 40C? Assume that all the ice melts and no heat is lost to the
surroundings. Take the specific heat capacity of tea to be 4200 J kg
1
K
1
.
Solution
1
1 1
2
1
Heat lost by the tea as it cools to 40 C
0.5 4200 (80 40)
84000 J
Heat needed to melt all the ice to water and raise the temperature to 40 C
, where is the mass of ice requir
F
Q
mc T
Q
mL mc T m
=
= A
=
=
=
= + A
5
5
ed
3.34 10 4200 (40 0)
5.02 10
m m
m
= +
=
Since no heat is lost to the surroundings:
1 2
5
84000 (5.02 10 )
0.167 kg
Q Q
m
m
=
=
=
TOPIC 1 HEAT AND TEMPERATURE W
23
You should attempt the following problems in the exercise to gauge your current
level of comprehension.
1. Why is water stored in a cool earthen jar cooler than water stored in a
plastic container?
2. Which of the following is more dangerous, exposure to steam or hot
water at the temperature of 100C? State the reasons for your answer.
ACTIVITY 1.6
1. What is the minimum amount of heat required to heat a 2.0 kg
aluminium block from a temperature of 30C to 100C?
2. Steam at 100C is passed over 1 kg of ice at a temperature of 0C.
Determine the amount of steam required to completely melt the
ice to water at 0C.
3. At 100C, the upper points of a resistance thermometer and a
constant volume gas thermometer are 75 Pa and 1.10 10
7
Pa,
respectively the lower points which corresponds to the freezing point
of ice (0C) are respectively 63 Pa and 8.00 10
6
Pa. If
measurements yield the values of 66 Pa and 9.76 10
6
Pa, determine
the temperatures which corresponds to these values.
4. Define the coefficient of linear expansion.
Why are there gaps in a railway track?
Workers are laying a steel rail track 20 m in length in a desert at
night. The temperature of the surroundings is 10C. What should be
the minimum distance of a gap between the rails so that the track is
still operational when the temperature soars to 50C during daytime?
[The coefficient of linear expansion for steel is 1.1 10
5
K
1
]
EXERCISE 1.3
X TOPIC 1 HEAT AND TEMPERATURE
24
- Temperature is the degree of warmth whereas heat is the transfer of thermal
energy.
- Temperature can be measured with thermometers. There are many types of
thermometers, for example mercury thermometers, constant volume gas
thermometers, platinum resistance thermometers and optical pyrometers.
- The heat content, specific heat and latent heat can be measured with
calorimeters.
- The Zeroth Law of Thermodynamics states that if a body C is in thermal
equilibrium with body A and B, then body A and B are in thermal equilibrium
with respect to each other even in the absence of any thermal contact between
them.
- Latent heat is the quantity of heat required to change the phase of 1 kg of a
substance without a change in temperature.
- Specific Heat is the amount of heat required to raise 1 kg of that substance by
1 K.
- Thermal Expansion is the increase (i.e. expansion) in length, area and volume
due to temperature increase.
Heat
Latent heat
Specific heat
Temperature
The zeroth law of thermodynamics
Thermal expansion
Thermometer
TOPIC 1 HEAT AND TEMPERATURE W
25
1. Which of the following statements is NOT true?
A. Heat is a form of energy.
B. The temperature of a body depends on how hot or cold it is.
C. The triple points of water is equal to 273.16 K.
D. The relation between the Celcius and Fahrenheit scales in
9
32
5
F C
T T =
.
2. What are SI units for the specific heat capacity of a body?
A. J kg
1
K
B. J kg
1
K
1
C. J kg
1
D. J K
1
3. A sphere has volume V at 25C. What is the increase in volume when it is
heated to 45C? The coefficient of volume expansion is .
A. 20V
B. 25V
C. 45V
D. 50V
4. A glass beaker holds exactly 1 L at 0C. What is the percentage increase in
its volume at 100C?
Given that the linear coefficient of glass is 8.3 10
6
K
1
.
A. 0.25 %
B. 0.28%
C. 0.31%
D. 0.33%
X TOPIC 1 HEAT AND TEMPERATURE
26
5. Nitrogen freezes at 346F. What is the Celsius equivalent of this
temperature?
A. 196C
B. 199C
C. 210C
D. 219C
1. State the difference between heat and temperature.
2. Define the Zeroth Law of Thermodynamics. Give a simple application of this
law.
3. Explain what is meant by thermal expansion.
4. When the temperature of a metallic disc is raised by 100C, its radius
increases by 1.5%.
(a) Calculate the coefficient of linear expansion for the disc.
(b) Give the percentage increase in the area of the disc.
Вам также может понравиться
- Understand Heat and Temperature with this GuideДокумент26 страницUnderstand Heat and Temperature with this GuideSJK(C) PHUI YINGОценок пока нет
- Chapter (1) Temperature and ThermometryДокумент4 страницыChapter (1) Temperature and ThermometryBǿ DYОценок пока нет
- Thermal Physics PDFДокумент87 страницThermal Physics PDFPriyanshu SharmaОценок пока нет
- Science 8: Most Essential Learning CompetenciesДокумент24 страницыScience 8: Most Essential Learning CompetenciesVeronica KimОценок пока нет
- SFG 3023 Chapter 1Документ67 страницSFG 3023 Chapter 1Nik AshrafОценок пока нет
- Physics I-21-22Документ50 страницPhysics I-21-22Ahmed BagradОценок пока нет
- TEMPERATURE AND THERMOMETRY: MEASURING HEATДокумент8 страницTEMPERATURE AND THERMOMETRY: MEASURING HEATElizabeth AnyegaОценок пока нет
- Phy CH 8 Final 9thДокумент28 страницPhy CH 8 Final 9thmastersahb302Оценок пока нет
- Thermal Physics Key ConceptsДокумент43 страницыThermal Physics Key ConceptsFarhan Mukhtiar YousafzaiОценок пока нет
- Thermometry Physics A LevelДокумент16 страницThermometry Physics A LevelNayana GaleaОценок пока нет
- Chapter Ten Lecture Ten Thermodynamics: TemperatureДокумент16 страницChapter Ten Lecture Ten Thermodynamics: TemperatureTony AtefОценок пока нет
- 05 Ley Cero Escalas TemperaturaДокумент32 страницы05 Ley Cero Escalas TemperaturaMichelle SerranoОценок пока нет
- SUMMARY - ThermodynamicsДокумент18 страницSUMMARY - Thermodynamicssethupanic macanic gamedzeОценок пока нет
- TemperatureДокумент6 страницTemperatureNur Khairiah Daimah SanupinОценок пока нет
- Heat Lecture NotesДокумент62 страницыHeat Lecture NotesAS HUMBLE PIANOОценок пока нет
- Physics IntroductionДокумент25 страницPhysics IntroductionhannaОценок пока нет
- Thermal Properties of Matter: Temperature and HeatДокумент24 страницыThermal Properties of Matter: Temperature and HeatChethan KumarОценок пока нет
- Understanding Thermal PrincipleДокумент7 страницUnderstanding Thermal PrincipleAngie Kong Su MeiОценок пока нет
- NCERT PH 2 Thermal Properties of MatterДокумент24 страницыNCERT PH 2 Thermal Properties of MatterkdsiddhantОценок пока нет
- Chapter-6 Temperature - HeatДокумент8 страницChapter-6 Temperature - Heat2220678Оценок пока нет
- Lec. 2Документ32 страницыLec. 2Ali. AboudОценок пока нет
- Physics For Engineers Tah Module PDFДокумент15 страницPhysics For Engineers Tah Module PDFFRANCES VISAYAОценок пока нет
- Temperature: Can Be Thought of AsДокумент5 страницTemperature: Can Be Thought of AsTarek Mohamed Ahmed AhmedОценок пока нет
- Chap03 TemperatureNHeatДокумент24 страницыChap03 TemperatureNHeatsamtomОценок пока нет
- Temperature MeasurementДокумент9 страницTemperature MeasurementMarco ConopioОценок пока нет
- Heat Model1Документ115 страницHeat Model1deoОценок пока нет
- Hermal Roperties OF Atter: Hapter LevenДокумент25 страницHermal Roperties OF Atter: Hapter LevensiddanshОценок пока нет
- Thermal Properties of MatterДокумент9 страницThermal Properties of MatterTrillionare HackОценок пока нет
- BSES-29-REVIEWERДокумент8 страницBSES-29-REVIEWERjerico.lapurgaОценок пока нет
- Thermal PhysicsДокумент24 страницыThermal PhysicsSuraj GopaulОценок пока нет
- CalorДокумент14 страницCaloreka123Оценок пока нет
- 11Документ24 страницы11anil.gelra5140100% (1)
- Chapter Four Heat and ThermodynamicsДокумент44 страницыChapter Four Heat and ThermodynamicsmesfinОценок пока нет
- TD Module 2Документ47 страницTD Module 2mujeebОценок пока нет
- Keph 203Документ24 страницыKeph 203Ayush JaiswalОценок пока нет
- Fluid and Thermal ExitДокумент25 страницFluid and Thermal Exitdavididosa40Оценок пока нет
- Thermal Energy Transfer and Equilibrium ExplainedДокумент12 страницThermal Energy Transfer and Equilibrium ExplainedBoedisantosoОценок пока нет
- Physics 73 Lecture Notes on Thermodynamics, Relativity and Quantum PhysicsДокумент80 страницPhysics 73 Lecture Notes on Thermodynamics, Relativity and Quantum Physics8ienneОценок пока нет
- 11 ChapterДокумент24 страницы11 ChapterShashwat SahayОценок пока нет
- Form Four Notes PDFДокумент23 страницыForm Four Notes PDFSanti NgoranОценок пока нет
- g484 Module 3 4 3 2 TemperatureДокумент6 страницg484 Module 3 4 3 2 Temperatureapi-236179294Оценок пока нет
- Section III 12 TemperatureДокумент27 страницSection III 12 Temperaturedanwilliams85Оценок пока нет
- Lesson 4.1 (Smtai 09) .Документ5 страницLesson 4.1 (Smtai 09) .Ilman MohamadОценок пока нет
- CHEMISTRYДокумент12 страницCHEMISTRYmurtada18851Оценок пока нет
- Chapter - 11 HeatДокумент22 страницыChapter - 11 HeatMuhammad Arif RattarОценок пока нет
- Thermodynamics and temperature scalesДокумент14 страницThermodynamics and temperature scalesCarl Angelo MartinОценок пока нет
- Phys 2 Lecture 01 Thermodynamics-1Документ19 страницPhys 2 Lecture 01 Thermodynamics-1Maruja TheaОценок пока нет
- Chapter-6 Temperature & Heat PDFДокумент5 страницChapter-6 Temperature & Heat PDFKoushik DewriОценок пока нет
- Heat and Temperature: Understanding Key ConceptsДокумент57 страницHeat and Temperature: Understanding Key ConceptsJeanette Valera JimenezОценок пока нет
- Grade 10 CH 6 To SendДокумент36 страницGrade 10 CH 6 To SendZynx DixonОценок пока нет
- Ch. 17 Biomedical Phy.Документ9 страницCh. 17 Biomedical Phy.Mahmoud Abu MayalehОценок пока нет
- Temperature, Heat & Thermal: Physics Thermodynamics Energy System Thermal Contact WorkДокумент12 страницTemperature, Heat & Thermal: Physics Thermodynamics Energy System Thermal Contact WorkAmirul ZahariОценок пока нет
- CH 11Документ11 страницCH 11vikram11032008Оценок пока нет
- HEAT SRGДокумент67 страницHEAT SRGBharat JadhavОценок пока нет
- Oleh: Dewi Ekawati Indah Dwiphayanti Laelatul Fitriani Juwita Chandra DewiДокумент31 страницаOleh: Dewi Ekawati Indah Dwiphayanti Laelatul Fitriani Juwita Chandra DewiNurulWardhani11Оценок пока нет
- Chapter-6 Temperature Heat PDFДокумент8 страницChapter-6 Temperature Heat PDFMohammad Fuad HasanОценок пока нет
- Experiment 5 Newton's Law of CoolingДокумент7 страницExperiment 5 Newton's Law of Coolingatif irshadОценок пока нет
- Pressure, Heat and Temperature - Physics for Kids - 5th Grade | Children's Physics BooksОт EverandPressure, Heat and Temperature - Physics for Kids - 5th Grade | Children's Physics BooksОценок пока нет
- ThermodynamicДокумент14 страницThermodynamicWanMardziyyahОценок пока нет
- ThermodynamicДокумент12 страницThermodynamicWanMardziyyahОценок пока нет
- EXPERIMENT Density of SolidДокумент4 страницыEXPERIMENT Density of SolidWanMardziyyahОценок пока нет
- Worksite Hazard Analysis 1-Hour LessonДокумент2 страницыWorksite Hazard Analysis 1-Hour LessonWanMardziyyahОценок пока нет
- September Semester 2010 Ebmp3103/Edmp3103 - Production Technology Assignment (30%)Документ5 страницSeptember Semester 2010 Ebmp3103/Edmp3103 - Production Technology Assignment (30%)WanMardziyyahОценок пока нет
- Advanced Steel Design PPT in PDFДокумент88 страницAdvanced Steel Design PPT in PDFaskcmiitmОценок пока нет
- Mechanism Design: A SeriesДокумент3 страницыMechanism Design: A Seriesamirmasood kholojiniОценок пока нет
- Correlation between viscosities in dynamic and steady flowДокумент3 страницыCorrelation between viscosities in dynamic and steady flowM.Çağrı AltındalОценок пока нет
- Che201 Test-1Документ4 страницыChe201 Test-1Fawziyyah AgboolaОценок пока нет
- Elastic Stability of Plates (Plate Buckling AnalysisДокумент10 страницElastic Stability of Plates (Plate Buckling Analysisruhul72Оценок пока нет
- Wind Tunnel Designs and Their Diverse Engineering Applications 14Документ2 страницыWind Tunnel Designs and Their Diverse Engineering Applications 14Dang Tien PhucОценок пока нет
- Liquid Drop Model of NucleusДокумент4 страницыLiquid Drop Model of NucleusHemanta BhattaraiОценок пока нет
- 9.design of JIB Crane Supporting StructuresДокумент14 страниц9.design of JIB Crane Supporting Structuresepe civilОценок пока нет
- FLUENT Tutorial 5 - BlowerДокумент26 страницFLUENT Tutorial 5 - BlowerKwanchai ChoicharoenОценок пока нет
- A Study On Piled Raft FoundationДокумент7 страницA Study On Piled Raft FoundationCostas SachpazisОценок пока нет
- Engineering Electromagnetics Solutions ManualДокумент3 страницыEngineering Electromagnetics Solutions Manualaamirjaved40Оценок пока нет
- CO2 Mollier Chart PDFДокумент1 страницаCO2 Mollier Chart PDFmarko quirozОценок пока нет
- Modern Physics PDFДокумент20 страницModern Physics PDFPrathm MittalОценок пока нет
- Assignment - 13: Name - Harsh Rai Branch - CE Sec-3M1 University Roll No - 1801000029Документ9 страницAssignment - 13: Name - Harsh Rai Branch - CE Sec-3M1 University Roll No - 1801000029Suraj PatwaОценок пока нет
- Principle of Operation of An AccelerometerДокумент5 страницPrinciple of Operation of An AccelerometersangeethsreeniОценок пока нет
- Astrological Concepts and Applications DiscussedДокумент2 страницыAstrological Concepts and Applications Discusseddevesh parwaniОценок пока нет
- Modeling of Dynamic Systems: An Introduction to Quantities, Models, and Model Solution MethodsДокумент666 страницModeling of Dynamic Systems: An Introduction to Quantities, Models, and Model Solution MethodsZahid RontyОценок пока нет
- Truss - 1 Detail 1: See Spot Purlin DetailДокумент1 страницаTruss - 1 Detail 1: See Spot Purlin DetailEarl Vergille Petiluna ReveloОценок пока нет
- Gear FailureДокумент27 страницGear FailureSheri KhosoОценок пока нет
- Chap05 StaticFailureДокумент9 страницChap05 StaticFailureFatih AŞCIОценок пока нет
- UNIVERSITY EXAMINATIONS STEAM PLANT III THEORYДокумент13 страницUNIVERSITY EXAMINATIONS STEAM PLANT III THEORYGarry Van der Beek100% (1)
- Vibration Analysis and Work-Rounding Mechanism in Centerless GrindingДокумент31 страницаVibration Analysis and Work-Rounding Mechanism in Centerless GrindingNatKThОценок пока нет
- Assignment 1 2Документ2 страницыAssignment 1 2Jeevan GОценок пока нет
- (Fundamental) Physics of Elementary Particles FPPv2012 0104Документ598 страниц(Fundamental) Physics of Elementary Particles FPPv2012 0104sollun3Оценок пока нет
- Designing Integral Bridges Without Movement JointsДокумент32 страницыDesigning Integral Bridges Without Movement Jointspmullins_11100% (5)
- Leonhard EulerДокумент14 страницLeonhard EulerAnonymous GRuHWbxJr9Оценок пока нет
- Wind TunnelsДокумент240 страницWind TunnelsshoshonkoОценок пока нет
- Experiment 7: Simple PendulumДокумент5 страницExperiment 7: Simple Pendulumapi-586722985Оценок пока нет
- ASTM D6641 - Standard Test Method For Compressive Properties of Polymer Matrix Composite Materials Using A Combined Loading Compression Test FixtureДокумент13 страницASTM D6641 - Standard Test Method For Compressive Properties of Polymer Matrix Composite Materials Using A Combined Loading Compression Test FixtureGiuseppeОценок пока нет
- ASE 445 Hypersonic Flow Part 1 OutlineДокумент40 страницASE 445 Hypersonic Flow Part 1 OutlineAtes Deniz PugarОценок пока нет