Академический Документы
Профессиональный Документы
Культура Документы
Chagas' Disease: Advances in Diagnosis and Management: Andrei C. Sposito, Jose A. F. Ramires
Загружено:
mike_helplineОригинальное название
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
Chagas' Disease: Advances in Diagnosis and Management: Andrei C. Sposito, Jose A. F. Ramires
Загружено:
mike_helplineАвторское право:
Доступные форматы
e303
C
h
a
g
a
s
D
i
s
e
a
s
e
:
A
d
v
a
n
c
e
s
i
n
D
i
a
g
n
o
s
i
s
a
n
d
M
a
n
a
g
e
m
e
n
t
C
H
A
P
T
E
R
e
3
7
Copyright 2008 The McGraw-Hill Companies. All rights reserved.
Chagas Disease:
Advances in Diagnosis
and Management
Andrei C. Sposito, Jose A. F. Ramires
INTRODUCTION
Chagasic cardiomyopathy is the major complication resulting from
in-
fection by
Trypanosoma cruzi,
occurring in up to 30% of individuals
who have a positive specic serology. The
T. cruzi
infection is related to
the close proximity between humans and triatomines carrying
T.
cruzi
and extends from the Southern United States
11
and Mexico to the
south of Argentina. According to the World Health Organization Tech-
nical Report, among individuals living in Latin American countries,
56 million are infected and 25 million are at risk of contracting the
infection (Chap. 206).
It usually takes 1020 years for the infection to manifest the disease
in a broad range of clinical presentations including heart failure, cardi-
ac arrhythmias, thromboembolism, and sudden death. Once estab-
lished, the signs of heart failure result in life expectancy being reduced
to
5 years. Therefore, strategies to identify the disease in the early
phase and characterize predictive signs and potential therapeutic tar-
gets have been intensely pursued. In this chapter, we discuss the results
from recent, novel research on the diagnosis and management of Cha-
gas disease.
LABORATORY DIAGNOSIS
Parasitemia
During the acute phase of the disease, parasites can easi-
ly be found by microscopic observation of fresh blood. Morphologic
characteristics of the parasite and differentiation from
T. rangeli
can
also be determined by staining the blood smears. When parasitemia is
low, procedures for parasite concentration or indirect methods (xeno-
diagnosis and hemoculture) can be used instead. Parasite concentra-
tion could be obtained either by microhematocrit or by the Strout
method. In the microhematocrit, blood is centrifuged and the buffy
coat examined by microscopy to visualize trypomastigote movements.
In the Strout method, blood cells are rst eliminated by precipitation
and centrifugation. The supernatant is then submitted to a second
centrifugation and the precipitate is examined as fresh blood.
The xenodiagnostic method consists of feeding uninfected triatom-
inae with blood from the patient under examination and then investi-
gating the intestinal contents of the insects some days later to search
for metacyclic trypomastigotes. Articial xenodiagnosis is preferred to
avoid inconveniences from direct contact between the triatomines and
patients skin. Recently, amplication of
T. cruzi
DNA target sequences
by polymerase chain reaction (PCR) has become a preferred method
for the detection of parasites in blood and tissues. Such PCR tech-
niques are especially helpful for the follow-up of chemotherapy for
T.
cruzi
. However, despite being more sensitive than xenodiagnosis and
hemoculture, this sensitivity is equally dependent on the magnitude of
the parasitemia.
Immunodiagnosis
Infected individuals soon develop antibodies, ini-
tially IgM and later IgG, against several epitopes of
T. cruzi
allowing the
indirect diagnosis of Chagas disease. Conventional immunodiagnostic
tests are available worldwide and are based on three main techniques:
hemagglutination, immunouorescence, and ELISA. Nonconventional
tests using recombinant chimeric proteins, synthetic peptides, or puri-
ed antigens have been developed to increase specicity and reduce
cross-reactivity with other infections and with autoimmune diseases.
In general, conventional tests have elevated sensitivity, and a positive
result in two conventional tests is sufcient to diagnose the infection. In
parallel with PCR for detecting
T. cruzi
parasitemia, conventional sero-
logic tests could also be used for the follow-up of patients undergoing
chemotherapy.
ANATOMIC DIAGNOSIS
Myocardial Fibrosis and Inflammation
T. cruzi
can be easily detected
in muscle cells and interstitial histiocytes of individuals in the acute
phase of Chagas disease. However, such a nding is rare in the chronic
phase of the disease. Hence, the main nding in the hearts of patients
with chronic Chagas disease is the presence of inammatory inl-
trates. Chagas myocarditis lesions are spread out over both ventricles
and in a large spectrum of severity, transforming myobers into -
brous tissue and featuring the changes in heart structure and function.
Accordingly, myocardial brosis caused by Chagas disease is a strong
marker of clinical impairment and ventricular dysfunction.
The histopathologic evaluation of myocardial brosis and inam-
mation can be obtained through a percutaneous and transvenous en-
domyocardial biopsy of the right ventricle. This diagnostic procedure
has been proved to be a powerful tool for both the prediction of clini-
cal outcome and estimation of the severity of myocardial damage in
Chagas disease and in other primary and secondary cardiac diseases.
In patients with Chagas disease who undergo heart transplantation,
endomyocardial biopsy is considered the gold standard technique in
the differentiation between allograft rejection and reactivation of the
disease. In such patients, however, the use of endomyocardial biopsy
has been limited by the mild, albeit signicant, intrinsic risk of the
procedure and the lack of evidence-based parameters that may guide
clinical decisions. Consequently, noninvasive methods have been de-
veloped to explore potential clinical benets from assessing the degree
of myocardial inammation and brosis in patients with Chagas disease.
In our group, the histopathologic ndings of myocardial inammation
via endomyocardial biopsy were compared with two noninvasive tech-
niques, gallium-67 myocardial uptake scintigraphy and MRI. Despite
both noninvasive techniques detecting Chagas myocarditis, MRI was
more accurate due to the higher spatial resolution.
Myocardial delayed enhancement by MRI has been proved to be ef-
cient in detecting myocardial brosis in ischemic and nonischemic
myocardial disease. In Chagas disease, delayed enhanced MRI can de-
tect even traces of myocardial brosis in individuals in the indetermi-
nate phase, that is, the period before presentation of cardiac electrical
or mechanical abnormalities.
3
Such rened accuracy would potential-
ly be helpful for characterization and treatment of arrhythmogenic
foci in patients with Chagas disease. Moreover, the severity of myo-
cardial brosis detected by MRI is proportional to the severity of myo-
cardial dysfunction and clinical symptoms.
In patients in the chronic phase of the disease, myocardial brosis is
found particularly at the apex and inferolateral regions of the left ven-
tricle. Ischemia, inammation, mechanical factors, and parasympa-
thetic nerve cell destruction are considered among potential
underlying mechanisms for these lesions. In these affected regions,
ventricular wall aneurysms would develop in a later stage of Chagas
cardiomyopathy, constituting a classic sign of the disease.
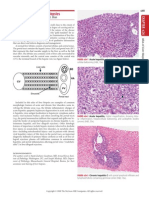
Figure e37-1
shows the typical apex lesion with severe myocardial brosis in a heart
examined at autopsy.
Myocardial Perfusion
Myocardial ischemia has been frequently re-
ported in patients with Chagas disease and normal coronary angio-
grams. In fact, these patients have chest pain associated with
electrocardiographic signs of ischemia simulating obstructive coro-
nary artery disease. From a pathophysiologic standpoint, vascular dys-
autonomia due to denervation and inammatory damage of the
microcirculation is considered the leading cause of myocardial is-
chemia in these patients.
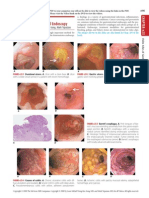
Figure e37-2
shows myocardial denervation
and ischemia by scintigraphic imaging; the denervation demonstrated
by the reduced cardiac uptake of
123
I-metaiodobenzylguanidine
(MIBG) is concordant with the perfusion decit, but is much larger,
suggesting that denervation is the initiating event.
In angiographic studies, endothelium-dependent and -independent
impairment in coronary vasodilation have been observed in patients
with Chagas disease.
4
With scintigraphic perfusion imaging using
thallium-201, transient and permanent myocardial perfusion abnor-
malities have been described in patients with the disease (Chaps. 222
e37
e304
P
A
R
T
1
8
e
-
C
h
a
p
t
e
r
s
f
r
o
m
I
n
t
e
r
n
a
t
i
o
n
a
l
A
d
v
i
s
o
r
y
E
d
i
t
o
r
s
Copyright 2008 The McGraw-Hill Companies. All rights reserved.
and
e20
). These studies demonstrate an elevated topographic correla-
tion between perfusion and wall motion abnormalities. Even though
prospective cohorts demonstrating a temporal association between
perfusion defects and the development of wall motion dysfunction are
lacking, it is plausible to consider that such perfusion defects would
represent an early sign of Chagas cardiomyopathy. In addition, these
radionuclide studies also indicate that the perfusion defects are pre-
dominantly in the apex and inferolateral regions, which are those most
affected by the inammatory damage and the autonomic denervation.
From a clinical perspective, caution must be taken to interpret the
presence of perfusion defects detected by either scintigraphy or MRI as
an indication of epicardial coronary disease in patients with Chagas dis-
ease. Actually, both the endothelium-dependent and -independent va-
sodilatory responses of coronary resistance vessels are also affected in
patients with idiopathic dilated cardiomyopathy and angiographically
normal coronary arteries. These patients have impairment of the vasodi-
lator responses to both metabolic and pharmacologic stimuli and an in-
creased sensitivity to vasoconstrictors. Such evidence indicates that the
segmental microvascular dysfunction observed in patients with Chagas
disease is unlikely to be pathogen-dependent but rather an early sign of
ventricular wall disease. Whether such perfusion disturbances also con-
tribute to the detriment of ventricular wall motion is presently unknown.
Myocardial Wall Motion
In the acute phase of the disease, pericardial
effusion and occasionally myocardial wall motion abnormalities have
been described. By denition, in the indeterminate phase, chronically
infected individuals remain a parasite reservoir without being affected
by the disease and consequently have a normal life expectancy. Hence,
the appearance of an abnormality of the ventricular wall reects pro-
gression of the disease and must be considered, accordingly, as the on-
set of the chronic phase of Chagas disease.
In the chronic phase, thinning, aneurysm formation, and wall mo-
tion dysfunction are the most frequent ndings detected on echocar-
diography. In keeping with necropsy, scintigraphic, and MRI studies,
these echocardiographic ndings are mostly observed in the apex and
inferobasal regions of the left ventricle. The segmental thinning of the
ventricular wall, particularly at the apex, promotes remodeling of the
left ventricle, which may increase mechanical tension and contribute
to aneurysm formation in this area. Nevertheless, abnormalities of
other left ventricular segments can also be found.
As the disease progresses, the affected segments of the ventricular
wall become hypokinetic, akinetic, or even dyskinetic. Frequently, dia-
stolic dysfunction occurs in an early phase of the disease, as shown in
Fig. e37-3
. The thinning of the apex becomes an aneurysm, and the
global systolic function of the left and right ventricles deteriorates. Ini-
tially, the systolic dysfunction may be apparent only under pharmaco-
logic stress by dobutamine or phenylephrine, characterizing the
reduction of systolic reserve. Echocardiography or MRI can accurately
detect these characteristics.
The right ventricle is rst and predominantly affected in the major-
ity of patients with Chagas disease. This may occur even in the ab-
sence of any detectable abnormality in the left ventricle. Accordingly,
in these patients, the development of heart failure is typically mani-
fested with a predominance of systemic over pulmonary congestion.
Because echocardiography has a low accuracy for detecting right ven-
tricular dysfunction, radionuclide angiography or MRI is preferred for
evaluation of this chamber.
CHAGAS DISEASE
ETIOLOGIC TREATMENT
Nitrofurans and nitroimidazole derivatives
(nifurtimox and benznidazole respectively) have been the cornerstones of
trypanosomicidal treatment in recent decades. These compounds seem to
exert their trypanosomicidal
action by the generation of superoxide radi-
cals causing oxidative
stress and cell death in susceptible parasites.
From the clinical point of view, the activity of treatment with both com-
pounds is evident in terms of parasite load reduction and serologic conver-
sion to negative in the acute phase of Chagas disease and in congenital
FIGURE e37-1
Photo of a heart from a patient with chronic chagasic
cardiac disease,
exhibiting a typical lesion at the left ventricle.
FIGURE e37-2
Myocardial denervation and ischemia detected by
scintigraphic imaging.
Above.
The denervation is demonstrated by
the reduced cardiac uptake of
123
l-metaiodobenzylguanidine (MIBG)
(
arrows
).
Below.
A permanent perfusion deficit with smaller size as
compared with the denervation is demonstrated by scintigraphic per-
fusion with
99m
Tc-sestamibi (MIBI).
FIGURE e37-3
Echocardiographic analysis of mitral inflow
by
pulse-wave Doppler showing diastolic function in a patient with Cha-
gas disease. Normally, the early flow velocity (E) is higher than the late
flow velocity (A), which is related to atrial contraction. In this case,
there is an inverted E/A relation, indicating the impairment of left ven-
tricular relaxation.
e305
C
h
a
g
a
s
D
i
s
e
a
s
e
:
A
d
v
a
n
c
e
s
i
n
D
i
a
g
n
o
s
i
s
a
n
d
M
a
n
a
g
e
m
e
n
t
C
H
A
P
T
E
R
e
3
7
Copyright 2008 The McGraw-Hill Companies. All rights reserved.
infection. In the indeterminate phase, new promising findings have been
reported particularly in children and young adults, showing long-lasting
disappearance of specific antibodies in 5898% of treated individuals to-
gether with a 1020% rate reduction of side effects. In general, treatment
with nitroimidazole derivatives, especially benznidazole, has been shown
to be effective more frequently in reducing parasitemia and specific anti-
body titers in individuals in the indeterminate phase. Whether this treat-
ment will prevent the development of cardiac or digestive complications
of the disease is still unclear. Large randomized controlled trials are re-
quired to define this issue.
The effect of trypanosomicidal therapies on parasite load or disease pro-
gression in patients in the chronic symptomatic phase of Chagas disease is
even less clear. The disappearance of specific antibodies is uncommon in the
chronic phase and may take up to 1020 years. Parasite DNA is present in
several tissues and may induce immune response and perhaps disease pro-
gression. Hence, the ideal therapeutic schema or duration for such chronic
patients is unknown. In addition, several adverse reactions, such as peripher-
al neuropathy and skin disorders, have been reported in a large proportion
of patients treated with both nifurtimox and benznidazole. Thus, there is in-
sufficient evidence to demonstrate the clinical benefit for trypanosomicidal
treatment in patients in the chronic phase of Chagas disease.
Novel potential targets for trypanosomicidal treatment have been in-
vestigated to reduce side effects and increase treatment efficacy. Sterol
biosynthesis inhibitors, protein prenylation inhibitors, protease inhibitors,
and phospholipid analogues are among potential chemotherapeutic
agents for this purpose. Although some of these compounds have already
demonstrated potent inhibitory activity in vitro against
T. cruzi
, clinical ben-
efits have not been proved thus far.
COMPLEMENTARY TREATMENT
In the acute phase, symptoms
disappear in up to 2 months. In rare cases of severe acute myocarditis, an
empirical combination of corticoid and trypanosomicidal treatments has
been attempted. In animal models, immunosuppressive therapy in combi-
nation with benznidazole has been demonstrated to be effective in atten-
uating the inflammatory response. However, insufficient studies in humans
have been done to define the ideal approach in these cases.
The aim of treatment in the chronic phase of Chagas disease is to atten-
uate symptoms and prevent complications. The most relevant cardiac com-
plications in the late chronic phase are heart failure and life-threatening
arrhythmias. The mortality attributable to Chagas disease is fundamentally
related to these two disorders. Even though few studies have compared the
efficacy of the treatment for heart failure in patients with and without Cha-
gas disease, the standards of clinical treatment are mostly the same. Heart
failure in both classes of patients responds equally to digitalis, diuretic, and
vasodilator therapy (Chaps. 227 and 231). The use of angiotensin-convert-
ing enzyme (ACE) inhibitors has been reported to reduce neurohormonal
activation, improving heart failure symptoms and nonlethal arrhythmias. In
an animal model, there is evidence that aldosterone blockade with spirono-
lactone attenuates myocardial remodeling and inflammatory infiltration
and may reduce mortality in Chagas cardiomyopathy.
8
As is the case for nonchagasic heart failure, the use of beta blockers is
also believed to be beneficial in patients with Chagas disease. In addition,
beta blockers may reduce the transmyocardial pressure gradient and at-
tenuate subendocardial ischemia, which is supposed to participate in the
deterioration of ventricular function in Chagas disease. The blockade of
sympathetic activity, which is typically augmented in these patients, may
help to attenuate ventricular remodeling and arrhythmias. Thus, despite
the lack of specific trials to verify this assumption, beta blocker use in pa-
tients with Chagas disease who also have heart failure is indicated.
There is some evidence that amiodarone can prevent complex arrhyth-
mias in patients with heart failure irrespective of the cause. In small studies
in patients with Chagas disease with sustained ventricular tachyarrhyth-
mias, amiodarone provided longer intervals free of arrhythmic events com-
pared with other antiarrhythmic drugs. The efficacy of amiodarone in
preventing ventricular tachyarrhythmias seems to be reduced in patients
with severe systolic dysfunction. In these patients, percutaneous catheter
ablation, implantation of implanted cardioverter/defibrillators (ICD) or sur-
gical procedures may be attempted.
Reentry is considered to be the major arrhythmogenic mechanism of
ventricular tachyarrhythmias in Chagas disease, it is usually in the perian-
eurysmatic zone or in focal areas of fibrosis in the inferolateral region of the
left ventricle. Surgical treatment consists of conventional aneurysmectomy
associated with endocardial or myocardial resection and/or isolation of
critical sites of reentry by endocardiectomy or cryoablation guided by elec-
trophysiologic mapping. Alternatively, interpapillary endomyocardial cryo-
ablation without electrophysiologic mapping has been attempted in
patients with sustained ventricular tachycardia and akinesia or dyskinesia
of the inferolateral region of the left ventricle, with efficacy in nearly 60%.
Because the mortality of the procedure is high, surgical ablation should be
considered only when systolic dysfunction is not severe, the overall surgi-
cal risk is low, and when other surgical procedures, such as aneurysmecto-
my, are not indicated.
Nonsurgical simultaneous epicardial and endocardial catheter ablation
has been introduced recently as an alternative approach in the treatment
of patients with Chagas disease and recurrent ventricular tachycardia.
10
Because critical sites of reentry may be endocardial, intramural, or epicar-
dial, this combined approach provides a higher efficacy for treating recur-
rent ventricular tachycardia. For patients with severe systolic dysfunction,
however, the insertion of an ICD is the treatment of choice, particularly in
those with left ventricular ejection fractions <30%. Symptomatic or high-
risk bradyarrhythmia is frequently manifested in these patients. Thus, im-
plantation of an ICD with pacing capability is often indicated. ICDs are
effective in preventing death due to ventricular tachyarrhythmias, but
frequent shocks triggered by tachycardias, life-threatening or not, can
lead to a reduced quality of life. Therefore, a combination of ICD, antiar-
rhythmic therapy, and catheter ablation appears to be ideal in these
patients.
A
CKNOWLEDGMENTS
The authors are indebted to Dr. Carlos E. Rochitte, Dr. Maria de Lurdes
Higuchi, Dr. Wilson Mathias, and Dr. Claudio Meneghetti for kindly
providing the gures for this chapter.
REFERENCES
1. Higuchi ML et al: The role of active myocarditis in the develop-
ment of heart failure in chronic Chagas disease: A study based on
endomyocardial biopsies. Clin Cardiol 10:665, 1987
2. Bocchi EA et al: Magnetic resonance imaging in chronic Chagas
disease: Correlation with endomyocardial biopsy findings and
Gallium-67 cardiac uptake. Echocardiography 15:279, 1998
3. Rochitte CE et al: Myocardial delayed enhancement by magnetic
resonance imaging in patients with Chagas disease: A marker of
disease severity. J Am Coll Cardiol 46:1553, 2005
4. Torres FW et al: Coronary vascular reactivity is abnormal in pa-
tients with Chagas heart disease. Am Heart J 129:995, 1995
5. Marin-Neto JA et al: Pathogenesis of chronic Chagas heart dis-
ease. Circulation 115:1109, 2007
6. Acquatella H et al: Limited myocardial contractile reserve and
chronotropic incompetence in patients with chronic Chagas dis-
ease: Assessment by dobutamine stress echocardiography. J Am
Coll Cardiol 33:522, 1999
7. Villar JC et al: Trypanocidal drugs for chronic asymptomatic
Trypanosoma cruzi
infection. Cochrane Database Syst Rev
2002(1):CD003463
8. Ramires FJ et al: Aldosterone antagonism in an inflammatory
state: Evidence for myocardial protection. J Renin Angiotensin
Aldosterone Syst 7:162, 2006
9. dAvila A et al: New perspectives on catheter-based ablation of
ventricular tachycardia complicating Chagas disease: Experimen-
tal evidence of the efficacy of near infrared lasers for catheter abla-
tion of Chagas VT. J Interv Cardiol Electrophysiol 7:23, 2002
10. Sosa E et al: Radiofrequency catheter ablation of ventricular tachy-
cardia guided by nonsurgical epicardial mapping in chronic Cha-
gasic heart disease. Pacing Clin Electrophysiol 22(1 Pt 1):128, 1999
11. Bern C et al: Evaluation and treatment of Chagas disease in the
United States. JAMA 298(18):2171, 2007
Вам также может понравиться
- Imaging in Peripheral Arterial Disease: Clinical and Research ApplicationsОт EverandImaging in Peripheral Arterial Disease: Clinical and Research ApplicationsОценок пока нет
- Spot Sign Predict Hematoma ExpansionДокумент7 страницSpot Sign Predict Hematoma ExpansionRonny RahadiОценок пока нет
- Clinical Cases in Chronic Thromboembolic Pulmonary HypertensionОт EverandClinical Cases in Chronic Thromboembolic Pulmonary HypertensionWilliam R. AugerОценок пока нет
- 1 Neurosurg Clin North Am 2010Документ168 страниц1 Neurosurg Clin North Am 2010darkmd100% (1)
- ChagasPaper2 - AAD MCA Mayo2020Документ12 страницChagasPaper2 - AAD MCA Mayo2020Maria Carolina Ayala RamírezОценок пока нет
- Cryptogenic Stroke: Clinical PracticeДокумент10 страницCryptogenic Stroke: Clinical PracticenellieauthorОценок пока нет
- Visualizing Cerebral Small Vessel Degeneration During Aging and Diseases Using2Документ15 страницVisualizing Cerebral Small Vessel Degeneration During Aging and Diseases Using2Sofía ValdésОценок пока нет
- Thesis Acute Coronary SyndromeДокумент8 страницThesis Acute Coronary Syndromeaflpcdwfunzfed100% (2)
- Written BiblioДокумент6 страницWritten BiblioHILLARY JOY ACOSTAОценок пока нет
- Cetin 2016Документ10 страницCetin 2016Della Puspita SariОценок пока нет
- Salim 2009Документ6 страницSalim 2009raden chandrajaya listiandokoОценок пока нет
- Yatsynovich 2017 Cardiac SarcoidosiДокумент64 страницыYatsynovich 2017 Cardiac SarcoidosiJohan ArocaОценок пока нет
- Comparison of Neurological Clinical Manifestation in Patients With Hemorrhagic and Ischemic StrokeДокумент5 страницComparison of Neurological Clinical Manifestation in Patients With Hemorrhagic and Ischemic StrokeDessy FarwasОценок пока нет
- Arteritis TakayasuДокумент7 страницArteritis TakayasuGina ButronОценок пока нет
- Clinical Relevance of Acute Cerebral Microinfarcts in Vascular Cognitive ImpairmentДокумент10 страницClinical Relevance of Acute Cerebral Microinfarcts in Vascular Cognitive Impairmentzefri suhendarОценок пока нет
- Cryptogenic Stroke 2016Документ10 страницCryptogenic Stroke 2016tatukyОценок пока нет
- Evolving Concepts of The Vulnerable Atherosclerotic Plaque and TheДокумент16 страницEvolving Concepts of The Vulnerable Atherosclerotic Plaque and TheKhánh Nguyễn NgọcОценок пока нет
- Factores para FNRДокумент10 страницFactores para FNRPOMYОценок пока нет
- The Usefulness of sST2 and Galectin-3 As Novel Biomarkers For Better Risk Stratification in Hypertrophic CardiomyopathyДокумент8 страницThe Usefulness of sST2 and Galectin-3 As Novel Biomarkers For Better Risk Stratification in Hypertrophic CardiomyopathyHugo ARОценок пока нет
- Jurnal 20Документ7 страницJurnal 20Zella ZakyaОценок пока нет
- Editor 'S Choice e Cerebral Hyperperfusion Syndrome After Carotid Artery Stenting: A Systematic Review and Meta-AnalysisДокумент12 страницEditor 'S Choice e Cerebral Hyperperfusion Syndrome After Carotid Artery Stenting: A Systematic Review and Meta-AnalysisanankastikОценок пока нет
- Coronary Artery Disease Literature ReviewДокумент4 страницыCoronary Artery Disease Literature Reviewaflsvwoeu100% (1)
- Thirty-Day Readmission Is Higher in Patients With Brainstem vs. Non-Brainstem Lacunar StrokeДокумент5 страницThirty-Day Readmission Is Higher in Patients With Brainstem vs. Non-Brainstem Lacunar StrokeijasrjournalОценок пока нет
- CardiologyДокумент11 страницCardiologyandreaОценок пока нет
- Eci 12222Документ9 страницEci 12222Andikaputra Brahma WidiantoroОценок пока нет
- Development and Validation of A Risk Score For Predicting Death in Chagas' Heart DiseaseДокумент8 страницDevelopment and Validation of A Risk Score For Predicting Death in Chagas' Heart Diseaserandom guyОценок пока нет
- Svensson2014 PDFДокумент7 страницSvensson2014 PDFJorge DahdalОценок пока нет
- TEP Systemic Thrombolysis For Pulmonary Embolism Evidence, Patient Selection, and Protocols For ManagementДокумент10 страницTEP Systemic Thrombolysis For Pulmonary Embolism Evidence, Patient Selection, and Protocols For Managementbenitez1228Оценок пока нет
- Eco Doppler Trascraneal 1Документ12 страницEco Doppler Trascraneal 1David Sebastian Boada PeñaОценок пока нет
- A Comparative Study of Platelet Indices in Acute Coronary SyndromeДокумент4 страницыA Comparative Study of Platelet Indices in Acute Coronary SyndromeMarcellia AngelinaОценок пока нет
- Thick and Diffuse Cisternal Clot Independently Predicts Vasospasm-Related Morbidity and Poor Outcome After Aneurysmal Subarachnoid HemorrhageДокумент9 страницThick and Diffuse Cisternal Clot Independently Predicts Vasospasm-Related Morbidity and Poor Outcome After Aneurysmal Subarachnoid HemorrhageVanessa LandaОценок пока нет
- Mendieta Et Al 2023 Determinants of Progression and Regression of Subclinical Atherosclerosis Over 6 YearsДокумент15 страницMendieta Et Al 2023 Determinants of Progression and Regression of Subclinical Atherosclerosis Over 6 YearsEnzo GonzalezОценок пока нет
- Ehac 811Документ3 страницыEhac 811Rositaka1991Оценок пока нет
- Viral Myocarditis: A Paradigm For Understanding The Pathogenesis and Treatment of Dilated CardiomyopathyДокумент7 страницViral Myocarditis: A Paradigm For Understanding The Pathogenesis and Treatment of Dilated CardiomyopathyLeslie Noelle GarciaОценок пока нет
- 2021 Special Care in Dentistry Detection of Calcified AtheromasДокумент6 страниц2021 Special Care in Dentistry Detection of Calcified AtheromasNATALIA SILVA ANDRADEОценок пока нет
- LeukemiaДокумент10 страницLeukemiaShapira alОценок пока нет
- Wang 2016Документ9 страницWang 2016Elisabeth TikalakaОценок пока нет
- Clonal Hematopoiesis and Risk For Atherosclerotic CardiovascularДокумент15 страницClonal Hematopoiesis and Risk For Atherosclerotic CardiovasculargiulioОценок пока нет
- Stroke Prevention and Treatment in Sickle Cell Disease: Robert J. Adams, MS, MDДокумент4 страницыStroke Prevention and Treatment in Sickle Cell Disease: Robert J. Adams, MS, MDMeshaki MbarukaОценок пока нет
- Features of Branch Occlusive Disease-Type Intracranial Atherosclerotic Stroke in Young PatientsДокумент7 страницFeatures of Branch Occlusive Disease-Type Intracranial Atherosclerotic Stroke in Young PatientsrajaririnОценок пока нет
- Increasingly Sensitive Assays For Cardiac TroponinsДокумент8 страницIncreasingly Sensitive Assays For Cardiac TroponinsAbel Espinoza MedallaОценок пока нет
- Rutman Et Al 2022 Incidental Vascular Findings On Brain Magnetic Resonance AngiographyДокумент13 страницRutman Et Al 2022 Incidental Vascular Findings On Brain Magnetic Resonance AngiographySofía ValdésОценок пока нет
- Markers For Inflammation and Oxidative Stress in Patients With Coronary Artery Disease and Microvascular Disease - Is There A Difference?Документ3 страницыMarkers For Inflammation and Oxidative Stress in Patients With Coronary Artery Disease and Microvascular Disease - Is There A Difference?TeodorОценок пока нет
- Diagnosis and Management of Pericardial EffusionДокумент15 страницDiagnosis and Management of Pericardial EffusionYani Mu Mu Utak-utukОценок пока нет
- ECV Hemorragico RADIOДокумент19 страницECV Hemorragico RADIOLuis DubonОценок пока нет
- Eurheartj Ehu357 FullДокумент8 страницEurheartj Ehu357 FullagneselimОценок пока нет
- Circulating Serum Markers and QRS Scar Score in Chagas Cardiomyopathy 2015Документ6 страницCirculating Serum Markers and QRS Scar Score in Chagas Cardiomyopathy 2015Laura montillaОценок пока нет
- Spontaneous Intracerebral HaemorrhageДокумент3 страницыSpontaneous Intracerebral Haemorrhageselvie87Оценок пока нет
- Anemia Aplastic JournalДокумент13 страницAnemia Aplastic JournalGenoveva Maditias Dwi PertiwiОценок пока нет
- 116 229 1 SMДокумент6 страниц116 229 1 SMsinlookerОценок пока нет
- H Bevan, K Sharma and W Bradley: Stroke in Young AdultsДокумент6 страницH Bevan, K Sharma and W Bradley: Stroke in Young Adultsarif 2006Оценок пока нет
- Cerebral Venous Sinus ThrombosisДокумент11 страницCerebral Venous Sinus ThrombosisdjcafОценок пока нет
- 19 TEP Tak2019Документ12 страниц19 TEP Tak2019Juan Carlos Uribe CaballeroОценок пока нет
- Correlation Between Neutrophil To Lymphocyte Ratio and Coronary Calcium Score in CT Angiography. NLR and Coronary CalcificationДокумент6 страницCorrelation Between Neutrophil To Lymphocyte Ratio and Coronary Calcium Score in CT Angiography. NLR and Coronary Calcificationinna mayaОценок пока нет
- Literature Review On Acute Coronary SyndromeДокумент7 страницLiterature Review On Acute Coronary Syndromegazqaacnd100% (1)
- The Comparative and Usefulness of Platelet Distribution Width in Acute Coronary SyndromeДокумент6 страницThe Comparative and Usefulness of Platelet Distribution Width in Acute Coronary SyndromeAndi Tiara S. AdamОценок пока нет
- Low Systemic Vascular ResistanceДокумент7 страницLow Systemic Vascular ResistanceMuhammad BadrushshalihОценок пока нет
- Nikolsky2007 PDFДокумент7 страницNikolsky2007 PDFPicha PichiОценок пока нет
- Myelodysplastic Syndromes: Diagnosis, Prognosis, and TreatmentДокумент11 страницMyelodysplastic Syndromes: Diagnosis, Prognosis, and TreatmentTataОценок пока нет
- High Prevalence of Subclinical Left Ventricular Dysfunction in Patients With Psoriatic ArthritisДокумент8 страницHigh Prevalence of Subclinical Left Ventricular Dysfunction in Patients With Psoriatic ArthritisEmanuel NavarreteОценок пока нет
- 2 N 5087Документ14 страниц2 N 5087mike_helplineОценок пока нет
- PNP General Purpose Amplifier: Absolute Maximum RatingsДокумент10 страницPNP General Purpose Amplifier: Absolute Maximum Ratingsmike_helplineОценок пока нет
- 2 N 3704Документ7 страниц2 N 3704mike_helplineОценок пока нет
- 2 N 3904Документ17 страниц2 N 3904mike_helplineОценок пока нет
- 2 N 3903Документ11 страниц2 N 3903mike_helplineОценок пока нет
- Applications: Mechanical DataДокумент2 страницыApplications: Mechanical DataelsenseisanОценок пока нет
- 2 N 5769Документ7 страниц2 N 5769m_umair72001Оценок пока нет
- Transistor NPN 2n222aДокумент8 страницTransistor NPN 2n222aRoberto Eduardo Tenorio RamosОценок пока нет
- 407px Abbey LincolnДокумент1 страница407px Abbey Lincolnmike_helplineОценок пока нет
- 413px-Memphis Slim, American Folk Blues Festival 1972 (Heinrich Klaffs Collection 72)Документ1 страница413px-Memphis Slim, American Folk Blues Festival 1972 (Heinrich Klaffs Collection 72)mike_helplineОценок пока нет
- 400px Peter BroetzmannДокумент1 страница400px Peter Broetzmannmike_helplineОценок пока нет
- 410px Jack TeagardenДокумент1 страница410px Jack Teagardenmike_helplineОценок пока нет
- 401px-Chris Biscoe PhotoДокумент1 страница401px-Chris Biscoe Photomike_helplineОценок пока нет
- 449px-Bob Cooper and June Christy (Gottlieb 13091)Документ1 страница449px-Bob Cooper and June Christy (Gottlieb 13091)mike_helplineОценок пока нет
- 458px-Boyd Raeburn 1946 (Gottlieb)Документ1 страница458px-Boyd Raeburn 1946 (Gottlieb)mike_helplineОценок пока нет
- 417px-Pharoah Sanders PhotoДокумент1 страница417px-Pharoah Sanders Photomike_helplineОценок пока нет
- 450px Conny BauerДокумент1 страница450px Conny Bauermike_helplineОценок пока нет
- 450px-DM OrangeJacket TieДокумент1 страница450px-DM OrangeJacket Tiemike_helplineОценок пока нет
- 468px Eddie JeffersonДокумент1 страница468px Eddie Jeffersonmike_helplineОценок пока нет
- 449px-Louis Jordan, New York, N.Y., CA. July 1946 (William P. Gottlieb 04721)Документ1 страница449px-Louis Jordan, New York, N.Y., CA. July 1946 (William P. Gottlieb 04721)mike_helplineОценок пока нет
- 453px-1995 Tony CoeДокумент1 страница453px-1995 Tony Coemike_helplineОценок пока нет
- 461px-Adrian Rollini and Allen Haulon, Between 1938 and 1948 (William P. Gottlieb 07491)Документ1 страница461px-Adrian Rollini and Allen Haulon, Between 1938 and 1948 (William P. Gottlieb 07491)mike_helplineОценок пока нет
- 456px-Lionel Hampton and Arnett Cobb, Aquarioum, NYC, CA. June 1946 (Gottlieb)Документ1 страница456px-Lionel Hampton and Arnett Cobb, Aquarioum, NYC, CA. June 1946 (Gottlieb)mike_helplineОценок пока нет
- 463px-Scott Hamilton & Ray BrownДокумент1 страница463px-Scott Hamilton & Ray Brownmike_helplineОценок пока нет
- Atlas of Liver Biopsies: Jules L. Dienstag, Atul K. BhanДокумент4 страницыAtlas of Liver Biopsies: Jules L. Dienstag, Atul K. Bhanmike_helplineОценок пока нет
- Atlas of Neuroimaging: Andre Furtado, William P. DillonДокумент36 страницAtlas of Neuroimaging: Andre Furtado, William P. Dillonmike_helplineОценок пока нет
- Video Atlas of Gastrointestinal Endoscopy: Louis Michel Wong-Kee-Song, Mark TopazianДокумент8 страницVideo Atlas of Gastrointestinal Endoscopy: Louis Michel Wong-Kee-Song, Mark Topazianmike_helplineОценок пока нет
- 478px-Lucky Thompson Hilda A. Taylor and Al McKibbonДокумент1 страница478px-Lucky Thompson Hilda A. Taylor and Al McKibbonmike_helplineОценок пока нет
- Primary Immunodeficiencies Associated With or Secondary To Other DiseasesДокумент2 страницыPrimary Immunodeficiencies Associated With or Secondary To Other Diseasesmike_helplineОценок пока нет
- Hpim E35.fm PDFДокумент16 страницHpim E35.fm PDFmike_helplineОценок пока нет
- Crime Scene Drawing January Incident 10501-10600Документ100 страницCrime Scene Drawing January Incident 10501-10600columbinefamilyrequest100% (2)
- Wetlands Denote Perennial Water Bodies That Originate From Underground Sources of Water or RainsДокумент3 страницыWetlands Denote Perennial Water Bodies That Originate From Underground Sources of Water or RainsManish thapaОценок пока нет
- FIREXДокумент2 страницыFIREXPausОценок пока нет
- Laboratory Methodsin ImmnunologyДокумент58 страницLaboratory Methodsin Immnunologyadi pОценок пока нет
- A Comprehensive Guide To HR Best Practices You Need To Know This Year (Infographic)Документ42 страницыA Comprehensive Guide To HR Best Practices You Need To Know This Year (Infographic)MALATHI MОценок пока нет
- An Etymological Dictionary of The Scottivol 2Документ737 страницAn Etymological Dictionary of The Scottivol 2vstrohmeОценок пока нет
- Gastric Emptying PresentationДокумент8 страницGastric Emptying Presentationrahul2kОценок пока нет
- Project TitleДокумент15 страницProject TitleadvikaОценок пока нет
- ICONS+Character+Creator+2007+v0 73Документ214 страницICONS+Character+Creator+2007+v0 73C.M. LewisОценок пока нет
- Intro To EthicsДокумент4 страницыIntro To EthicsChris Jay RamosОценок пока нет
- Ignorance Is The Curse of God. Knowledge Is The Wing Wherewith We Fly To Heaven."Документ3 страницыIgnorance Is The Curse of God. Knowledge Is The Wing Wherewith We Fly To Heaven."Flori025Оценок пока нет
- Field Assignment On Feacal Sludge ManagementДокумент10 страницField Assignment On Feacal Sludge ManagementSarah NamyaloОценок пока нет
- Hypnosis ScriptДокумент3 страницыHypnosis ScriptLuca BaroniОценок пока нет
- Feuerhahn Funeral Bullet 17 March 2015Документ12 страницFeuerhahn Funeral Bullet 17 March 2015brandy99Оценок пока нет
- Chapter 15 (Partnerships Formation, Operation and Ownership Changes) PDFДокумент58 страницChapter 15 (Partnerships Formation, Operation and Ownership Changes) PDFAbdul Rahman SholehОценок пока нет
- APICS-Houston Newsletter Sept 2012Документ16 страницAPICS-Houston Newsletter Sept 2012Christopher SeifertОценок пока нет
- Philippine Housing Industry: Regulatory ReviewДокумент42 страницыPhilippine Housing Industry: Regulatory ReviewAl MarzolОценок пока нет
- The BreakupДокумент22 страницыThe BreakupAllison CreaghОценок пока нет
- Government by Algorithm - Artificial Intelligence in Federal Administrative AgenciesДокумент122 страницыGovernment by Algorithm - Artificial Intelligence in Federal Administrative AgenciesRone Eleandro dos SantosОценок пока нет
- LIM Gr7 Q4W3Документ9 страницLIM Gr7 Q4W3Eto YoshimuraОценок пока нет
- Genomics - FAOДокумент184 страницыGenomics - FAODennis AdjeiОценок пока нет
- De Luyen Thi Vao Lop 10 Mon Tieng Anh Nam Hoc 2019Документ106 страницDe Luyen Thi Vao Lop 10 Mon Tieng Anh Nam Hoc 2019Mai PhanОценок пока нет
- Bootstrap DatepickerДокумент31 страницаBootstrap DatepickerdandczdczОценок пока нет
- EMI - Module 1 Downloadable Packet - Fall 2021Документ34 страницыEMI - Module 1 Downloadable Packet - Fall 2021Eucarlos MartinsОценок пока нет
- Curriculum Vitae: Personal InformationДокумент3 страницыCurriculum Vitae: Personal InformationMira ChenОценок пока нет
- PT - Science 5 - Q1Документ4 страницыPT - Science 5 - Q1Jomelyn MaderaОценок пока нет
- Adverbs Before AdjectivesДокумент2 страницыAdverbs Before AdjectivesJuan Sanchez PrietoОценок пока нет
- SULTANS OF SWING - Dire Straits (Impresión)Документ1 страницаSULTANS OF SWING - Dire Straits (Impresión)fabio.mattos.tkd100% (1)
- KalamДокумент8 страницKalamRohitKumarSahuОценок пока нет
- PCI Bank V CA, G.R. No. 121413, January 29, 2001Документ10 страницPCI Bank V CA, G.R. No. 121413, January 29, 2001ademarОценок пока нет
- By the Time You Read This: The Space between Cheslie's Smile and Mental Illness—Her Story in Her Own WordsОт EverandBy the Time You Read This: The Space between Cheslie's Smile and Mental Illness—Her Story in Her Own WordsОценок пока нет
- Summary: Outlive: The Science and Art of Longevity by Peter Attia MD, With Bill Gifford: Key Takeaways, Summary & AnalysisОт EverandSummary: Outlive: The Science and Art of Longevity by Peter Attia MD, With Bill Gifford: Key Takeaways, Summary & AnalysisРейтинг: 4.5 из 5 звезд4.5/5 (42)
- Think This, Not That: 12 Mindshifts to Breakthrough Limiting Beliefs and Become Who You Were Born to BeОт EverandThink This, Not That: 12 Mindshifts to Breakthrough Limiting Beliefs and Become Who You Were Born to BeРейтинг: 2 из 5 звезд2/5 (1)
- Raising Mentally Strong Kids: How to Combine the Power of Neuroscience with Love and Logic to Grow Confident, Kind, Responsible, and Resilient Children and Young AdultsОт EverandRaising Mentally Strong Kids: How to Combine the Power of Neuroscience with Love and Logic to Grow Confident, Kind, Responsible, and Resilient Children and Young AdultsРейтинг: 5 из 5 звезд5/5 (1)
- The Age of Magical Overthinking: Notes on Modern IrrationalityОт EverandThe Age of Magical Overthinking: Notes on Modern IrrationalityРейтинг: 4 из 5 звезд4/5 (24)
- Summary: The Psychology of Money: Timeless Lessons on Wealth, Greed, and Happiness by Morgan Housel: Key Takeaways, Summary & Analysis IncludedОт EverandSummary: The Psychology of Money: Timeless Lessons on Wealth, Greed, and Happiness by Morgan Housel: Key Takeaways, Summary & Analysis IncludedРейтинг: 5 из 5 звезд5/5 (80)
- The Obesity Code: Unlocking the Secrets of Weight LossОт EverandThe Obesity Code: Unlocking the Secrets of Weight LossРейтинг: 4 из 5 звезд4/5 (6)
- The Body Keeps the Score by Bessel Van der Kolk, M.D. - Book Summary: Brain, Mind, and Body in the Healing of TraumaОт EverandThe Body Keeps the Score by Bessel Van der Kolk, M.D. - Book Summary: Brain, Mind, and Body in the Healing of TraumaРейтинг: 4.5 из 5 звезд4.5/5 (266)
- Raising Good Humans: A Mindful Guide to Breaking the Cycle of Reactive Parenting and Raising Kind, Confident KidsОт EverandRaising Good Humans: A Mindful Guide to Breaking the Cycle of Reactive Parenting and Raising Kind, Confident KidsРейтинг: 4.5 из 5 звезд4.5/5 (169)
- ADHD is Awesome: A Guide to (Mostly) Thriving with ADHDОт EverandADHD is Awesome: A Guide to (Mostly) Thriving with ADHDРейтинг: 5 из 5 звезд5/5 (1)
- Why We Die: The New Science of Aging and the Quest for ImmortalityОт EverandWhy We Die: The New Science of Aging and the Quest for ImmortalityРейтинг: 4 из 5 звезд4/5 (3)
- Gut: the new and revised Sunday Times bestsellerОт EverandGut: the new and revised Sunday Times bestsellerРейтинг: 4 из 5 звезд4/5 (392)
- The Courage Habit: How to Accept Your Fears, Release the Past, and Live Your Courageous LifeОт EverandThe Courage Habit: How to Accept Your Fears, Release the Past, and Live Your Courageous LifeРейтинг: 4.5 из 5 звезд4.5/5 (253)
- The Ritual Effect: From Habit to Ritual, Harness the Surprising Power of Everyday ActionsОт EverandThe Ritual Effect: From Habit to Ritual, Harness the Surprising Power of Everyday ActionsРейтинг: 3.5 из 5 звезд3.5/5 (3)
- When the Body Says No by Gabor Maté: Key Takeaways, Summary & AnalysisОт EverandWhen the Body Says No by Gabor Maté: Key Takeaways, Summary & AnalysisРейтинг: 3.5 из 5 звезд3.5/5 (2)
- Sleep Stories for Adults: Overcome Insomnia and Find a Peaceful AwakeningОт EverandSleep Stories for Adults: Overcome Insomnia and Find a Peaceful AwakeningРейтинг: 4 из 5 звезд4/5 (3)
- Dark Psychology & Manipulation: Discover How To Analyze People and Master Human Behaviour Using Emotional Influence Techniques, Body Language Secrets, Covert NLP, Speed Reading, and Hypnosis.От EverandDark Psychology & Manipulation: Discover How To Analyze People and Master Human Behaviour Using Emotional Influence Techniques, Body Language Secrets, Covert NLP, Speed Reading, and Hypnosis.Рейтинг: 4.5 из 5 звезд4.5/5 (110)
- To Explain the World: The Discovery of Modern ScienceОт EverandTo Explain the World: The Discovery of Modern ScienceРейтинг: 3.5 из 5 звезд3.5/5 (51)
- Mindset by Carol S. Dweck - Book Summary: The New Psychology of SuccessОт EverandMindset by Carol S. Dweck - Book Summary: The New Psychology of SuccessРейтинг: 4.5 из 5 звезд4.5/5 (328)
- Outlive: The Science and Art of Longevity by Peter Attia: Key Takeaways, Summary & AnalysisОт EverandOutlive: The Science and Art of Longevity by Peter Attia: Key Takeaways, Summary & AnalysisРейтинг: 4 из 5 звезд4/5 (1)
- Cult, A Love Story: Ten Years Inside a Canadian Cult and the Subsequent Long Road of RecoveryОт EverandCult, A Love Story: Ten Years Inside a Canadian Cult and the Subsequent Long Road of RecoveryРейтинг: 4 из 5 звезд4/5 (44)
- Summary: The Myth of Normal: Trauma, Illness, and Healing in a Toxic Culture By Gabor Maté MD & Daniel Maté: Key Takeaways, Summary & AnalysisОт EverandSummary: The Myth of Normal: Trauma, Illness, and Healing in a Toxic Culture By Gabor Maté MD & Daniel Maté: Key Takeaways, Summary & AnalysisРейтинг: 4 из 5 звезд4/5 (9)