Академический Документы
Профессиональный Документы
Культура Документы
Solutions - : Solute Solvent
Загружено:
Lawn940 оценок0% нашли этот документ полезным (0 голосов)
22 просмотров4 страницыchemistry notes
Оригинальное название
1354313032_2012_Chemistry_Notes
Авторское право
© © All Rights Reserved
Доступные форматы
DOCX, PDF, TXT или читайте онлайн в Scribd
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документchemistry notes
Авторское право:
© All Rights Reserved
Доступные форматы
Скачайте в формате DOCX, PDF, TXT или читайте онлайн в Scribd
0 оценок0% нашли этот документ полезным (0 голосов)
22 просмотров4 страницыSolutions - : Solute Solvent
Загружено:
Lawn94chemistry notes
Авторское право:
© All Rights Reserved
Доступные форматы
Скачайте в формате DOCX, PDF, TXT или читайте онлайн в Scribd
Вы находитесь на странице: 1из 4
MODULE 3: WATER Chapters: 10 14
Chapter 10: Water on Earth
- Only substance found in 3 states
- In BIOsphere, LITHOsphere, HYDROsphere, ATMOsphere
- Essential transport system (eg. Minerals, vitamins)
- heat transfer systems
- essential chemical in many reactions (eg. Photosynthesis)
Solutions
- Homogenous mixtures
Solute solid which is dissolved
Solvent substance which does the dissolving
Saturated no more solute will dissolve in current conditions
Unsaturated more solute is able to be dissolved in current conditions / does not contain maximum
amount of solute
Density = m / V
Chapter 11: Molecular Structure (of water) + Hydrogen Bonding
Molecular Shapes
- electron pairs arranged as far apart as possible = minimise repulsion
Linear
Can be Polar, eg.
HCl
Can be Non-Polar
eg. H2
Non-Polar
Triangular Planar (triangle with central atom)
Bent Shaped
Polar eg. H
2
O
Tetrahedral (triangular pyramid, with central atom)
Polar Bonds covalent, uneven charge distribution
Electronegativity
- determines bond type
- large different = ionic
- moderate difference = polar covalent
- no difference = non-polar covalent
Polar Molecules:
- contain one or more polar bonds
- Not symmetrical
MODULE 3: WATER Chapters: 10 14
Intermolecular Forces
Hydrogen Bonding strong dipole-dipole between H and O, N or F
Dipole-Dipole attraction between oppositely slightly charged ends of polar molecules
Dispersion weakest, non-polar molecules, temporary dipoles due to constant movement of electrons,
usually gases
Surface Tension molecules at surface have an overall downwards attractive force, creates tension
Viscosity resistance to flow
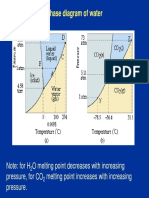
M.P/B.P of Water higher than expected due to strong HYDROGEN bonding
Chapter 12: Water as a Solvent
Solvent Dissolves a Solute
- Intermolecular forces between solvent broken
- Intermolecular or Ionic bonds broken
- Intermolecular between solvent + solute form
Like Dissolves Like
- Polar solvents dissolve polar solutes
- Non-polar solvents dissolve non-polar solutes
- Water = polar and ionic compounds
- Solubility depends = solute molecular structure + bonding
- Polar or non-polar + size of particles eg. Does water have the muscle power to physically separate
the solute particles
Ionic Usually soluble Eg. NaCl, CuSO
4
C.M which form H bonds Soluble Eg. Glucose, HF
Polar + Non-Polar C.M Slightly Soluble solubility
increases with polarity
Eg. CO
2
, N
2
Large C.M Insoluble Eg. Cellulose
C.N Insoluble
Dissociation Ionic solid separates into ions (as dissolves)
Ionisation C.M dissolves to form ions
Electrolyte produces ions when dissolved = conductor solution
MODULE 3: WATER Chapters: 10 14
Chapter 13: Soluble and Insoluble Salts
Precipitation Reaction two solutions mix, insoluble solid forms
Precipitate - solid which forms from solution of two soluble solutions
Table of Solubilities
GENERALLY SOLUBLE EXCEPTIONS (ions)
Sodium Na
+
,
Potassium K
+
Ammonium NH
4
+
Nitrates NO
3
-
- All
Sulphates SO
4
2-
Sr, Ba, Pb ions insoluble
Ca, Ag slightly soluble
Chloride Cl
-
Ag insoluble
Pb slightly soluble
Bromine Br
-
Ag insoluble
Pb slightly soluble
Iodine I
-
Ag, Pb insoluble
Equilibrium
- Rate of forward and reverse reactions equal
- No observable change
- Eg. In saturated solutions
Concentration
- Diluted solution = # of moles same, concentration decreases
C = n/V
Concentration = number of moles (solute) / volume
(Mol L
-1
)
(mol) (L)
Chapter 14: Heat Capacity of Water
Specific Heat Capacity
- Amount of heat energy needed to raise 1 gram of substance by one Kelvin/degrees
- J/g/K or Jg
-1
K
-1
Water = 4.18 Jg
-1
K
-1
GENERALLY INSOLUBLE EXCEPTIONS
Carbonates CO
3
2-
All solubles
Phosphates PO
4
3-
All solubles
Oxides O
2-
All solubles , Ba soluble
Ca slightly soluble
Hydroxides OH
-
All solubles , Ba soluble
Ca slightly soluble
EXOTHERMIC
Energy is released
Decrease in enthalpy of system
Increase in surroundings temp.
Combustion reactions heat + light released
ENDOTHERMIC
Energy is absorbed (from surroundings)
Increase in enthalpy of system
Decrease in surroundings temp.
Photosynthesis, thermal decomp. reactions
MODULE 3: WATER Chapters: 10 14
Combustion Reactions oxidation reactions
Enthalpy stored heat energy
- H = H (products/final) H (reactants/initial)
- -H exothermic
- +H endothermic
Energy Change
H = -mCT H = energy (J) m= mass
C = specific heat capacity
T = temp. change
- Change in enthalpy system
- Shows exo. or endo.
Heat of solution
- Enthalpy change when solute dissolves in solvent
- Endothermic separating solute particles
- Endothermic separating water molecules
- Exothermic hydration process
o Thermal pollution excess heat released into enviro.
o Gases become less soluble as water temp. increases
Вам также может понравиться
- Biology Exam 4 Study GuideДокумент12 страницBiology Exam 4 Study GuideKhusbu PatelОценок пока нет
- Combining Chemicals - Fun Chemistry Book for 4th Graders | Children's Chemistry BooksОт EverandCombining Chemicals - Fun Chemistry Book for 4th Graders | Children's Chemistry BooksОценок пока нет
- Chemistry Final ReviewДокумент12 страницChemistry Final Reviewhayleyguillou3272100% (1)
- GCSE Chemistry Revision: Cheeky Revision ShortcutsОт EverandGCSE Chemistry Revision: Cheeky Revision ShortcutsРейтинг: 4.5 из 5 звезд4.5/5 (3)
- Beiru Arab University SolutionsДокумент74 страницыBeiru Arab University SolutionsMoh AmmОценок пока нет
- IGCSE Chemistry Definitions - Learn Key TermsДокумент5 страницIGCSE Chemistry Definitions - Learn Key Termsjenifer100% (1)
- Chemistry NotesДокумент31 страницаChemistry NotesDaniel LiОценок пока нет
- Phase Diagram of WaterДокумент29 страницPhase Diagram of WaterSaif UllahОценок пока нет
- Q=Mxcxδt: Df1 - Getting Energy From FuelsДокумент6 страницQ=Mxcxδt: Df1 - Getting Energy From FuelskjОценок пока нет
- Acids and BasesДокумент1 страницаAcids and BasesWilson Pedro Tamega JuniorОценок пока нет
- Chemistry ReviewДокумент8 страницChemistry ReviewDulwin Jayalath100% (2)
- 2 ChemistryДокумент7 страниц2 ChemistryAlexandra EscalonaОценок пока нет
- Properties of WaterДокумент154 страницыProperties of WaterGayathri AnandОценок пока нет
- Unit 7Документ49 страницUnit 7Ivy CustodioОценок пока нет
- Intermolecular Forces and Properties of LiquidsДокумент9 страницIntermolecular Forces and Properties of LiquidsAmar Poñado BagacinaОценок пока нет
- Reviewer For Chemistry 2 2ND Semester 1ST QuarterДокумент8 страницReviewer For Chemistry 2 2ND Semester 1ST QuarterElla VelgaОценок пока нет
- Chapter 13 Key Properties of SolutionsДокумент16 страницChapter 13 Key Properties of SolutionsSurya PrakashОценок пока нет
- Chapter 10 StudentДокумент19 страницChapter 10 StudentGorgi PavlovОценок пока нет
- Chem 1B Potma Midterm II Review Packet Key PointsДокумент14 страницChem 1B Potma Midterm II Review Packet Key Pointssarah_choi_21Оценок пока нет
- Chemistry Interview Questions Segregated (2)Документ4 страницыChemistry Interview Questions Segregated (2)nhempire1717Оценок пока нет
- Unit-4 - Chapters (1,2,3,4)Документ10 страницUnit-4 - Chapters (1,2,3,4)Farah AounОценок пока нет
- The chemistry of water molecule and its role as solventДокумент6 страницThe chemistry of water molecule and its role as solventEva MoonОценок пока нет
- Biochemistry NotesДокумент90 страницBiochemistry Notespatialokkumar100% (2)
- 2 Water As Universal Solvent and CoolentДокумент10 страниц2 Water As Universal Solvent and CoolentZaifi KhanОценок пока нет
- Unit V Solutions: Solution ChemistryДокумент7 страницUnit V Solutions: Solution ChemistryCharlie SobcovОценок пока нет
- Chapter 11: States of Matter and IMF (40Документ9 страницChapter 11: States of Matter and IMF (40JohnnyMoyaОценок пока нет
- Properties, Structure & Separation TechniquesДокумент8 страницProperties, Structure & Separation TechniquesMatthew ChengОценок пока нет
- Chemistry Glossary For A2Документ21 страницаChemistry Glossary For A2s_s_i_hassaanОценок пока нет
- Chapter 4 SolutionДокумент22 страницыChapter 4 SolutionFiraol MamoОценок пока нет
- Colligative Properties of Electrolytes Vs Nonelectrolytes and Introduction To ThermodynamicsДокумент15 страницColligative Properties of Electrolytes Vs Nonelectrolytes and Introduction To ThermodynamicsEdd VillamorОценок пока нет
- AP Chemistry Properties of Solutions: Important TermsДокумент17 страницAP Chemistry Properties of Solutions: Important TermsLuc LeОценок пока нет
- Surface ChemistryДокумент17 страницSurface ChemistryFourte GuОценок пока нет
- Biological Molecules Chapter on Water and CarbohydratesДокумент30 страницBiological Molecules Chapter on Water and CarbohydratesAlicia LamОценок пока нет
- Acids, bases and chemical terms definedДокумент49 страницAcids, bases and chemical terms definedmujieОценок пока нет
- MolarityДокумент7 страницMolarityMacxieОценок пока нет
- Genchem CheatsheetДокумент2 страницыGenchem CheatsheetLevi San JoseОценок пока нет
- Chemistry Unit 5 Notes - Part 1Документ5 страницChemistry Unit 5 Notes - Part 1Aly-Salman TejaniОценок пока нет
- Topic Name: Ruochen Wu Teacher: Dr. Williams Class: Chemistry B4 Date: 4/3/13 Questions/Main Ideas: NotesДокумент5 страницTopic Name: Ruochen Wu Teacher: Dr. Williams Class: Chemistry B4 Date: 4/3/13 Questions/Main Ideas: NotesRuochen WuОценок пока нет
- IntroДокумент3 страницыIntroMary Rose AllinegОценок пока нет
- As Chemistry Unit 1 NotesДокумент8 страницAs Chemistry Unit 1 NoteshamzazzОценок пока нет
- AP Biology Semester 1 ReviewДокумент44 страницыAP Biology Semester 1 ReviewGeelonSo100% (1)
- Chemistry 2Документ6 страницChemistry 2YURI ISHIDAОценок пока нет
- Chapter ThirteenДокумент19 страницChapter ThirteenFarah AttallahОценок пока нет
- Yr 11 Chemistry Exam NotesДокумент13 страницYr 11 Chemistry Exam NotesadfknaljhОценок пока нет
- Chem NotesДокумент11 страницChem NotesAОценок пока нет
- Ebbing 12Документ20 страницEbbing 12Ra MilОценок пока нет
- The Physics of Liquid WaterДокумент24 страницыThe Physics of Liquid WaterAbdul Rahim KaladiОценок пока нет
- Enthalpy of Solution Part 2 EdexcelДокумент2 страницыEnthalpy of Solution Part 2 EdexcelKevin The Chemistry TutorОценок пока нет
- Solubilidad Explicada RSCДокумент8 страницSolubilidad Explicada RSCSebastian Finger CaraccioliОценок пока нет
- Final Study GuideДокумент8 страницFinal Study GuidePaula Larios AguilarОценок пока нет
- WATER Is The Solvent of Choice For BiologicalДокумент15 страницWATER Is The Solvent of Choice For Biologicalvicbart11Оценок пока нет
- Chemistry I Study GuideДокумент11 страницChemistry I Study GuideDeepti SailappanОценок пока нет
- Solubility Xplained: Emist1 Y, Gam Idge UnivДокумент8 страницSolubility Xplained: Emist1 Y, Gam Idge Univroza maiyarniОценок пока нет
- Chemistry Eoc Study Guide (11x17)Документ2 страницыChemistry Eoc Study Guide (11x17)api-254514513Оценок пока нет
- Chemistry Final Study GuideДокумент27 страницChemistry Final Study Guidesana lazfaОценок пока нет
- POLARITY OF A MOLECULE TO ITS PROPERTIESДокумент25 страницPOLARITY OF A MOLECULE TO ITS PROPERTIESCherry-Ann BernardezОценок пока нет
- Chemistry Terms and DefinitionsДокумент6 страницChemistry Terms and DefinitionsbesamundoОценок пока нет
- CHEF126 Note (1-3) UNITEN FOUNDATION CHEMISTRY 2Документ5 страницCHEF126 Note (1-3) UNITEN FOUNDATION CHEMISTRY 2Filarius Peter UsopОценок пока нет
- Reactions of Key Organic GroupsДокумент35 страницReactions of Key Organic GroupsMatthew HallОценок пока нет
- Lect 17Документ26 страницLect 17vatsalp3Оценок пока нет
- Experiment 40B Light, Energy and SpectraДокумент11 страницExperiment 40B Light, Energy and SpectraAdnan SheraziОценок пока нет
- Mechanics of Composite Materials and LaminatedДокумент139 страницMechanics of Composite Materials and LaminatedKhoi Le100% (1)
- Calibration Curve of MBДокумент140 страницCalibration Curve of MBSandip KadoliОценок пока нет
- Thermal properties of ammonium nitrateДокумент4 страницыThermal properties of ammonium nitratesj_scribdОценок пока нет
- A Method To Model Wood by Using ABAQUS Finite Element SoftwareДокумент58 страницA Method To Model Wood by Using ABAQUS Finite Element SoftwareAnonymous 7MdZQn1Оценок пока нет
- Ci33 321 Aisc Design Guide 1 - Column Base Plates - 2nd EditionДокумент16 страницCi33 321 Aisc Design Guide 1 - Column Base Plates - 2nd EditionALFA ENGINEERINGОценок пока нет
- Spacetime Singularity & Poincare's Balayage: Mathematical Renormalization of Newtonian Potentials Using Nonlinear Singular Elliptic and Parabolic Equations. Carlos C. ArandaДокумент13 страницSpacetime Singularity & Poincare's Balayage: Mathematical Renormalization of Newtonian Potentials Using Nonlinear Singular Elliptic and Parabolic Equations. Carlos C. ArandaCarlos Cesar ArandaОценок пока нет
- Emissivity Measurement of Radiating SurfacesДокумент4 страницыEmissivity Measurement of Radiating Surfacesashish100% (1)
- Chapter 13Документ41 страницаChapter 13Zahurul IslamОценок пока нет
- Homework 3 Vector Algebra and CalculusДокумент3 страницыHomework 3 Vector Algebra and CalculusSwathi BDОценок пока нет
- Potential Gradient - Wikipedia, The Free EncyclopediaДокумент4 страницыPotential Gradient - Wikipedia, The Free EncyclopediaMaria MarreroОценок пока нет
- Development of A New Family of Normalized Modulus Reduction and Materials Dumping Curves Darendeli PhD-2008)Документ25 страницDevelopment of A New Family of Normalized Modulus Reduction and Materials Dumping Curves Darendeli PhD-2008)omar45Оценок пока нет
- Drilling Fluids Flow BehaviorДокумент44 страницыDrilling Fluids Flow BehaviorIsmail IqbalОценок пока нет
- .. Armin Hermann, The Genesis of Quantum Theory (1899-1913)Документ12 страниц.. Armin Hermann, The Genesis of Quantum Theory (1899-1913)P. R. SREENIVASANОценок пока нет
- PLAXIS - 3D2018 Tutorial Lesson 09 PDFДокумент14 страницPLAXIS - 3D2018 Tutorial Lesson 09 PDFMarwan HMОценок пока нет
- Mass Transfer CoefficientsДокумент3 страницыMass Transfer CoefficientsjuandiegoCOОценок пока нет
- Antalgin 2Документ11 страницAntalgin 2lindaОценок пока нет
- DFT Review for Transition Metal ChemistryДокумент60 страницDFT Review for Transition Metal ChemistryGiliandroFariasОценок пока нет
- The Bending Stress Flexure Formula: Formula. We First Write An Expression For The Bending Moment Produced by TheДокумент2 страницыThe Bending Stress Flexure Formula: Formula. We First Write An Expression For The Bending Moment Produced by TheborahajayОценок пока нет
- ARCHIMEDES OF SYRACUSE GRP 3-1Документ12 страницARCHIMEDES OF SYRACUSE GRP 3-1Marvin kakindaОценок пока нет
- Principles of ship stabilityДокумент102 страницыPrinciples of ship stabilityDeepak Kumar75% (4)
- CO oxidation over Ag/Co/Ce mixed oxide catalystsДокумент15 страницCO oxidation over Ag/Co/Ce mixed oxide catalystssencanlisОценок пока нет
- Essential Soil Tests for Building FoundationsДокумент6 страницEssential Soil Tests for Building FoundationsJustin MusopoleОценок пока нет
- Chang Electrochemistry PDFДокумент1 страницаChang Electrochemistry PDFggk2013Оценок пока нет
- Adiabatic Flow in a Duct with FrictionДокумент7 страницAdiabatic Flow in a Duct with FrictioncroprobosОценок пока нет
- 2014 - JPCL - Art - Perovskite Charge Trapping EPRДокумент6 страниц2014 - JPCL - Art - Perovskite Charge Trapping EPRLiang FrankОценок пока нет
- Nuclear Physics: DAE SymposiumДокумент579 страницNuclear Physics: DAE SymposiumESОценок пока нет
- Filled Polyamide 12 Using The Multi Jet Fusion Printing ProcessДокумент21 страницаFilled Polyamide 12 Using The Multi Jet Fusion Printing Processpat151Оценок пока нет
- Standard Test Methods For Maximum Index Density and Unit Weight of Soil Using A Vibratory (ASTM D4253)Документ5 страницStandard Test Methods For Maximum Index Density and Unit Weight of Soil Using A Vibratory (ASTM D4253)April Joy PerezОценок пока нет