Академический Документы
Профессиональный Документы
Культура Документы
The Big Picture
Загружено:
Anonymous oUoJ4A8xОригинальное название
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
The Big Picture
Загружено:
Anonymous oUoJ4A8xАвторское право:
Доступные форматы
When a chemical system achieves equilibrium, it is through a process of energy transfer
between the components, with energetic parts constantly giving energy away. This means that
the probability distribution should favor situations where the energy is spread throughout the
system and disfavor situations where any system component has a great deal of energy. The
exact form of the probability distribution is an exponential function:
or
This equation means that the probability of finding a particle in state i or x (use i for discrete
distributions and x for continuous ones) is proportional to the exponential of -E (minus the
energy of the state) divided by the product of k
B
(Boltzmann's constant) and T (the
temperature in Kelvins). What is meant by a state? Each state corresponds to a set of possible
measured values. For an atom in an ideal gas, each state has particular values of the three
velocity components u
x
, u
y
, and u
z
. States of a molecule can be indexed by the same three
velocity components, plus vibrational and rotational energy levels. Since we know that any
probability distribution must be normalized, we can determine the constants C and C'
explicitly:
or
(The denominator in each case is called the "partition function".) This probability distribution
describes how energy is distributed at equilibrium for an amazingly wide variety of chemical
and physical systems. This probability distribution (in discrete and continuous forms) is the
Maxwell-Boltzmann distribution.
The Big Picture
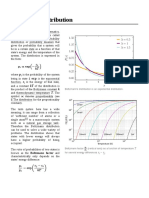
The Maxwell-Boltzmann distribution as shown above gives the probability of finding the
system in any particular state. It applies to the ideal gas, among many other systems. For
the case of the ideal gas, we also want to know the probability of finding an atom with a
particular speed, regardless of direction. The Maxwell-Boltzmann speed distribution
provides this information.
)(((((((((((((((((((\
In physics, particularly statistical mechanics, the MaxwellBoltzmann
distribution or Maxwell distribution describes particle speeds in gases, where the
particles move freely without interacting with one another(except for very
brief elastic collisions in which they may exchange momentum and kinetic energy,
but do not change their respective states of intramolecular excitation, as a function of
the temperature of the system, the mass of the particle, and speed of the particle.)
It is a probability distribution for the speed of a particle constituting the gas -
the magnitude of its velocity vector( meaning that for a given temperature, the
particle will have a speed selected randomly from the distribution, but is more likely
to be within one range of some speeds than others)
The MaxwellBoltzmann distribution applies to ideal gases close to thermodynamic
equilibrium with negligible quantum effects and at non-relativistic speeds.
The distribution is named after James Clerk Maxwell and Ludwig Boltzmann. While
the distribution was first derived by Maxwell in 1860 on basic grounds,[4] Boltzmann
later carried out significant investigations into the physical origins of this distribution.
2 Distributions (various forms)
o 2.1 Distribution for the momentum vector
o 2.2 Distribution for the energy
o 2.3 Distribution for the velocity vector
o 2.4 Distribution for the speed
o 2.5 Distribution for relative speed
o 2.6 Typical speeds
BoseEinstein statistics
In quantum statistics, BoseEinstein statistics (BE statistics) is one of two possible
ways in which a collection of non-interacting indistinguishable particles may occupy a
set of available discrete energy states, at thermodynamic equilibrium.
The aggregation of particles in the same state, which is a characteristic of particles
obeying BoseEinstein statistics, accounts for the cohesive streaming of laser
light and the frictionless creeping of superfluid helium. The theory of this behaviour
was developed (192425) by Satyendra Nath Bose, who recognized that a collection
of identical and indistinguishable particles can be distributed in this way. The idea
was later adopted and extended by Albert Einstein in collaboration with Bose.
The BoseEinstein statistics apply only to those particles not limited to single
occupancy of the same statethat is, particles that do not obey the Pauli
exclusion principle restrictions. Such particles have integer values of spin and are
named bosons, after the statistics that correctly describe their behaviour. There must
also be no significant interaction between the particles.
Concept[edit]
At low temperatures, bosons behave differently from fermions (which obey the FermiDirac statistics)
in that an unlimited number of them can "condense" into the same energy state. This apparently
unusual property also gives rise to the special state of matter Bose Einstein Condensate. Fermi
Dirac and BoseEinstein statistics apply when quantum effects are important and the particles are
"indistinguishable". Quantum effects appear if the concentration of particles satisfies,
where N is the number of particles and V is the volume and n
q
is the quantum concentration, for
which the interparticle distance is equal to the thermal de Broglie wavelength, so that
the wavefunctions of the particles are barely overlapping. FermiDirac statistics apply to
fermions (particles that obey the Pauli exclusion principle), and BoseEinstein statistics
apply to bosons. As the quantum concentration depends on temperature, most systems at high
temperatures obey the classical (MaxwellBoltzmann) limit unless they have a very high density,
as for a white dwarf. Both FermiDirac and BoseEinstein become MaxwellBoltzmann
statistics at high temperature or at low concentration.
BE statistics was introduced for photons in 1924 by Bose and generalized to atoms
by Einstein in 192425.
The expected number of particles in an energy state i for BE statistics is
with
i
> and where n
i
is the number of particles in state i, g
i
is the degeneracy of state i,
i
is the energy of the ith state, is thechemical potential, k is the Boltzmann constant, and T is
absolute temperature. For comparison, the average number of fermions with energy given
by FermiDirac particle-energy distribution has a similar form,
BE statistics reduces to the RayleighJeans Law distribution for ,
namely .
*******************************************************************************************************************88
The partition function is the most important quantity in statistical thermodynamics.
, it indicates how particles in an assembly are partitioned among the
various energy levels of an atom or molecule. The partition function can also, of course,
be calculated by summing over energy states rather than energy levels, i.e.,
Вам также может понравиться
- Boltzmann DistributionДокумент7 страницBoltzmann Distributionhobson616Оценок пока нет
- s m 1.pdf...................................Документ24 страницыs m 1.pdf...................................mahmadidres095Оценок пока нет
- Comparison Between - MB - BE - FDДокумент5 страницComparison Between - MB - BE - FDSoumajit DasОценок пока нет
- Comparison Between Maxwell-Boltzmann, Bose-Einstein and Fermi - Dirac StatisticsДокумент5 страницComparison Between Maxwell-Boltzmann, Bose-Einstein and Fermi - Dirac StatisticsMansoorОценок пока нет
- Physical Applications of The MaxwellДокумент7 страницPhysical Applications of The MaxwellRahul MalhotraОценок пока нет
- Maxwell-Boltzmann Statistics - WikipediaДокумент7 страницMaxwell-Boltzmann Statistics - WikipediaamarmotОценок пока нет
- Bose-Einstein Statistics - WikipediaДокумент5 страницBose-Einstein Statistics - WikipediaSubhas Chandra GangulyОценок пока нет
- Chaos and Quantum Thermalization: Department of Physics University of California Santa Barbara, CA 93106Документ30 страницChaos and Quantum Thermalization: Department of Physics University of California Santa Barbara, CA 93106Anonymous UjdVtuTtqОценок пока нет
- Lect 1Документ43 страницыLect 1Prateek DasОценок пока нет
- Statistical Physics Part IIДокумент39 страницStatistical Physics Part IIfrankythebroОценок пока нет
- Classical Statistics and Quantum StatisticsДокумент12 страницClassical Statistics and Quantum StatisticsGayathri Shunmugiah100% (1)
- SUMSEM2-2018-19 ECE1006 ETH VL2018199000757 Reference Material I 02-Jun-2019 Statistical Mechanics Bose Einstein and Fermi Dirac DistributionsДокумент18 страницSUMSEM2-2018-19 ECE1006 ETH VL2018199000757 Reference Material I 02-Jun-2019 Statistical Mechanics Bose Einstein and Fermi Dirac Distributionsaghil ashokan aghilОценок пока нет
- Nicholas Bigelow - Spins Mixed UpДокумент2 страницыNicholas Bigelow - Spins Mixed UpPomac232Оценок пока нет
- TRM 9Документ82 страницыTRM 9BerhanuОценок пока нет
- Maxwell-Boltzmann Speed DistributionДокумент8 страницMaxwell-Boltzmann Speed DistributionlamyantingОценок пока нет
- On The Transformation of Light Into Heat in Solids IIДокумент19 страницOn The Transformation of Light Into Heat in Solids IIkuhnyfe878100% (1)
- Boltzmann Constant Wiki CCДокумент8 страницBoltzmann Constant Wiki CCjammer999Оценок пока нет
- WM-Lecture-1 180917Документ13 страницWM-Lecture-1 180917Atika AlamОценок пока нет
- BoseДокумент4 страницыBoseQasim AliОценок пока нет
- Fermi Condensates: Markus Greiner, Cindy A. Regal, and Deborah S. JinДокумент9 страницFermi Condensates: Markus Greiner, Cindy A. Regal, and Deborah S. JinHarryОценок пока нет
- Maxwell-Boltzmann DistributionДокумент5 страницMaxwell-Boltzmann DistributionChristian Alic KelleyОценок пока нет
- (Wiki) PhononДокумент24 страницы(Wiki) Phononxyzzy012Оценок пока нет
- The Theory of Relativity: And Other EssaysОт EverandThe Theory of Relativity: And Other EssaysРейтинг: 4.5 из 5 звезд4.5/5 (11)
- PHYSICS-2 Theoretical Questions For Final 2014Документ59 страницPHYSICS-2 Theoretical Questions For Final 2014Meirhan ZhalelОценок пока нет
- Three Additional States of Matter - Plasma, Bose-Einstein Condensate and Fermionic CondensateДокумент6 страницThree Additional States of Matter - Plasma, Bose-Einstein Condensate and Fermionic CondensateCRIZ CATIBOGОценок пока нет
- Quantum Phase Transitions Without Thermodynamic Limits: Keywords: Microcanonical Equilibrium Continuous Phase TransitionДокумент10 страницQuantum Phase Transitions Without Thermodynamic Limits: Keywords: Microcanonical Equilibrium Continuous Phase TransitionacreddiОценок пока нет
- Physical ChenistryДокумент146 страницPhysical ChenistrychemasimОценок пока нет
- AT - Unit 4Документ56 страницAT - Unit 4mayilОценок пока нет
- Einstein - On The Theory of Radiation (1917)Документ15 страницEinstein - On The Theory of Radiation (1917)Ple NaibrudaОценок пока нет
- Susanne La Mura - The Bose-Einstein Condensate: How Information Technology Helps To Push On Physical AchievementsДокумент3 страницыSusanne La Mura - The Bose-Einstein Condensate: How Information Technology Helps To Push On Physical AchievementsLomewcxОценок пока нет
- Lynden-Bell and Tsallis Distributions For The HMF Model: T E P J BДокумент15 страницLynden-Bell and Tsallis Distributions For The HMF Model: T E P J Bbatenas1Оценок пока нет
- A New Relativistic High Temperature Bose-Einstein CondensationДокумент22 страницыA New Relativistic High Temperature Bose-Einstein CondensationDon RiggsОценок пока нет
- Think Physics: Beginner's Guide to an Amazingly Wide Range of Fundamental Physics Related QuestionsОт EverandThink Physics: Beginner's Guide to an Amazingly Wide Range of Fundamental Physics Related QuestionsОценок пока нет
- Plasma (Physics) : Common PlasmasДокумент17 страницPlasma (Physics) : Common PlasmasjspsakjhОценок пока нет
- Michael A. Levin and Xiao-Gang Wen - A Unification of Light and Electrons Through String-Net Condensation in Spin ModelsДокумент8 страницMichael A. Levin and Xiao-Gang Wen - A Unification of Light and Electrons Through String-Net Condensation in Spin ModelsSwertyyОценок пока нет
- Unified Field Theory in a Nutshell1: The Quest for the Theory of EverythingОт EverandUnified Field Theory in a Nutshell1: The Quest for the Theory of EverythingОценок пока нет
- LecturesДокумент221 страницаLecturesHannah TurnerОценок пока нет
- Glos S Ary: A - E: Ads/CftДокумент32 страницыGlos S Ary: A - E: Ads/CftNicole LacraОценок пока нет
- StatisticalPhysics Part1 HandoutДокумент27 страницStatisticalPhysics Part1 HandoutMauro LaraОценок пока нет
- Pauli Exclusion PrincipleДокумент5 страницPauli Exclusion Principlea4477728Оценок пока нет
- Laws and PrinciplesДокумент11 страницLaws and PrinciplesJowayne HudsonОценок пока нет
- Spontaneous Symmetry Breaking in Quantum Field TheoryОт EverandSpontaneous Symmetry Breaking in Quantum Field TheoryРейтинг: 1 из 5 звезд1/5 (1)
- Quantum Phase Transition From A Super Uid To A Mott Insulator in A Gas of Ultracold AtomsДокумент6 страницQuantum Phase Transition From A Super Uid To A Mott Insulator in A Gas of Ultracold AtomsAsai BaraОценок пока нет
- Superstatistics: C. Beck, E.G.D. CohenДокумент9 страницSuperstatistics: C. Beck, E.G.D. CohenbatenasОценок пока нет
- Classical and Quantum Ideal Gases: Abstract Bose and Einstein's Prediction of Bose-Einstein Condensation Came OutДокумент24 страницыClassical and Quantum Ideal Gases: Abstract Bose and Einstein's Prediction of Bose-Einstein Condensation Came Outksva2326Оценок пока нет
- Heavy Ion Collisions ExplainedДокумент5 страницHeavy Ion Collisions ExplainedAakash ShahОценок пока нет
- Periodic Boundary Condition Induced Breakdown of The EquipartitioДокумент10 страницPeriodic Boundary Condition Induced Breakdown of The Equipartitioreza mirzakhaniОценок пока нет
- What is Charge? – The Redefinition of Atom - Energy to Matter ConversionОт EverandWhat is Charge? – The Redefinition of Atom - Energy to Matter ConversionОценок пока нет
- OLIVIER Statistical Physics - Distribution Functions - OS - 2020Документ33 страницыOLIVIER Statistical Physics - Distribution Functions - OS - 2020steveОценок пока нет
- Negative Mass and Negative Refractive Index in Atom Nuclei - Nuclear Wave Equation - Gravitational and Inertial Control: Part 1: Gravitational and Inertial Control, #1От EverandNegative Mass and Negative Refractive Index in Atom Nuclei - Nuclear Wave Equation - Gravitational and Inertial Control: Part 1: Gravitational and Inertial Control, #1Оценок пока нет
- Bose-Einstein CondensateДокумент20 страницBose-Einstein CondensateNirmal BhowmickОценок пока нет
- Design Techincal LGH-F RX5-E 201206 3Документ105 страницDesign Techincal LGH-F RX5-E 201206 3Anonymous oUoJ4A8xОценок пока нет
- 1.fluid Mechanics & MachinesДокумент1 страница1.fluid Mechanics & MachinesAnonymous oUoJ4A8xОценок пока нет
- Part 1-Airconditioning-Ventilation-UserGuide - 1Документ20 страницPart 1-Airconditioning-Ventilation-UserGuide - 1Anonymous oUoJ4A8xОценок пока нет
- Student and employee performance increases with improved ventilationДокумент20 страницStudent and employee performance increases with improved ventilationAnonymous oUoJ4A8xОценок пока нет
- 1.fluid Mechanics & MachinesДокумент1 страница1.fluid Mechanics & MachinesAnonymous oUoJ4A8xОценок пока нет
- Doared Uor, Dnod, D:Od9: Karnataka Pollution ControlДокумент7 страницDoared Uor, Dnod, D:Od9: Karnataka Pollution ControlAnonymous oUoJ4A8xОценок пока нет
- File - Upload: Recharge Your PDF Brochures With FlowpaperДокумент5 страницFile - Upload: Recharge Your PDF Brochures With FlowpaperAnonymous oUoJ4A8xОценок пока нет
- 148Документ1 страница148Anonymous oUoJ4A8xОценок пока нет
- 3Документ4 страницы3Anonymous oUoJ4A8xОценок пока нет
- STP Technical Bid PDFДокумент350 страницSTP Technical Bid PDFvipin KesharwaniОценок пока нет
- 28247Документ8 страниц28247shekharОценок пока нет
- Latest Dated 23.01.20 Progressive Lawyers SocietyДокумент298 страницLatest Dated 23.01.20 Progressive Lawyers SocietyAnonymous oUoJ4A8xОценок пока нет
- SJ Series P1 HitachiДокумент60 страницSJ Series P1 HitachiJohnDОценок пока нет
- 3DX Super & 4DX BrochureДокумент4 страницы3DX Super & 4DX BrochureAnonymous oUoJ4A8xОценок пока нет
- Doared Uor, Dnod, D:Od9: Karnataka Pollution ControlДокумент7 страницDoared Uor, Dnod, D:Od9: Karnataka Pollution ControlAnonymous oUoJ4A8xОценок пока нет
- Membranes: Membrane Technologies in Wastewater Treatment: A ReviewДокумент28 страницMembranes: Membrane Technologies in Wastewater Treatment: A ReviewIbrahim DewaliОценок пока нет
- Classification of Lab Vent Des LevelsДокумент38 страницClassification of Lab Vent Des LevelsMahmood Eijaz100% (1)
- Simple Sewage: Treatment SolutionsДокумент10 страницSimple Sewage: Treatment SolutionsAnonymous oUoJ4A8xОценок пока нет
- Test Equipment Solutions DatasheetДокумент5 страницTest Equipment Solutions DatasheetAnonymous oUoJ4A8xОценок пока нет
- Chapter 316: Dialysis CenterДокумент16 страницChapter 316: Dialysis CenterAnonymous oUoJ4A8xОценок пока нет
- Web - Upload Gtcs 1718juneДокумент3 страницыWeb - Upload Gtcs 1718juneAnonymous oUoJ4A8xОценок пока нет
- EMC Magazine Latest 1Документ46 страницEMC Magazine Latest 1Anonymous oUoJ4A8xОценок пока нет
- Strategic assessment of solid waste management in Kerala citiesДокумент9 страницStrategic assessment of solid waste management in Kerala citiesAnonymous oUoJ4A8xОценок пока нет
- Earth Resistivity Report 2017Документ9 страницEarth Resistivity Report 2017ali omarОценок пока нет
- China AND Football: World Sport'S Newest SuperpowerДокумент13 страницChina AND Football: World Sport'S Newest SuperpowerAnonymous oUoJ4A8xОценок пока нет
- Comparative Advantage As A Success Factor in Football Clubs: Evidence From The English Premier League (EPL)Документ23 страницыComparative Advantage As A Success Factor in Football Clubs: Evidence From The English Premier League (EPL)Anonymous oUoJ4A8xОценок пока нет
- AIFF Constitution DefinitionsДокумент61 страницаAIFF Constitution DefinitionsAnonymous oUoJ4A8xОценок пока нет
- Selection of Main CB and Branch CB Version 22-8-12Документ25 страницSelection of Main CB and Branch CB Version 22-8-12lehaphuong03Оценок пока нет
- INTERNET STANDARDSДокумент18 страницINTERNET STANDARDSDawn HaneyОценок пока нет
- FireДокумент1 страницаFireThunder BirdОценок пока нет
- 1.1 Motivation: X X 2 I J 2 X X X XДокумент4 страницы1.1 Motivation: X X 2 I J 2 X X X XpincoОценок пока нет
- Physics FieldsДокумент731 страницаPhysics FieldsDaisyОценок пока нет
- Matrices and Determinants-03-ExerciseДокумент28 страницMatrices and Determinants-03-ExerciseRaju SinghОценок пока нет
- Ch01 PDFДокумент33 страницыCh01 PDFphooolОценок пока нет
- Dibrugarh University Entrance Examination For M.Sc. in Physics Question PapersДокумент12 страницDibrugarh University Entrance Examination For M.Sc. in Physics Question PapersPeas and Carrots100% (2)
- Thermal PhysicsДокумент9 страницThermal Physicssrivastava_binduОценок пока нет
- QFTlecturesДокумент96 страницQFTlecturesJosé JiménezОценок пока нет
- (Baker A.) Representations of Finite Groups (BookFi)Документ80 страниц(Baker A.) Representations of Finite Groups (BookFi)mastermathОценок пока нет
- Advanced Engineering Mathematics AssignmentДокумент4 страницыAdvanced Engineering Mathematics Assignmentmahdi najafzadehОценок пока нет
- Into The Cool: Energy Flow, Thermodynamics, and Life: Articles You May Be Interested inДокумент2 страницыInto The Cool: Energy Flow, Thermodynamics, and Life: Articles You May Be Interested inemanuel sanchesОценок пока нет
- 2.3 Properties of Determinants CramersДокумент4 страницы2.3 Properties of Determinants CramersChloeОценок пока нет
- Mech LND 18.0 M03 Modal AnalysisДокумент26 страницMech LND 18.0 M03 Modal AnalysisradhouanhmОценок пока нет
- ROM For Nonlinear Structural AnalysisДокумент23 страницыROM For Nonlinear Structural AnalysisLê QuỳnhОценок пока нет
- Hartman-Grobman Theorem and Normal FormsДокумент18 страницHartman-Grobman Theorem and Normal FormslucassecoОценок пока нет
- Heat Conduction From A Sphere To A Stagnant FluidДокумент3 страницыHeat Conduction From A Sphere To A Stagnant FluidMitesh WaghelaОценок пока нет
- Vcde Tutorial Sem-Ii 2022-23Документ12 страницVcde Tutorial Sem-Ii 2022-23Sruthi ChallapalliОценок пока нет
- Lyapunov Stability Analysis of Nonlinear SystemsДокумент28 страницLyapunov Stability Analysis of Nonlinear SystemsGurpreet KaurОценок пока нет
- Statistical Physics Solution ManualДокумент105 страницStatistical Physics Solution Manualgeorgeattack100% (4)
- LO.10 IS PhyДокумент30 страницLO.10 IS PhyAbo Alphotoh GamingОценок пока нет
- MAT100 Semester 1 Cosurse Outline 2023Документ10 страницMAT100 Semester 1 Cosurse Outline 2023chinseuchisomo11Оценок пока нет
- 4 - Conics PDFДокумент11 страниц4 - Conics PDFARADHYA SHARMAОценок пока нет
- Introduction To Heat Transfer: Reference BooksДокумент67 страницIntroduction To Heat Transfer: Reference BooksVaishnavi MandhaniОценок пока нет
- The Ideal - Gas Equation of StateДокумент13 страницThe Ideal - Gas Equation of StateAudu SanusiОценок пока нет
- Lec - 4 Vectors-MatricesДокумент24 страницыLec - 4 Vectors-MatriceskolterarsonОценок пока нет
- Elektrostatik dan Elektrodinamika Konsep DasarДокумент1 страницаElektrostatik dan Elektrodinamika Konsep DasarMelfi NenchiОценок пока нет
- General Thermodynamics and Heat Transfer: MEC121 1 Semester Atty. Edgar Alan DonascoДокумент30 страницGeneral Thermodynamics and Heat Transfer: MEC121 1 Semester Atty. Edgar Alan DonascoMikay OrillanedaОценок пока нет
- 05 - Second Law of Thermodynamic - The Carnot CycleДокумент9 страниц05 - Second Law of Thermodynamic - The Carnot CycleFabio BosioОценок пока нет
- Turbulence Models: Dr.N.V.Mahalakshmi Asst Professor, Department of Mechanical EngineeringДокумент24 страницыTurbulence Models: Dr.N.V.Mahalakshmi Asst Professor, Department of Mechanical EngineeringinfomechОценок пока нет
- Section Course OutlineДокумент3 страницыSection Course OutlineAdnan AKhtarОценок пока нет